Abstract
Objective
We investigated mechanisms involved in iron (Fe) transport by DMT1 (endosomal Fe(II) exporter, encoded by the Nramp2 gene) using wild-type Chinese hamster ovary (CHO) cells and Nramp2-transfected CHO cells, as well as reticulocytes from normal and mk/mk mice that have a defect in DMT1.
Materials and Methods
CHO cells and reticulocytes were incubated with 59Fe bound to various ligands. The radioiron was present in its Fe(II) or Fe(III) forms or bound to transferrin (Tf), and the internalized 59Fe measured under varying experimental conditions. Additionally, 125I-Tf interaction with reticulocytes was investigated and 59Fe incorporation into their heme was determined.
Results
Hyperexpression of DMT1 in CHO cells greatly increases their capacity to acquire ferrous iron. Although CHO-Nramp2 cells showed an increase in Fe(III) uptake as compared to CHO cells, they transported Fe(III) with much lower efficacy than Fe(II). In addition to their defect in Fe uptake, mk/mk reticulocytes also showed a decrease in Tf receptor levels.
Conclusions
Given that CHO cells acquire iron from Fe(II)-ascorbate with much higher rates than from Fe(III)-Tf, Tf-receptor levels represent the rate-limiting step in their iron uptake. As Fe(III) transport by CHO-Nramp2 cells can be inhibited by the impermeable oxidant K3Fe(CN)6, a membrane ferric reductase is probably needed for reduction of Fe(III) to Fe(II), which is then transported by DMT1. DMT1 is not a limiting factor in Fe acquisition by normal reticulocytes and their heme synthesis.
Virtually all organisms possess an absolute requirement for iron because of its unsurpassed catalytic versatility. However, the chemical properties of iron that allow for its usefulness are also responsible for its potential toxicity and, hence, iron metabolism is tightly controlled at both the organismal and cellular levels [1–4]. In vertebrates, iron is transported within the body between sites of absorption, storage, and utilization by the plasma glycoprotein transferrin [1] which binds ferric iron very tightly, but reversibly. Delivery of iron to most cells occurs following the binding of transferrin (Tf) to Tf receptors [5] on the cell membrane. Extracellular -Tf is bound by the membrane-bound Tf receptors and internalized via receptor-mediated endocytosis into an endosome. Iron is released from Tf following a decrease in endosomal pH (reviewed in [1]) and is then transported across the endosomal membrane by DMT1 [6,7]. DMT1 (also known as Nramp2, DCT1 {divalent cation transporter [8]} or Slc11a2), is encoded by a gene that belongs to the “natural resistance macrophage-associated protein” (Nramp)-family of genes identified by Gros and coworkers [9]. Mutations of Nramp2 cause decreased iron uptake by erythroid cells (and possibly other cells) in mice with microcytic anemia (mk/mk) [6] and in anemic Belgrade (b/b) rats [7]. Additionally, several recent reports have demonstrated that DMT1 mutations cause hypochromic microcytic anemias in human patients [10–14]. Because the substrate for DMT1 is ferrous iron [8], reduction of Tf-borne Fe(III) must occur in endosomes. Importantly, Ohgami et al. [15] have recently identified a gene, Steap3 (six transmembrane, epithelial antigen of the prostate 3), whose product is a compelling candidate for endosomal ferric reductase. The protein coded by this gene is highly expressed in hematopoietic tissues, is present in endosomes and colocalizes with Tf, Tf receptors, and DMT1.
Immature erythroid cells are the most avid consumers of iron, most of which is used for hemoglobin synthesis. Although normally all of this iron is delivered via the Tf receptor pathway [16], in vitro experiments revealed that immature erythroid cells can also acquire non-Tf-bound iron in its ferrous form [17,18]. It is likely that the transmembrane Fe2+ transport system in developing erythroid cells reflects the activity of DMT1. Physiologically, all iron in the circulation is Tf-bound and, hence, DMT1 expressed at the plasma membrane of erythroid and other cells has no substrate. Hence, in normal individuals DMT1 can assume its function of Fe2+-transporter only following its recruitment into endosomes where it colocalizes with Tf [19,20].
In this study, we investigated the mechanisms involved in iron transport by DMT1, exploiting Chinese hamster ovary (CHO) cells transfected with mouse Nramp2 gene as well as reticulocytes from wild-type and mk/mk mice. We found that hyperexpression of DMT1 in CHO cells dramatically increases their capacity to acquire ferrous iron. Although Nramp2-transfected CHO cells also display an increase in Fe(III) uptake, it is highly likely that the iron can be transported only following its reduction to Fe(II). Both wild-type Nramp2-transfected CHO cells and normal reticulocytes acquire Fe(II) at rates much higher than that at which iron is taken up physiologically from Tf. These observations suggest that Tf-receptor levels represent a limiting factor in iron uptake by all three cell types examined in this study. Reticulocytes from mk/mk mice show not only a defect in Fe(II) uptake, but also a decrease in iron uptake from Tf and a dramatic inhibition of iron incorporation into heme.
Materials and methods
Cells
CHO cells were transfected with mouse Nramp2 gene as described by Gruenheid et al. [19]. Immunological staining using anti-DMT1 antibody demonstrated that transfected cells (referred to as CHO-Nramp2 cells) exhibited a stable and high expression of DMT1 at the plasma membrane [19]. CHO cells were cultured in minimum essential medium containing 10% fetal calf serum at 37°C in a CO2-incubator; medium for the growth of CHO-Nramp2 cells was supplemented with 1 mg/mL G418.
Reticulocytosis was induced by injecting CD1 mice with neutralized phenylhydrazine (intraperitoneally) at a dose of 50 mg/kg/day for 3 continuous days. On the 3rd or 4th day following the last injection, blood was taken from the heart under ether anesthesia using heparin as anticoagulant, and red blood cells (−45% reticulocytes) were washed three times with ice-cold phosphatebuffered saline (PBS) at 4°C. Mature erythrocytes (containing virtually no reticulocytes) were obtained from untreated CD1 mice, by collecting the bottom quarter of packed red blood cells during the washing procedure.
In some experiments, untreated homozygous mk/mk mice (kindly provided by Dr. Mark Fleming, Harvard University) were used as a source of reticulocytes; control reticulocytes were collected from their heterozygous or wild-type (+/?) counterparts treated with phenylhydrazine as described here. In experiments comparing iron or Tf uptake by reticulocytes from mk/mk and +/? animals, a special effort was made to adjust concentration of reticulocytes in both samples to the same level. This was confirmed by measurement of RNA content [21] in reticulocyte samples from mk/mk and +/? animals.
59Fe uptake by CHO cells
59Fe(II) uptake
Reduction of 59Fe(III) to 59Fe(II) was accomplished using ascorbate as described by Egyed [17] using some modifications. Briefly, 56FeSO4 (10-fold excess) was added to 59FeCl3 in 0.1 M HCl (Amersham, UK), after which the ascorbate solution, deoxygenated by nitrogen gas, was added to make a final iron-to-ascorbate ratio of 1:44. After additional dilution with an appropriate incubation buffer, the 59Fe(II)-ascorbate mixture was used (within 20 minutes) for uptake experiments. Using 1,10-phenanthroline and ferozine, that specifically bind Fe(II) to form spectrophotometrically detectable complexes, we demonstrated that iron remained reduced in the incubation buffer for at least 2 hours.
Preparation of CHO and CHO-Nramp2 cells for 59Fe(II) uptake studies
Cells were collected following treatment with 0.25% trypsin, 1 mM ethylenediamine tetraacetic acid (GIBCO, Toronto, Ontario, Canada), and approximately 8 × 105 cells/well were seeded into six-well plates (Nunclon; VWR Canlab, Mississauga, Ontario, Canada). After about 16 hours preincubation in the medium described here, cells were washed twice with warm PBS, followed by addition of 2 mL deoxygenated warm incubation buffer (25 mM Tris, 25 mM Mes, 140 mM NaCl, 5.4 mM KCl, 5 mM glucose, 1.8 mM CaCl2, pH 5.0 – 7.4) that was prepared as described by Fleming et al. [7], except that MgSO4 was omitted; MgSO4 was found to slightly inhibit Fe(II) uptake by CHO-Nramp2 cells. After adding 59Fe(II)-ascorbate at the indicated concentrations, the uptake was initiated by immediately transferring the plates to the CO2 incubator (37°C), with gentle shaking for 10 minutes. The 59Fe uptake was terminated by three washes with ice-cold PBS. Cells were detached and the membrane-associated 59Fe was removed using 30-minute incubation (4°C) in 2 mL PBS containing 1 mg/mL pronase plus 5 mM ethylenediamine tetraacetic acid [22]. After an additional two washes with PBS, the 59Fe radioactivities in both cell pellets and supernatants were counted using 1282 Compugamma gamma counter (LKB Instruments, Pleasant Hill, CA, USA). The former represented intracellular 59Fe, while the latter accounted for the membrane-bound 59Fe. As no significant difference in either cell number or protein contents was observed between CHO and CHO-Nramp2 cells after 16 hours of preincubation, calculations for Fe uptake were based on the cell numbers at the time of seeding, and expressed as pmole Fe/106 cels. All 59Fe uptake measurements were performed in duplicates; initial control experiments revealed that the 59Fe uptake at 4°C was negligible.
Non-Tf 59Fe(III) uptake
59Fe(III)-citrate (ratio of 1:100) was prepared as described previously [22] and 59Fe(III) uptake experiments were conducted as described for 59Fe(II) uptake studies.
59Fe uptake from 59Fe-Tf
59Fe2-Tf was prepared as described by Martinez-Medellin and Schulman [23]. 59Fe uptake was performed (CO2-incubator, 37°C) in minimum essential medium supplemented with 25 mM HEPES (pH 7.4), 10 mM NaHCO3, and 1% bovine serum albumin, either in the presence of different concentrations of 59Fe2-Tf for 1 hour or in the presence of 2.5 µM 59Fe2-Tf for different time intervals. The 59Fe uptake was terminated by washing cells (three times) with ice-cold PBS. Membrane-associated 59Fe-Tf was removed by pronase (1 mg/mL) treatment (30 minutes/4°C) after which the cells were collected and washed twice with icecold PBS. The radioactivity in cell pellets was counted and the rates of iron uptake expressed as pmoles per 106 cells.
In some experiments intracellular 59Fe distribution in CHO cells, following their incubation with either 59Fe(II)-ascorbate or 59Fe2-Tf, was examined. Lysates of CHO cells were metabolically labeled with 59Fe, separated on 3% to 20% polyacrylamide gradient gels in the presence of Triton X-100 and 59Fe was detected by autoradiography as previously described [24,25]. Briefly, the cell pellet was resuspended in 80 µL lysing solution containing 0.14 M NaCl, 0.1 M HEPES, 1.5% Triton X-100, and 1 mM phenylmethylsulfonyl fluoride (pH 7.4) at 4°C. After vigorous vortexing, the lysates were centrifuged using a microcentrifuge for 15 minutes (4°C). The supernatants (Triton X-100 soluble) were carefully transferred into clean tubes. The 59Fe radioactivities in both the supernatant and pellet (Triton X-100 insoluble) fractions were counted for 59Fe radioactivity. The total supernatants were subjected to gradient gel electrophoresis, after which the gel was dried and autoradiographed, the 59Fe-containing bands cut, and their 59Fe radioactivities measured using a gamma counter. Human 59Fe2-Tf and murine-59Fe ferritin were used as markers and included in the gel in separate lanes. Following the autoradiography, three 59Fe-containing bands could be distinguished; two of them comigrated with 59Fe-Tf and 59Fe-ferritin markers, respectively. Moreover, the identities of 59Fe-Tf and 59Fe-ferritin were confirmed by supershifts of their respective bands using anti-Tf or anti-ferritin antibodies. In addition, 59Fe-radioactivity was also found in a diffuse rapidly migrating band, corresponding to Y-band identified previously in reticulocytes treated with heme synthesis inhibitors [24]; when desferrioxamine was added to the samples before electrophoresis, most of the 59Fe radioactivity found in the rapidly migrating band Y disappeared.
59Fe uptake by reticulocytes and erythrocytes
59Fe(II) uptake was measured using 59Fe(II)-ascorbate (see above) added to a suspension of erythrocytes or reticulocytes (hematocrit −20%) in buffer containing 270 mM sucrose, 4 mM Pipes (pH 4.5 – 7.4) [26].
59Fe uptake from 59Fe-Tf was measured by incubating cells (hematocrit −20%) in minimum essential medium containing 25 mM HEPES (pH 7.4), 10 mM NaHCO3 and 1% bovine serum albumin. At indicated time intervals, cells were thoroughly washed in ice-cold PBS and total cell 59Fe-radioactivity counted. 59Fe incorporated into heme was measured following heme extraction from 59Fe-labeled cells using acid methyl ethyl ketone [27].
125I-Tf cycle in reticulocytes
Iron-saturated 125I-Tf was prepared using Iodo-Bead (Pierce, Rockford, IL, USA) according to manufacturer’s procedure. Iron-saturated Tf and Na125I were obtained from Sigma (St Louis, MO, USA) and ICN Biomedical (Irving, CA, USA), respectively. The rates of cell surface-associated 125I-Tf internalization were measured by incubation of reticulocytes in presence of 2 µM 125I-Tf at 37°C in the same buffer used for 59Fe uptake studies. Samples were taken at different time intervals and immediately transferred into ice-cold PBS to terminate the uptake. After three washes, cell pellets (50 µL) were resuspended in 400 µL 0.25% pronase and incubated at 4°C for 30 minutes to remove the membrane-associated 125I-Tf. 125I-Tf radioactivity in the washed cells represented internalized fraction.
To measure intracellular125I-Tf release, reticulocytes were first incubated in the presence of 2 µM of 125I-Tf at 37°C for 30 minutes. Following three washes with ice-cold PBS at 4°C to eliminate non–cell-associated 125I-Tf, cell pellets were resuspended in the buffer supplemented with 2 µM 56Fe-Tf. The intracellular 125I-Tf release was initiated by warming up the samples at 37°C. Samples were taken at different time intervals and immediately transferred into ice-cold PBS to terminate the release. After centrifugation, the 125I-Tf radioactivities were measured in both cell pellets and supernatant that contained released 125I-Tf.
Scatchard analysis of 125I-Tf binding to TfR was performed as described previously [28]. Briefly, reticulocytes were incubated in the presence of increasing concentrations of 125I-Tf for 2 hours, followed by three washed with ice-cold PBS to remove the unbound 125I-Tf. The radioactivity in cell pellet represented the fraction of cell surface Tf receptor-associated 125I-Tf. The nonspecific binding was obtained in the presence of 50-fold excess of 56Fe2-Tf.
Data analysis
For all data herein, the average of duplicate samples is presented; differences in duplicate samples were <5%. Each experiment was repeated at least three times and representative results are presented. “Day-to-day” variability and logistical constraints generally precluded sophisticated statistical analysis; however, the deviation in relative changes between replicate experiments was typically <3%.
Results
Fe(II) uptake by CHO cells
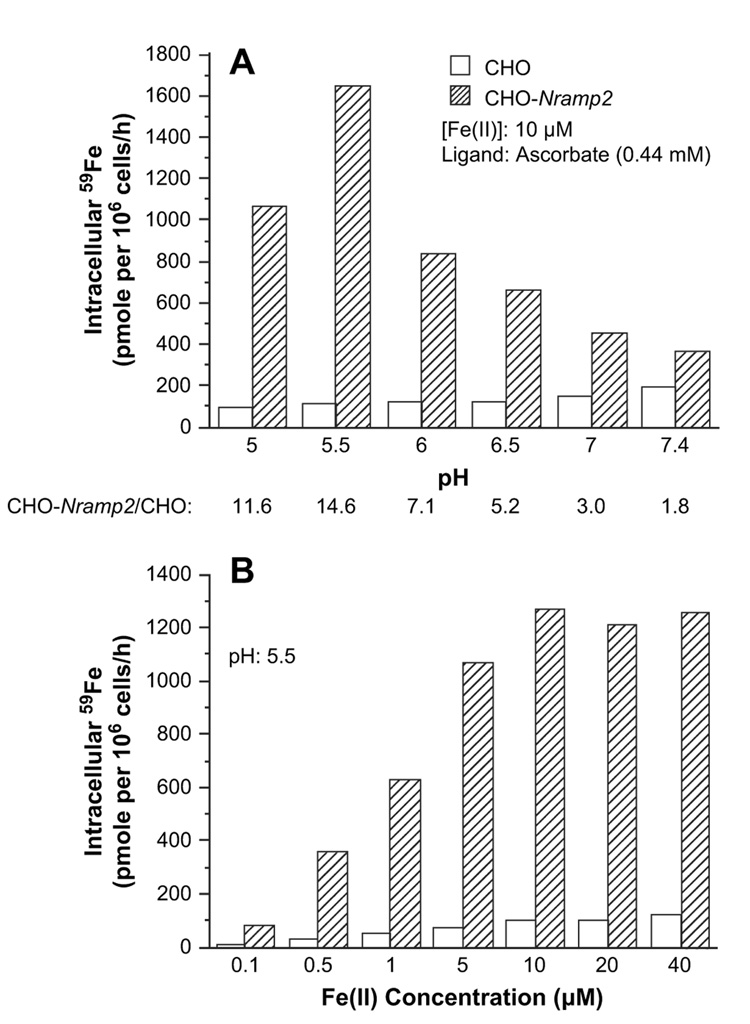
As Fe(II) transport via DMT1 is coupled with proton transport [8], we examined the effect of pH on 59Fe(II) uptake by CHO-Nramp2 cells and CHO cells. Figure 1A shows that in the pH ranging from 5.0 to 7.4, CHO-Nramp2 cells, as compared to wild-type CHO cells, exhibit considerably higher uptake of 59Fe(II). The rate of Fe(II) uptake by CHO-Nramp2 cells is highest at pH 5.5, and at this pH the cells take up about 15-fold more Fe(II) than their untransfected counterparts (Fig. 1A). In both cell types the uptake of Fe(II) is saturable at about 10 µM iron, at which concentration the CHO-Nramp2 cells take up 12.5-fold more Fe(II) than the CHO cells (Fig. 1B). At both saturating and lower concentrations of Fe(II), its uptake by both CHO and CHO-Nramp2 cells is linear for at least 60 minutes (Table 1).
Figure 1.
Ferrous iron uptake by Chinese hamster ovary (CHO) and CHO-Nramp2 cells. (A) pH-dependent uptake of Fe(II). (B) Concentration-dependent uptake of Fe(II).
Table 1.
Kinetics of Fe(II) uptake by CHO and CHO-Nramp2 cells
| Intracellular 59Fe (pmole per 106 cells)* |
||||||
|---|---|---|---|---|---|---|
| Time of incubationy† | 15 minutes | 30 minutes | 60 minutes | |||
| [Fe(II)] | CHO | CHO-Nramp2 | CHO | CHO-Nramp2 | CHO | CHO-Nramp2 |
| 0.1 µM | 0.1 | 23.0 | 3.4 | 45.3 | 4.4 | 73.1 |
| 1.0 µM | 13.1 | 158.3 | 21.3 | 375.5 | 34.9 | 716.8 |
| 10.0 µM | 26.3 | 288.8 | 46.8 | 854.4 | 102.5 | 1814.0 |
CHO = Chinese hamster ovary.
Indicated values represent averages of duplicates
pH of the incubation mixture was 5.5.
Fe(II) uptake by both CHO and CHO-Nramp2 cells is temperature-dependent since incubation at 4°C inhibited the uptake by >97%. Incubation of cells in the presence of rotenone (10 µM) inhibited Fe(II) uptake by CHO-Nramp2 cells by >70%, indicating that Fe(II) transport via DMT1 is an adenosine triphosphate-dependent process (not shown).
As DMT1 expressed in oocytes seems to transport not only Fe2+, but also numerous other divalent metal ions [8], we next investigated the effects of other metals on 59Fe(II) uptake by CHO-Nramp2 cells. Table 2 shows that a variety of divalent metals interfere with ferrous iron uptake by CHO-Nramp2 cells and the order of their inhibitory effects is as follows: Cu > Cd > Co > Mn > Ni. These results confirm that DMT1 displays a broad substrate selectivity [8]. However, we did not observe any significant inhibitory effect of Zn2+ and Pb2+ on ferrous iron uptake by CHO-Nramp2. This finding is in conflict with the observation of Gunshin et al. [8], who reported that DCT1/ DMT1 mediates cellular uptake of Zn2+ and Pb2+. Although some divalent metals (Cd2+, Co2+, Mn2+) inhibited ferrous iron uptake by nontransfected CHO cells, Cu2+, Ni2+, Mg2+, and Zn2+ did not inhibit this process (Table 2); however, Cu2+, at 5 µM and higher concentrations, inhibited ferrous iron uptake by CHO-Nramp2 cells. Even more surprisingly, Cu2+ and Pb2+ stimulated Fe(II) uptake by nontransfected CHO cells (Table 2). It is possible that CHO cells, similarly as immature erythroid cells [26], have two ferrous iron transport systems one of which is represented by DMT1. The second Fe(II) transport system in erythroid cells becomes prominent at higher iron concentrations and is stimulated by KCl, PbCl, LiCl, and CsCl [26]. Experiments are being planned to investigate whether DMT1-independent transport system can account for Pb2+-induced stimulation of Fe(II) uptake by CHO cells.
Table 2.
Effects of various divalent metals on Fe(II) uptake*
| Metals (µM) | CHO cells | CHO-Nramp2 cells |
|---|---|---|
| Cu2+ | ||
| (0.1) | 120.5 | 107.9 |
| (0.5) | 153.1 | 106.9 |
| (1.0) | 164.7 | 109.6 |
| (5.0) | 211.1 | 9.2 |
| (10.0) | 230.5 | 14.2 |
| (50.0) | 505.9 | 25.9 |
| Cd2+ | ||
| (10.0) | 68.3 | 42.1 |
| (50.0) | 36.6 | 12.1 |
| Co2+ | ||
| (10.0) | 91.1 | 85.4 |
| (50.0) | 66.2 | 47.9 |
| Mn2+ | ||
| (10.0) | 95.1 | 92.6 |
| (50.0) | 69.4 | 53.8 |
| (400.0) | 30.9 | 12.5 |
| Ni2+ | ||
| (10.0) | 99.5 | 98.4 |
| (50.0) | 96.5 | 80.4 |
| Mg2+ | ||
| (10.0) | 95.4 | 93.5 |
| (50.0) | 96.1 | 93.5 |
| Zn2+ | ||
| (10.0) | 96.2 | 99.8 |
| (50.0) | 99.7 | 94.9 |
| Pb2+ | ||
| (10.0) | 213.3 | 95.0 |
| (50.0) | 641.0 | 120.0 |
CHO = Chinese hamster ovary.
Percentages of controls incubated (60 minutes) in the absence of metals.
59Fe(II), 10 µM; ascorbate, 0.44 mM; pH 5.5
Fe(III) uptake by CHO cells
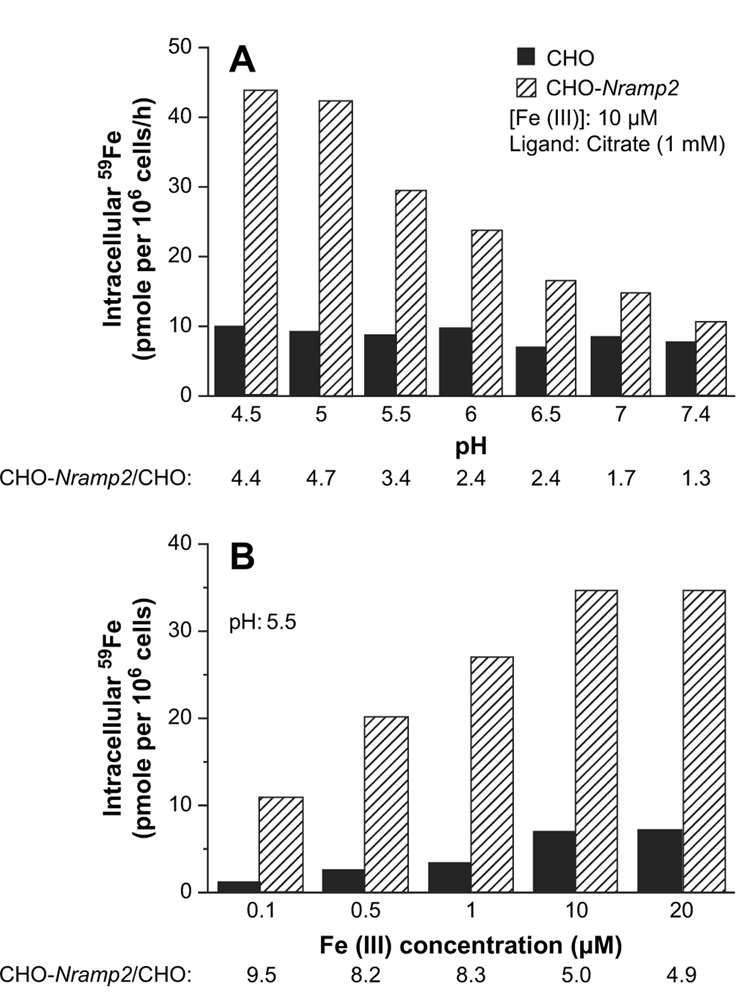
In the next set of experiments, we investigated whether a high expression of DMT1 in CHO cells affects the rate of Fe(III) uptake. Figure 2 demonstrates that Fe(III) uptake, as compared to Fe(II) uptake, exhibits some similarities but also several remarkable differences. Fe(III) uptake by CHO-Nramp2 is higher than that by CHO cells and shows pH dependency, although the maximum uptake occurs at pH as low as 4.5 (Fig. 2A). Fe(III) uptake by both cell lines is saturable at about 10 µM iron concentration (Fig. 2B). However, the quantitative difference between Fe(III) uptake in Nramp2-transfected vs wild-type CHO cells is less prominent than is the case for Fe(II) uptake. Even more importantly, the ratio of Fe(II):Fe(III) uptake by CHO-Nramp2 cells is 57 and 40 at pH 5.5 and 7.4, respectively (compare Fig. 1A and Fig. 2A), indicating that Fe(III) is transported with much lower efficacy.
Figure 2.
Ferric iron uptake by Chinese hamster ovary (CHO) and CHO-Nramp2 cells. (A) pH-dependent uptake of Fe(III). (B) Concentration-dependent uptake of Fe(III).
Fe(III) uptake is temperature-dependent (not shown) as well as adenosine triphosphate-dependent because it can be inhibited by rotenone (Table 3). Importantly, potassium ferricyanide (K3Fe(CN)6), in concentrations as low as 1 µM, causes 70% inhibition of Fe(III) uptake by CHO-Nramp2 cells (Table 3), decreasing the uptake almost to the level seen in wild-type CHO cells. In a control experiment, KCN (1 mM) had virtually no effect on Fe(III) uptake (not shown). Potassium ferricyanide serves as an extracellular “electron sink” and is known to inhibit a membrane ferric-reductase [29,30]. We conclude that DMT1 can transport Fe(III) only following its reduction to Fe(II), a process likely to be mediated by the plasma membrane ferric reductase inhibitable by K3Fe(CN)6. Cu2+ significantly inhibited, and ascorbate dramatically stimulated, Fe(III) uptake by both cell lines (Table 3), providing further support for the idea that reduction of iron is necessary and that DMT1 is involved in the transport.
Table 3.
Effects of various factors on Fe(III) uptake*
| Reagent (µM) | CHO cells | CHO-Nramp2 cells |
|---|---|---|
| Rotenone | ||
| 10.0 | 87.9 | 46.1 |
| 20.0 | 86.7 | 58.4 |
| 40.0 | 70.0 | 52.6 |
| Cu2+ | ||
| 1.0 | 94.5 | 50.2 |
| 5.0 | 91.9 | 20.6 |
| 10.0 | 76.8 | 20.2 |
| 51.0 | 71.1 | 10.4 |
| 100.0 | 48.3 | 6.7 |
| K3Fe(CN)6 | ||
| 0.5 | 87.9 | 46.1 |
| 1.0 | 90.2 | 26.5 |
| 3.3 | 85.3 | 30.1 |
| 10.0 | 85.1 | 25.4 |
| 1000.0 | 59.9 | 19.9 |
| Ascorbate | ||
| 440 | 1138.7 | 2826.0 |
Percentages of controls incubated (60 minutes) in the absence of reagents.
59Fe(III), 10 µM; citrate, 1 mM; pH 5.5.
Tf-derived Fe uptake by CHO cells
As expected, both CHO and CHO-Nramp2 cells were capable of acquiring 59Fe from 59Fe2-Tf by a temperature- and energy-dependent process, and the uptake of 59Fe was linear for at least 3 hours (not shown). CHO and CHO-Nramp2 cells took up Tf-borne iron with identical rates, leading to the accumulation of approximately 13 pmoles Fe/106 cells during a 1-hour incubation at pH 7.4 (Table 4).
Table 4.
59Fe uptake from 59Fe2-Tf*
| Iron uptake (pmol/106 cells/h) |
|||
|---|---|---|---|
| Experiment 1† | Experiment 2† | Average | |
| CHO cells | 14.9 | 12.1 | 13.5 |
| CHO-Nramp2 cells | 14.1 | 11.4 | 12.7 |
CHO = Chinese hamster ovary; Fe = iron; Tf = transferrin.
10 µM Tf; 20 µM Fe.
Indicated values represent averages of duplicates
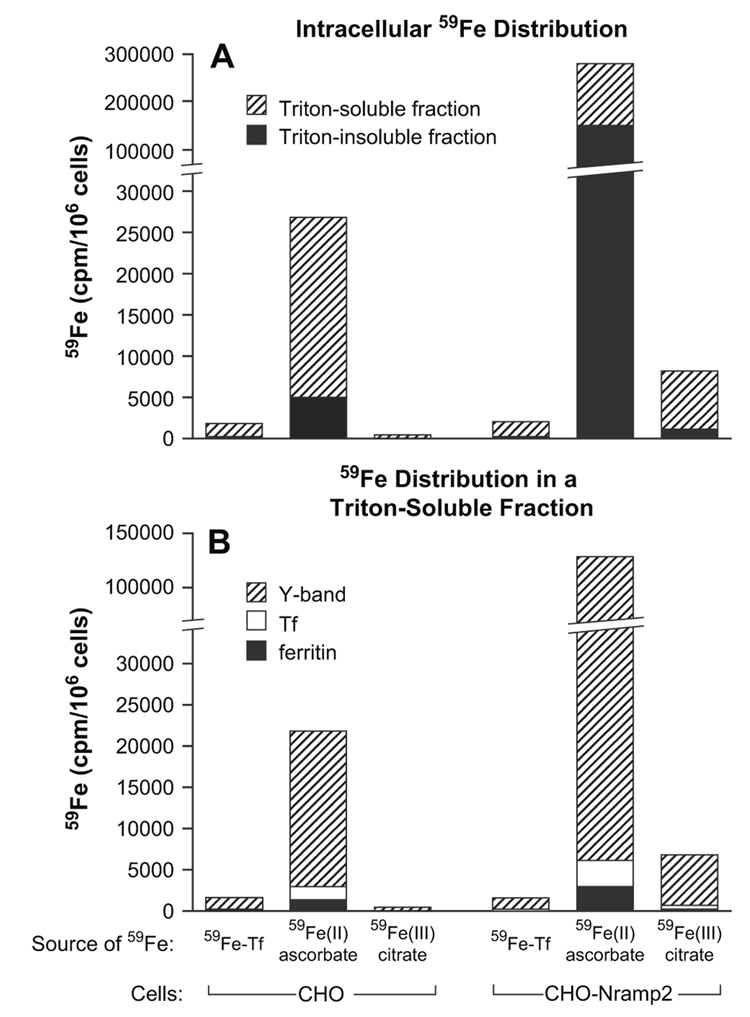
Figure 3 shows that about 80% of 59Fe taken up by wildtype CHO cells, following 1-hour incubation with 59Fe-Tf, can be found in a Triton X-100–soluble fraction, 10% of which appears in ferritin. On the other hand, after 1-hour incubation of CHO-Nramp2 cells with 59Fe(II)-ascorbate, only 45% of 59Fe is in Triton X-100-soluble fraction, and 2% of this 59Fe appears in ferritin; the vast majority of 59Fe is found in an as yet unidentified fraction with fast mobility upon native electrophoresis [24,25]; 59Fe in this fraction can be readily chelated by desferrioxamine.
Figure 3.
59Fe distribution in Chinese hamster ovary (CHO) or CHO-Nramp2 cells incubated with various sources of iron. Cells were incubated with 59Fe2-transferrin (Tf) (5 µM), 59Fe(II)-ascorbate (10 µM) or 59FeIII)-citrate (10 µM) for 1 hour, washed, and cell pellets solubilized as described in Materials and Methods. 59Fe radioactivity was measured in Triton X- 100 insoluble and soluble fractions (A), following which soluble fractions were subjected to native gradient gel electrophoresis and the 59Fe radioactivity in Y-band, Tf and ferritin (B) measured as described in Materials and Methods.
Fe uptake by mouse reticulocytes and erythrocytes
As expected (Table 5), mature erythrocytes are unable to take up iron from Tf, but they have some, though very limited, capacity to incorporate 59Fe from 59Fe(II)-ascorbate. As compared to erythrocytes, reticulocytes take up ferrous iron (likely via DMT1) with about a 14-fold higher efficiency. Reticulocytes acquire iron from ferrous-ascorbate much more efficiently (eightfold) than from Fe2-Tf. Most (86%) of Tf-borne iron is used for heme synthesis, whereas only 8% of the iron derived from Fe(II)-ascorbate appears in heme (Table 5). Even more importantly, absolute amount of iron used for heme synthesis is about 30% lower when the cells are offered non-Tf, Fe(II), as a substrate (Table 5).
Table 5.
Iron uptake by mouse reticulocytes and erythrocytes
| Source of 59Fe | 59Fe incorporation (pmoles/106 cells/h) | |||
|---|---|---|---|---|
| Reticulocytes |
Mature erythrocytes |
|||
| Total | Heme | Total | Heme | |
| 59Fe-Tf (10 µM) | 6.5 | 5.6 | 0.9 | 0 |
| 59Fe-ascorbate (20 µM) | 52.8 | 4.0 | 3.6 | 0 |
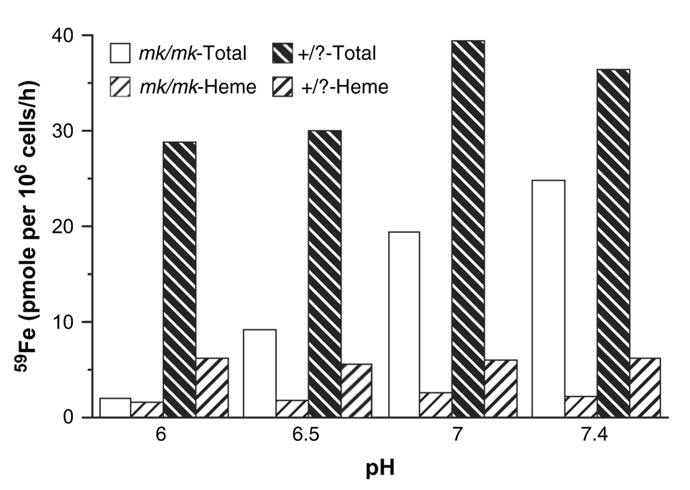
Fe(II) uptake by normal reticulocytes follows a pattern of pH-dependency, with a maximumuptake at pH 7 (Fig. 4) that is different from that described for CHO-Nramp2 cells (Fig. 1A). However, it should be noted that CHO-Nramp2 cells were incubated in a buffer containing only salt that caused hemolysis of reticulocytes below pH 7. Therefore, for ferrous uptake studies, reticulocytes were incubated in a buffer containing a high concentration of sucrose [26] that did not cause hemolysis. Figure 4 shows that reticulocytes derived from mk/mk mice exhibited a significant decrease in their capacity to acquire ferrous iron, and the uptake decreased progressively with decreasing pH.
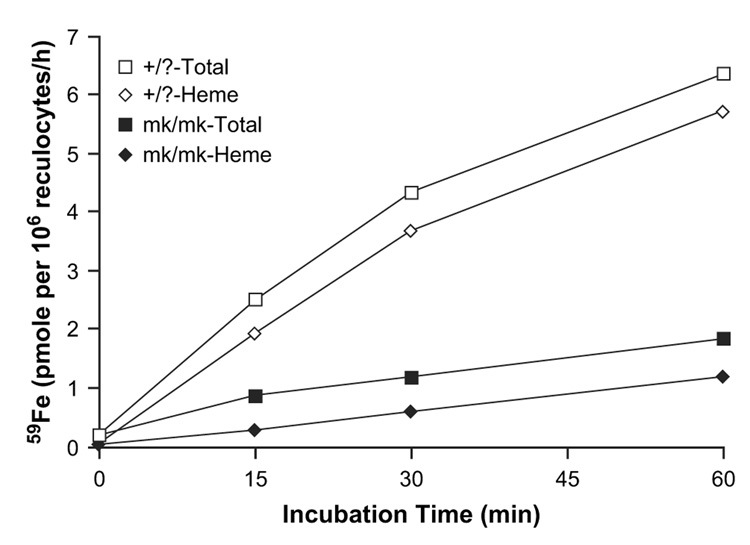
Figure 4.
59Fe(II) uptake by reticulocytes obtained from mk/mk and +/? reticulocytes. The cells were incubated with 59Fe(II)-ascorbate (20 µM and 0.88 mM, respectively) for 60 minutes at 37°C. 59Fe-heme was extracted using acid methyl ethyl ketone [27].
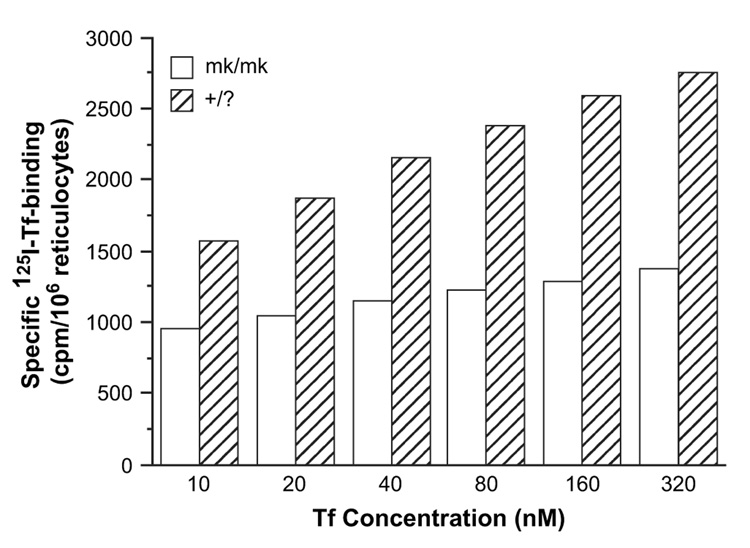
Reticulocytes from mk/mk mice, as compared to those from +/? animals, show about a 70% lower rate of iron uptake from Tf and use iron for heme synthesis less efficiently; after a 1-hour incubation wild-type reticulocytes used about 90% 59Fe for heme synthesis while reticulocytes from mk/mk mice used only about 65% (Fig. 5). Experiments were next designed to investigate whether the decrease in iron uptake from Tf by mk/mk reticulocytes could be accounted for only by defective DMT1 [6] or whether the reticulocytes from mk/mk animals had additional defects. We examined the rate of 125I-Tf internalization, 125I-Tf release and the affinity of Tf to Tf receptors and found no difference in these parameters in reticulocytes from mk/mk mice as compared to those from +/? animals (not shown). However, we unexpectedly found that the amount of 125I-Tf bound to surface receptors in reticulocytes from mk/mk mice was significantly reduced (Fig. 6), suggesting either an overall decrease in Tf receptor levels or a decrease in the presence of receptors at the cell surface.
Figure 5.
59Fe uptake from 59Fe-transferrin (Tf) (10 µM) by reticulocytes from mk/mk and +/? reticulocytes. 59Fe-heme was extracted using acid methyl ethyl ketone [27].
Figure 6.
Specific 125I-transferrin (Tf) binding to membranes of reticulocytes from mk/mk and +/? mice.
Discussion
DMT1 has recently been identified as the first transmembrane iron transporter [3,4,6–8]. It plays a dual role in transporting inorganic iron across the apical membrane of enterocytes as well as in translocating Tf-derived iron through endosomal membrane. In the first part of this study we investigated the uptake of iron from various sources by wild-type CHO cells and those expressing high levels of DMT1 in order to further characterize this transporter’s function and evaluate its involvement in overall cellular iron metabolism.
We have demonstrated that DMT1 efficiently transports ferrous iron into the cells that express high levels of this protein. The transport is temperature-, energy-, and pH-dependent. The highest iron-transporting activity occurs at pH 5.5, which is the pH needed for iron release from Tf in endosomes. These results corroborate previous findings of Gunshin et al. [8]. We also confirmed that several divalent metals (Cu, Cd, Co, Mn, Ni) are likely to be transported by DMT1 because they inhibit its Fe(II)-transporting activity. However, in contrast with the earlier report [8], we did not find inhibition of Fe(II) uptake by CHO-Nramp2 cells in the presence of Zn2+ and Pb2+. This discrepancy suggests that cell-specific factors (oocytes, ref. [8] or CHO cells, this study) may modulate the transport function of DMT1.
We also compared CHO cells and CHO-Nramp2 cells for their capacity to take up Fe(III) from ferric citrate. Somewhat surprisingly, we demonstrated that CHO-Nramp2 cells, as compared to their untransfected counterparts, have about fivefold higher uptake of iron offered in its ferric form. However, it is highly unlikely that DMT1 transports Fe(III) directly, and this conclusion is based on the following observations: Ferric iron transport by CHO-Nramp2 cells can be inhibited by the impermeable oxidant potassium ferricyanide, that inhibits the plasma membrane ferric reductase [29,30]. This result strongly suggests that Fe(III) has to be first reduced to Fe(II), which can then be transported by DMT1. Additionally, Cu2+, which inhibits Fe(II) transport by DMT1, significantly inhibits iron uptake (offered as ferric-citrate) by CHO-Nramp2 cells. It is possible that at least some ferric iron, taken up by CHO-Nramp2 cells, is reduced by Steap3; although most of this protein is localized intracellularly, a small fraction of Steap3 appears on the plasma membranes (Dr. Mark Fleming, personal communication). However, it is unknown whether this reductase is inhibitable by ferricyanide. In any case, these results are relevant to the mechanism of tissue iron uptake of non–transferrin-bound iron that is present in plasma of patients with severe iron overload [31–33].
Importantly, CHO and CHO-Nramp2 cells take up iron from Tf with identical rates (Table 4) that are much lower than the rates with which these cells take up ferrous iron (Fig. 1). These results strongly indicate that iron-transporting capacity of DMT1, even in wild-type CHO cells, greatly exceeds the iron-transporting capacity of the Tf-receptor pathway. In other words, Tf-receptor levels represent the rate-limiting step in iron uptake via the physiological Tf-dependent pathway. Moreover, intracellular 59Fe distribution is grossly disturbed when 59Fe is offered in the form of 59Fe(II)-ascorbate, in particular in CHO-Nramp2 cells (Fig. 3). About 85% of 59Fe taken up (60 minutes) from 59Fe2-Tf by wild-type CHO cells can be recovered in the Triton X-100–soluble fraction. However, CHO-Nramp2 cells following their incubation (60 minutes) with 59Fe(II)-ascorbate contain only 45% of their 59Fe in a Triton X100-soluble fraction, most of which (95%) can be found in the ill-defined fraction Y [24,25] with a high mobility following polyacrylamide gel electrophoresis (Fig. 3). In CHO-Nramp2 cells, provided with 59Fe(II), radioiron probably accumulates in this fraction, since the uptake of iron greatly exceeded the iron-storing capacity of ferritin; as compared with CHO cells, CHO-Nramp2 cells supplied with 59Fe(II) show a 6.5-fold increase of 59Fe-radioactivity in the fraction Y, but only a twofold increase in ferritin (Fig. 3).
In the second part of this study we examined uptake of iron, using either Fe2-Tf or Fe(II)-ascorbate as sources, by reticulocytes obtained from wild-type or mk/mk mice. Reticulocytes, as compared to mature erythrocytes, take up ferrous iron with about 15-fold higher efficacy, suggesting that DMT1 either disappears or becomes inactive with reticulocyte maturation (Fig. 4). Although reticulocytes can incorporate some 59Fe into heme when incubated with 59Fe(II)-ascorbate, the efficiency with which they utilize for heme synthesis 59Fe derived from 59Fe2-Tf, is significantly higher. This indicates that the Tf-receptor pathway plays an important role in the efficient targeting of iron towards mitochondria. As is the case with CHO cells, levels of Tf receptors in reticulocytes seem to represent the rate-limiting step in iron acquisition from Tf because the maximum iron uptake from Fe(II)-ascorbate is about eightfold higher than that from Fe2-Tf.
As expected [6,34] reticulocytes from mk/mk mice, as compared to those from normal mice, exhibit a defect in Fe(II) uptake that is most prominent at pH 6. The decrease is so dramatic that it could produce a limiting step for iron uptake via the Tf-receptor pathway. In fact, iron uptake from Tf is also dramatically decreased in reticulocytes from mk/mk mice (Fig. 5), but the decreased DMT1 activity is probably not the only factor responsible for this defect. Our finding of lower cell surface binding of 125I-Tf (Fig. 6), suggests that there may be a decrease in total Tf receptors levels in these mice. This would be consistent with our previous finding that inhibitors of heme synthesis decrease Tf receptor expression in induced murine erythroleukemia cells [35]. However, Garrick et al. have previously shown an increase in Tf receptor levels in reticulocytes from Belgrade rats [28]. Further evidence is needed to determine whether the total levels of Tf receptors are indeed decreased in mk/mk reticulocytes or if there is another cause for our observation of decreased surface Tf binding, such as retention of a subpopulation of noncycling Tf receptors within the cells.
Acknowledgments
Supported by grants from Canadian Institutes of Health Research (MT-14100) (to PP), the National Institutes of Health grant DK080765 (to A–S Z) and the National Institute of Allergy and Infections Diseases grant (AI 35327) (to P.G.). P.G. is a Senior Scientist of Canadian Institutes of Health Research. The authors thank Sandy Fraiberg for excellent editorial assistance and Dr. Alex Sheftel and Shan Soe-Lin for helpful comments.
References
- 1.Richardson DR, Ponka P. The molecular mechanisms of the metabolism and transport of iron in normal and neoplastic cells. Biochim Biophys Acta. 1997;1331:1–40. doi: 10.1016/s0304-4157(96)00014-7. [DOI] [PubMed] [Google Scholar]
- 2.Eaton JW, Qian M. Molecular bases of cellular iron toxicity. Free Radic Biol Med. 2002;32:833–840. doi: 10.1016/s0891-5849(02)00772-4. [DOI] [PubMed] [Google Scholar]
- 3.Hentze MW, Muckenthaler MU, Andrews NC. Balancing acts: molecular control of mammalian iron metabolism. Cell. 2004;117:285–297. doi: 10.1016/s0092-8674(04)00343-5. [DOI] [PubMed] [Google Scholar]
- 4.De Domenico I, McVey Ward D, Kaplan J. Regulation of iron acquisition and storage: consequences for iron-linked disorders. Nat Rev Mol Cell Biol. 2008;9:72–81. doi: 10.1038/nrm2295. [DOI] [PubMed] [Google Scholar]
- 5.Ponka P, Lok CN. The transferrin receptor: role in health and disease. Int J Biochem Cell Biol. 1999;31:1111–1137. doi: 10.1016/s1357-2725(99)00070-9. [DOI] [PubMed] [Google Scholar]
- 6.Fleming MD, Trencor CC, 3rd, Su MA, et al. cytic anaemia mice have a mutation in Nramp2, a candidate iron transporter gene. Nat Genet. 1997;16:383–386. doi: 10.1038/ng0897-383. [DOI] [PubMed] [Google Scholar]
- 7.Fleming MD, Romano MA, Su MA, Garrick LM, Garrick MD, Andrews NC. Nramp2 is mutated in the anemic Belgrade (b) rat: evidence of a role for Nramp2 in endosomal iron transport. Proc Natl Acad Sci U S A. 1998;95:1148–1153. doi: 10.1073/pnas.95.3.1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gunshin H, Mackenzie B, Berger UV, et al. Cloning and characterization of a mammalian proton-coupled metal-ion transporter. Nature. 1997;388:482–488. doi: 10.1038/41343. [DOI] [PubMed] [Google Scholar]
- 9.Cellier M, Prive G, Belouchi A, et al. Nramp defines a family of membrane proteins. Proc Natl Acad Sci U S A. 1995;92:10089–10093. doi: 10.1073/pnas.92.22.10089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mims MP, Guan Y, Pospisilova D, et al. Identification of a human mutation of DMT1 in a patient with microcytic anemia and iron overload. Blood. 2005;105:1337–1342. doi: 10.1182/blood-2004-07-2966. [DOI] [PubMed] [Google Scholar]
- 11.Priwitzerova M, Nie G, Sheftel AD, Pospisilova D, Divoky V, Ponka P. Functional consequences of the human DMT1 (SLC11A2) mutation on protein expression and iron uptake. Blood. 2005;106:3985–3987. doi: 10.1182/blood-2005-04-1550. [DOI] [PubMed] [Google Scholar]
- 12.Iolascon A, d’Apolito M, Servedio V, Cimmino F, Piga A, Camaschella C. Microcytic anemia and hepatic iron overload in a child with compound heterozygous mutations in DMT1 (SCL11A2) Blood. 2006;107:349–354. doi: 10.1182/blood-2005-06-2477. [DOI] [PubMed] [Google Scholar]
- 13.Beaumont C, Delaunay J, Hetet G, Grandchamp B, de Montalembert M, Tchernia G. Two new human DMT1 gene mutations in a patient with microcytic anemia, low ferritinemia, and liver iron overload. Blood. 2006;107:4168–4170. doi: 10.1182/blood-2005-10-4269. [DOI] [PubMed] [Google Scholar]
- 14.Iolascon A, Camaschella C, Pospisilova D, Piscopo C, Tchernia G, Beaumont C. Natural history of recessive inheritance of DMT1 mutations. J Pediatr. 2008;152:136–139. doi: 10.1016/j.jpeds.2007.08.041. [DOI] [PubMed] [Google Scholar]
- 15.Ohgami RS, Campagna DR, Greer EL, et al. Identification of a ferri reductase required for efficient transferrin-dependent iron uptake on erythroid cells. Nat Genet. 2005;37:1264–1269. doi: 10.1038/ng1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ponka P. Tissue-specific regulation of iron metabolism ad heme synthesis: distinct control mechanisms in erythroid cells. Blood. 1997;89:1–25. [PubMed] [Google Scholar]
- 17.Egyed A. Carrier mediated iron transport through erythroid cell membrane. Br J Haematol. 1988;68:483–486. doi: 10.1111/j.1365-2141.1988.tb04241.x. [DOI] [PubMed] [Google Scholar]
- 18.Morgan EH. Membrane transport of non-transferrin-bound iron by reticulocytes. Biochim Biophys Acta. 1988;943:428–439. doi: 10.1016/0005-2736(88)90374-4. [DOI] [PubMed] [Google Scholar]
- 19.Gruenheid S, Canonne-Hergaux F, Gauthier S, Hackam DJ, Grinstein S, Gros P. The iron transport protein Nramp2 is an integral membrane glycoprotein that colocalizes with transferrin in recycling endosomes. J Exp Med. 1999;189:831–841. doi: 10.1084/jem.189.5.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Canonne-Hergaux F, Zhang AS, Ponka P, Gros P. Characterization of the iron transporter DMT1 (NRAMP2/DCT1) in red blood cells of normal and anemic mk/mk mice. Blood. 2001;98:3823–3830. doi: 10.1182/blood.v98.13.3823. [DOI] [PubMed] [Google Scholar]
- 21.Schneider WC. Determination of nucleic acids in tissues by pentose analysis. Methods Enzymol. 1957;3:680–684. [Google Scholar]
- 22.Graham RM, Morgan EH, Baker E. Ferric citrate uptake by cultured rat hepatocytes is inhibited in the presence of transferrin. Eur J Biochem. 1998;253:139–145. doi: 10.1046/j.1432-1327.1998.2530139.x. [DOI] [PubMed] [Google Scholar]
- 23.Martinez-Medellin J, Schulman HM. The kinetics of iron and transferring incorporation into rabbit erythroid cells and the nature of stromal-bound iron. Biochim Biophys Acta. 1972;264:272–274. doi: 10.1016/0304-4165(72)90291-7. [DOI] [PubMed] [Google Scholar]
- 24.Richardson DR, Ponka P, Vyoral D. Distribution of iron in reticulocytes after inhibition of heme synthesis with succinylacetone: examination of the intermediates involved in iron metabolism. Blood. 1996;87:3477–3488. [PubMed] [Google Scholar]
- 25.Vyoral D, Petrak J, Hradilek A. Separation of cellular iron containing compounds by electrophoresis. Biol Trace Elem Res. 1998;61:263–275. doi: 10.1007/BF02789087. [DOI] [PubMed] [Google Scholar]
- 26.Hodgson LL, Quail EA, Morgan EH. Iron transport mechanisms in reticulocytes and mature erythrocytes. J Cell Physiol. 1995;162:181–190. doi: 10.1002/jcp.1041620204. [DOI] [PubMed] [Google Scholar]
- 27.Teale FW. Cleavage of the haem-protein link by acid methyl-ketones. Biochim Biophys Acta. 1959;35:543. doi: 10.1016/0006-3002(59)90407-x. [DOI] [PubMed] [Google Scholar]
- 28.Garrick MD, Gniecko K, Liu Y, Cohan DS, Garrick LM. Transferrin and the transferrin cycle in Belgrade rat reticulocytes. J Biol Chem. 1993;268:14867–14874. [PubMed] [Google Scholar]
- 29.Sun IL, Navas P, Crane FL, Morre DJ, Low H. NADH diferric transferring reductase in liver plasma membrane. J Biol Chem. 1987;262:15915–15921. [PubMed] [Google Scholar]
- 30.Sun IL, Crane FL, Grebing C, Low H. Properties of a transplasma membrane electron transport system in HeLa cells. J Bioenerg Biomembr. 1984;16:583–595. doi: 10.1007/BF00743247. [DOI] [PubMed] [Google Scholar]
- 31.Hershko C, Graham G, Bates GW, Rachmilewitz EA. Non-specific serum iron in thalassaemia: an abnormal serum iron fraction of potential toxicity. Br J Haematol. 1978;40:255–263. doi: 10.1111/j.1365-2141.1978.tb03662.x. [DOI] [PubMed] [Google Scholar]
- 32.Brissot P, Loréal O. Role of non-transferrin-bound iron in the pathogenesis of iron overload and toxicity. Adv Exp Med Biol. 2002;509:45–53. doi: 10.1007/978-1-4615-0593-8_3. [DOI] [PubMed] [Google Scholar]
- 33.Cabantchik ZI, Breuer W, Zanninelli G, Cianciulli P. LPI-labile plasma iron in iron overload. Best Pract Res Clin Haematol. 2005;18:277–287. doi: 10.1016/j.beha.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 34.Su MA, Trenor CC, Fleming JC, Fleming MD, Andrews NC. The G185R mutation disrupts function of the iron transporter Nramp2. Blood. 1998;92:2157–2163. [PubMed] [Google Scholar]
- 35.Chan RY, Seiser C, Schulman HM, Kuhn LC, Ponka P. Regulation of transferrin receptor mRNA expression. Distinct regulatory features in erythroid cells. Eur J Biochem. 1994;220:683–692. doi: 10.1111/j.1432-1033.1994.tb18669.x. [DOI] [PubMed] [Google Scholar]