Abstract
Human follicle development requires the recruitment of primordial follicles into a cohort of growing follicles from which one follicle is selected to ovulate a mature oocyte. During this developmental process, complex endocrine and intraovarian paracrine signals create a changing intrafollicular hormonal mileu. With this microenvironment, appropriate cumulus cell-oocyte signaling governs oocyte developmental competence, defined as the ability of the oocyte to complete meiosis and undergo fertilization, embryogenesis and term development. Many of these mechanisms are perturbed in polycystic ovary syndrome (PCOS), a heterogeneous syndrome characterized by ovarian hyperandrogenism, hyperinsulinemia from insulin resistance and reduced fecundity. In addition to these endocrinopathies, PCOS also is characterized by paracrine dysregulation of follicle development by intraovarian proteins of the transforming growth factor β (TGFβ) family. Consequently, PCOS patients undergoing ovarian stimulation for in vitro fertilization (IVF) are at increased risks of impaired oocyte developmental competence, implantation failure and pregnancy loss. Recent data demonstrate links between endocrine/paracrine factors and oocyte gene expression in PCOS and suggest that new clinical strategies to optimize developmental competence of PCOS oocytes should target correction of the entire follicle growth and oocyte development process.
Keywords: Polycystic ovary syndrome, follicle development, hyperandrogenism, hyperinsulinemia, oocyte maturation
Introduction
Human follicle development is an ordered process, in which primordial follicles are recruited into a cohort of growing follicles, from which one follicle is selected to ovulate a mature oocyte. Theca cell-granulosa cell interactions, intraovarian paracrine signals and oocyte secreted factors control preantral and early antral follicle development, and are regulated by several factors 1, including transforming growth factor β (TGFβ)-related proteins 2-4. Circulating gonadotropins and intraovarian paracrine signals govern subsequent antral follicle development. Throughout folliculogenesis, a changing intrafollicular microenvironment established by various proteins, steroids, energy metabolites, cytokines and growth factors 5 appropriately coordinates follicle growth and oocyte development 6. Within this intrafollicular microenvironment, appropriate cumulus cell-oocyte signaling induces the acquisition of oocyte developmental competence, defined as the ability of the oocyte to complete meiosis and undergo fertilization, embryogenesis and term development 7.
Many of these mechanisms are perturbed in polycystic ovary syndrome (PCOS), a heterogeneous syndrome characterized by luteinizing hormone (LH) hypersecretion, ovarian hyperandrogenism, polycystic ovaries, hyperinsulinemia from insulin resistance and reduced fecundity 8. PCOS patients undergoing ovarian stimulation for in vitro fertilization (IVF) have increased risks of impaired oocyte developmental competence, implantation failure and pregnancy loss 9-11. Moreover, obese PCOS patients experience low oocyte fertilization and failure of embryos to implant in their own uterus or those of their surrogates, implying impaired development competence of some PCOS oocytes 12. This chapter addresses crucial endocrine and intraovarian paracrine mechanisms governing follicular development and discusses how PCOS-related alterations of these mechanisms may impair oocyte developmental competence.
Follicular Development
The primordial follicle comprises an oocyte arrested in meiotic prophase I and surrounded by squamous granulosa cells (i.e., a germinal vesicle [GV] stage oocyte). When the primordial follicle initiates growth, its oocyte undergoes changes in messenger ribonucleic acid (mRNA) expression as its squamous granulosa cells enlarge into a single layer of cuboidal granulosa cells (i.e., primary follicle) 13, 14. With continued granulosa cell proliferation into several layers, the secondary follicle is formed. Theca cells organize into distinct layers around the follicle and establish mesenchymal-epithelial cell interactions that promote development of the follicle and its oocyte.
Primordial follicle growth is only minimally dependent upon follicle-stimulating hormone (FSH) and is primarily influenced by paracrine/endocrine factors 14. Granulosa cell-derived paracrine factors either can activate resting primordial follicles (e.g., kit ligand, transforming growth factor-α, epidermal growth factor) or can inhibit them (i.e., anti-mullerian hormone [AMH]), and may originate locally or from neighboring growing follicles responsive to FSH 1, 14,15. Oocyte-derived factors (growth differentiation factor 9 [GDF9)], bone morphogenetic protein 15 [BMP15]) also control follicle growth 2-4. These granulosa cell-oocyte interactions cause preantral follicles to develop over several months; to acquire FSH, estrogen and androgen receptors; and to become physiologically coupled by gap junctions 14, 16, 17.
Antral follicle formation is accompanied by diminished oocyte growth (reaching a maximum diameter of 140 μm), creation of extracellular fluid and differentiation of granulosa cell layers into mural and cumulus cell subpopulations 13, 14. Human antral follicles 2-5 mm in size become responsive to FSH 16, while those 6-8 mm in size acquire aromatase activity 18, allowing androgens produced by LH-stimulated theca cells to undergo aromatization to estrogens by FSH-stimulated granulosa cells. Estrogen production is facilitated by granulosa cell-derived paracrine regulation of theca cell P450 c17 activity 19, with inhibins and insulin-like growth factor (IGF)1 stimulating aromatizable androgen production, and follistatin binding activin and inhibiting its androgen-suppressing effect 20. With the ability of FSH to induce LH receptors on granulosa cells, allowing them to respond to both gonadotropins, the maturing follicle continues growth and steroidogenesis despite declining FSH levels in the circulation before the midcycle LH surge 16, 21.
With the onset of the midcycle LH surge, the preovulatory follicle shifts steroidogenesis from androgen and estrogen to progesterone production during final oocyte maturation 16. In reproductive-aged women, hundreds of primordial follicles initiate growth, 10-20 selectable antral follicles remain at the beginning of the normal cycle, but just one normally proceeds to ovulation 13. Follicular growth from primordial to preovulatory stage takes approximately 6 months, with the final 2 weeks of follicular development dependent upon changes in circulating gonadotropin levels 16.
Oocyte maturation
In mammals, developmentally competent oocytes have the necessary molecular components to complete meiosis, enter the mitotic cell cycle, create an embryonic genome, modify its chromatin structure, and transcribe the correct genes to begin the developmental program 22. These processes depend upon maternal mRNAs, proteins, and other molecular components of the oocyte acquired during its development. Acquisition of oocyte developmental competence occurs progressively throughout oocyte growth and maturation 23, 24 and is closely coordinated with follicular development 6.
With recruitment of primordial follicles into the growing preantral follicle pool, mammalian GV oocytes undergo intensive mRNA synthesis 25 and other structural as well as functional changes 23, 24, 26. Transcripts synthesized at this time are either used during oocyte growth or stored as ribonucleoproteins for later use during oocyte maturation and early preimplantation embryogenesis 22, 25. Intense RNA transcription is completed with maximal oocyte growth 26, 27, at which time oocytes are competent to complete meiotic maturation, but have not acquired full developmental competence.
Oocytes undergo further maturation with follicle growth 28. Transition from the GV-stage to the metaphase II-stage of mouse oocyte development is associated with transcriptional silencing and selective destruction of transcripts involving meiotic arrest, oxidative phosphorylation, energy production, protein synthesis and metabolism 29; post-transcriptional mRNA modifications and post-translational protein alterations in several species, however, continue to metaphase II 30, 31. Bovine oocytes exposed to increasing levels of estradiol (E2) and growth factors during follicle development also show morphological changes in lipid accumulation and nucleolar vacuolization 32. Protein synthesis essential for embryogenesis occurs predominantly before GV breakdown in bovine oocytes 31, being further modified by steroids in maturing ovine and porcine oocytes 23, 33, 34. Consequently, in cattle and sheep, in vitro matured oocytes from large antral follicles are more competent to develop into blastocysts than similarly-matured oocytes from small antral follicles 35, 36, with FSH also enhancing oocyte developmental competence in primates 37, 38. A similar relationship between follicle size and oocyte developmental competence likely exists for in vivo matured oocytes in humans 39-41.
With the preovulatory LH surge, the mammalian oocyte undergoes GV breakdown (metaphase I) and produces a haploid oocyte (metaphase II), at which time transcription and protein synthesis decline to basal levels 31, and the oocyte becomes capable of fertilization and initial embryonic development. Progression of the oocyte to the first meiotic metaphase (i.e., nuclear [meiotic] maturation) occurs together with cytoplasmic maturation, which involves recruitment and post-transcriptional modifications of mRNAs, translation of dormant transcripts, and post-translational modifications of proteins essential for fertilization and embryogenesis 30,42. Bidirectional cumulus cell-oocyte signaling via gap junctions also is essential for mammalian oocyte developmental competence 6, and may depend upon increasing progesterone levels 34.
PCOS and oocyte development
Beyond our basic understanding of oocyte physiology, there are several reasons why the relationship between PCOS and oocyte developmental competence remains unclear. First, the microenvironment of each follicle is unique and has its own effect on the developing oocyte 43. Second, multiple embryos are transferred simultaneously into the uterus during IVF, confusing relationships between follicle fluid steroid levels and embryo implantation. Finally, studies of oocyte developmental competence are limited by ethical and experimental constraints on the use of human oocytes and embryos for biomedical research. Therefore the following discussion of oocyte development in PCOS is based upon indirect markers of oocyte developmental competence, which include studies of oocyte gene expression, correlations between follicle fluid steroid levels and oocyte development in vivo, effects of enzymatic disruption of steroidogenesis on oocyte development in vivo, and actions of sex steroids on oocyte development in vitro.
Ovarian hyperandrogenism
Androgens promote early follicle growth in primates. Testosterone administration to adult female rhesus monkeys increases the number of primary, growing preantral and small antral follicles and the proliferation of granulosa cells within them by acting through its own receptor 44, 45. Androgen treatment in such monkeys also increases mRNA expression of FSH receptor, IGF1 receptor and IGF1 in granulosa cells 46, 47, while enhancing IGF1 and IGF1 receptor mRNA expression in primordial follicle oocytes 48.
Human preantral follicles also express mRNA for androgen receptor 17 and grow in response to androgen exposure since antral follicle numbers in women positively correlate with serum androstenedione levels 49, 50. As a result, intrinsic ovarian hyperandrogenism in PCOS 51 is accompanied by 1) abnormal oocyte gene expression in preantral follicles (see TGFβ-related genes), 2) hyperandrogenism in small antral follicles 52 and 3) development of PCO morphology 53, 54. The collective data strongly suggest that PCOS exerts complex effects on the oocyte that begin with early preantral follicle growth and continue during later follicle development.
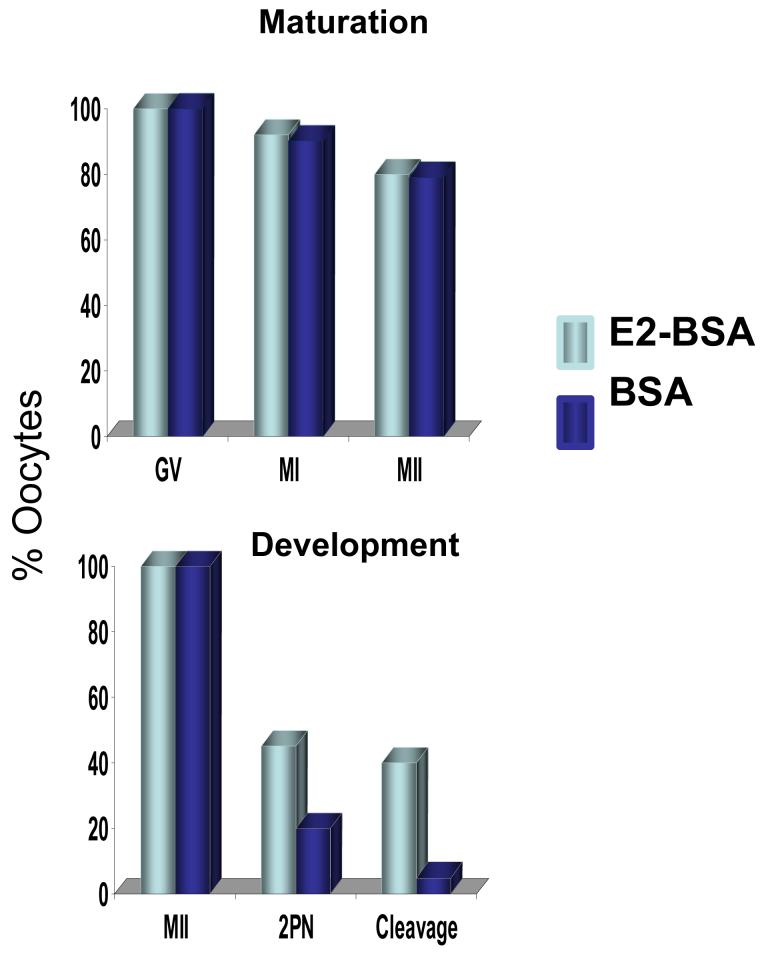
Androgen also interferes with E2-dependent signaling mechanisms accompanying oocyte cytoplasmic maturation. Exposure of cultured immature human oocytes to E2 bound to albumen increases fertilization and cleavage rates of in vitro matured oocytes, without affecting nuclear maturation (Figure 1) 55. Such E2 action on in vitro matured human oocytes is accompanied by increased oscillations of intracellular free calcium, which are antagonized by androgen 56. Consequently E2/androgen ratios to which immature human oocytes are exposed in the follicular phase appear to affect the quality of mature human oocytes obtained through IVF. In support of this, pregnancy outcome by IVF is related more to the E2/androgen ratio than to the absolute amount of E2 in the follicle 57.
Figure 1.
Human oocytes matured in vitro with and without E2. (With permission: Tesarik J, Mendoza C. Nongenomic effects of 17β-estradiol on maturing human oocytes: relationship to oocyte developmental potential. J Clin Endocrinol Metab 1995;80:1438-1443)
Moreover, small PCOS follicles have elevated 5α-reductase activity, which increases 5α-reduced androgens to levels capable of inhibiting granulosa cell aromatase activity in vitro and harming oocytes through limited E2 production 58, 59. As an animal model of PCOS, adult female rhesus monkeys exposed to prenatal androgen excess in early gestation and later subjected to FSH therapy show increased 5α-reductase and decreased aromatase activities in E2-deficient follicles 43 accompanied by impaired blastocyst development after combined rhFSH/human chorionic gonadotropin therapy 60. Consistent with E2-enhanced oocyte development in human and nonhuman primates 55, 61, low E2 production in IVF patients with 17α-hydroxylase deficiency is associated with in vitro embryonic developmental arrest 62. Conversely, follicle fluid E2 content in IVF patients positively correlates with oocyte fertilization, cleavage and implantation 62, with E2 levels in follicles containing in vitro fertilized oocytes being higher in women who conceive versus those who do not after embryo transfer 57, 62.
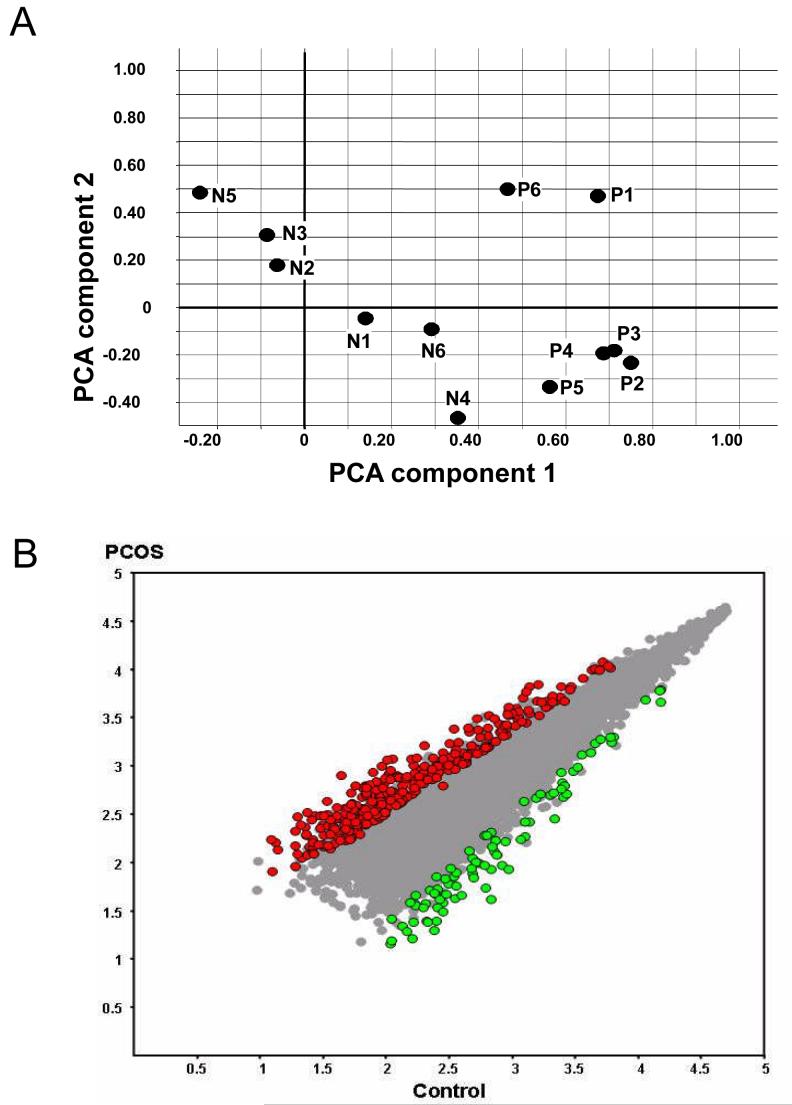
In PCOS patients undergoing ovarian stimulation for IVF, terminally differentiated follicles remain hyperandogenic 63 and contain meiotically-competent (metaphase II) oocytes with distinctly abnormal gene expression profiles (Figure 2) 64. Many of these differentially expressed genes in PCOS involve signal transduction, transcription, deoxyribonucleic acid and RNA processing and the cell cycle. Many of them also share promoter sequences containing putative transcription factor binding sites with sequence homology for androgen receptor, peroxisome proliferating receptor gamma and/or peroxisome proliferating receptor gamma-retinoid X receptor binding sites 64. These findings combined with androgen receptor 65 and insulin receptor mRNA expression 66 in human cumulus and mural granulosa cells provide rational physiological mechanisms by which endocrine factors can normally regulate appropriate follicle growth and oocyte development. Moreover, such cumulus and mural granulosa cell receptor expression also provides the physiological bases by which PCOS-related hyperandrogenism or adiposity-dependent insulin resistance perturbs cumulus-oocyte signaling.
Figure 2.
Gene expression profiles of 6 normal (N1-N6) and PCOS (P1-P6) oocytes. A) Principal Component Analysis, B) Differentially expressed mRNAs in PCOS vs. normal oocytes. (With permission: Wood JR, Dumesic DA, Abbott DH, et al. Molecular abnormalities in oocytes from women with polycystic ovary syndrome revealed by microarray analysis. J Clin Endocrinol Metab 2007;92:705-713)
Hyperinsulinemia and premature follicle luteinization
Insulin binds to its own receptors located on theca cells, surrounding stroma, granulosa cells and oocytes 66, 67 to promote follicle recruitment 68 and to stimulate theca cell 69 as well as granulosa cell steroidogenesis 70. Insulin stimulates theca cell androgen production by stimulating 17a-hydroxylase activity, amplifying LH- and IGF1-stimulated androgen production, elevating serum free testosterone levels through decreased hepatic sex hormone-binding globulin production, and enhancing serum IGF1 bioactivity through suppressed IGF-binding protein production 69. Insulin also enhances FSH-induced upregulation of LH receptors in granulosa cells and increases their ability to produce P4 in response to LH 71, 72.
Insulin sensitivity in PCOS patients is intrinsically impaired from abnormal post-receptor signal transduction, reducing insulin-mediated glucose uptake without affecting steroidogenesis 69. As a result, PCOS patients have insulin resistance independent and additive with that of obesity, with combined PCOS and obesity synergistically impairing glucose-insulin homeostasis and promoting ovarian steroidogenesis. This is presumably why hyperinsulinemia from insulin resistance in PCOS enhances androgen production 69 and also induces premature granulosa cell luteinization 70, 71, leading to arrest of cell proliferation and follicle growth. Consequently, small antral PCOS follicles exhibit P4 hypersecretion and overexpress LH receptors 21, 73 and also show an exaggerated shift in steroidogenesis from E2 to progesterone production 71. A comparable steroidogenic shift occurs in early-treated prenatally androgenized adult female rhesus monkeys undergoing gonadotropin therapy for IVF, in which combined LH hypersecretion and relative insulin excess at oocyte retrieval is accompanied by impaired blastocyst development 60. The further observation that insulin together with FSH upregulates LH receptor expression in cultured mouse cumulus-oocyte complexes and reduces blastocyst development 72 provides additional evidence that insulin excess might perturb oocyte development through altered cumulus-oocyte signaling.
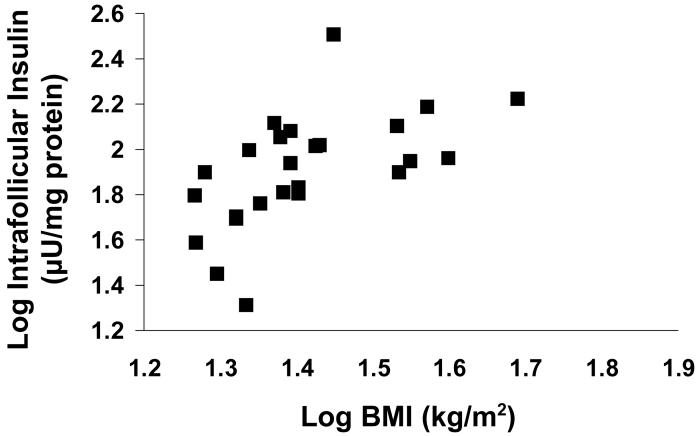
Consistent with this hypothesis, the amount of insulin present in the human follicle is determined by BMI and fasting serum insulin levels, and is highest in women with impaired glucose tolerance (Figure 3) 66. Therefore, the use of the metformin to improve insulin sensitivity has been proposed as a strategy to improve follicular growth and oocyte development in PCOS. In one of two prospective, randomized, double blind studies, pretreatment of PCOS patients with metformin preceding GnRH analog/rhFSH therapy for IVF did not affect ovarian responsiveness to FSH therapy nor pregnancy outcome 74. In the other, metformin therapy to PCOS women lowered serum fasting insulin, total and free testosterone as well as E2 levels at oocyte retrieval, enhanced clinical pregnancy and livebirth rates, and diminished the risk of severe ovarian hyperstimulation syndrome 75. In a recent double-blind, randomized study powered to examine live birth rate, however, metformin lacked superiority over clomiphene citrate in achieving live-birth in 626 infertile PCOS patients, making the current use of metformin to improve developmental competence of PCOS oocytes controversial76.
Figure 3.
Correlation between intrafollicular insulin levels and BMI in women undergoing ovarian stimulation for IVF. (With permission: Dumesic DA, Schramm RD, Abbott DH. Early Origins of Polycystic Ovary Syndrome (PCOS). Reprod Fertil Dev 2005;17:349-360)
TGFβ-related proteins
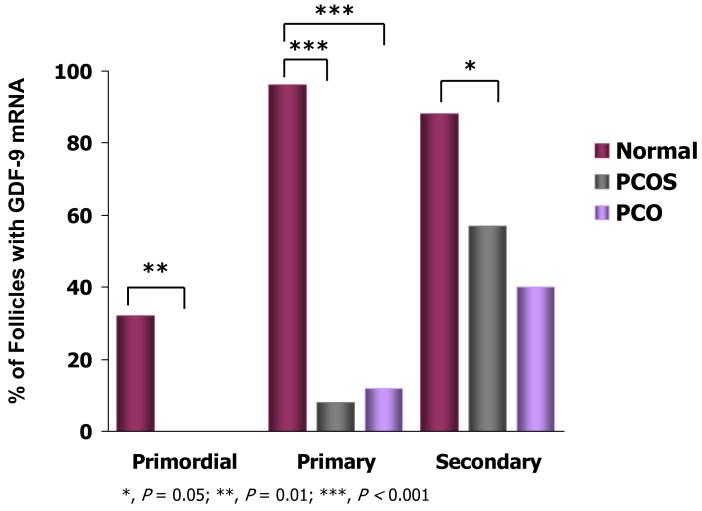
Follicle growth and oocyte development are regulated by several proteins of the TGFβ family, including activins, inhibins, AMH, GDF9 and BMP15 77. Many of these factors are produced by the oocyte (i.e., GDF9 and BMP15) and its surrounding granulosa cells (i.e., activins, inhibins, AMH) and interact with each other to coordinate granulosa cell-oocyte signaling. As an oocyte-secreted factor, GDF9 promotes granulosa cell proliferation and preantral growth 78-80, while its deficiency in mice impairs granulosa cell proliferation and causes follicular arrest at the primary follicle stage 78. In humans, GDF9 induces the growth of human ovarian follicles in vitro 81. Oocyte GDF9 expression normally begins in humans at the primordial-primary follicle transition and increases with preantral follicle growth 82, 83. Reduced GDF9 mRNA levels in PCOS oocytes from initiation of primordial follicle growth through the small antral follicle stage of development accompanies impaired follicle growth, presumably from altered granulosa cell-oocyte signaling (Figure 4) 54, 83.
Figure 4.
Percent human preantral follicles with GDF9 mRNA Expression.. *, P<0.05; **, P<0.01; ***, P<0.001. (With permission: Filho FLT, Baracat EC, Lee TH, et al. Aberrant expression of growth differentiation factor-9 in oocytes of women with polycystic ovary syndrome. J Clin Endocrinol Metab 2002;87:1337-1344)
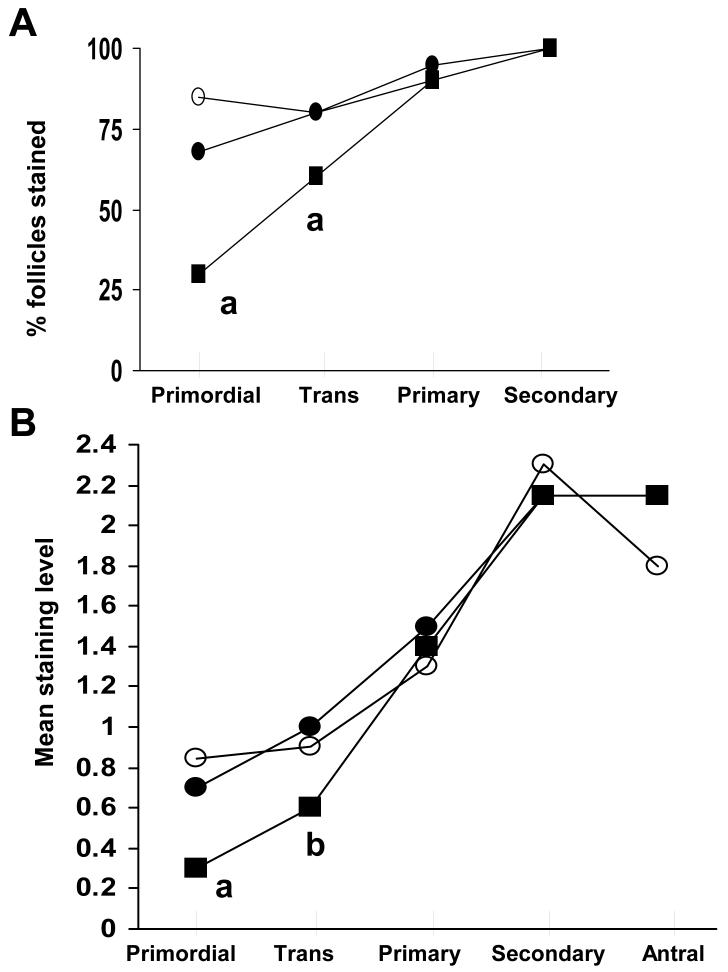
As another TGFβ family member, AMH is normally produced by granulosa cells of growing follicles 77, 84. Low AMH levels occur in primordial and primary follicles, increase to maximal levels in large preantral and small antral stages, and then decline during final follicular maturation 84-87. In vitro rodent studies show that AMH inhibits primordial follicle growth 15, while its deficiency has the opposite effect 88, suggesting that AMH produced by growing follicles inhibits growth of adjacent primordial follicles 84, 89. Histological examination of human ovaries shows reduced AMH levels in primordial and transitional follicles of PCOS patients, implicating relative AMH deficiency as an additional factor involved with abnormal growth of the primordial follicle and its oocyte (Figure 5) 84.
Figure 5.
A) Percent human follicles with AMH staining and B) mean intensity of AMH staining. a, P<0.005 vs. normal ovaries; b, P<0.005 vs. ovulatory PCO ovaries; c, P<0.005, d, P<0.05 vs. normal and ovulatory PCO ovaries. (With permission: Stubbs SA, Hardy K, Da Silva-Buttkus P, et al. Anti-mullerian hormone protein expression is reduced during the initial stages of follicle development in human polycystic ovaries. J Clin Endocrinol Metab 2005;90:5536-5543 Copyright 2005, The Endocrine Society)
Granulosa cell-derived inhibins and activins are dimeric glycoproteins. Inhibins consist of an α-subunit covalently joined by disulfide links to either a βA-subunit (inhibin A) or a βB-subunit (inhibin B) and suppress FSH synthesis. Dimerization of β subunits produces 3 forms of activin (activin A [βA-βA], activin AB βA-βB] and activin B [βB-βB]) that enhance FSH secretion 90. Follistatin, a glycoprotein structurally unrelated to the TGFβ superfamily, binds activin to inhibit its action 90. Collectively, activins promote follicular development by enhancing granulosa cell responsiveness to FSH, suppressing androgen synthesis and stimulating oocyte maturation, while inhibins produced by the dominant follicle stimulate theca cell androgen production for E2 synthesis 90, 91. In some PCOS patients, low serum activin A and high serum follistatin levels are observed 92, 93, while in others the normal intrafollicular shift from an activin-dominant to an inhibin-dominant microenvironment during follicle growth 94 is impaired 95, 96. The clinical implications of abnormal intraovarian activin and inhibin production from PCOS on oocyte development remain uncertain.
Conclusion
Polycystic ovary syndrome is characterized by ovarian hyperandrogenism, hyperinsulinemia from insulin resistance and paracrine dysregulation of several TGFβ-related proteins, all of which can perturb the intrafollicular environment. Acting directly or indirectly through cumulus cell-oocyte signaling, the abnormal intrafollicular environment induced by PCOS has the capacity to perturb cytoplasmic and/or nuclear maturation of the oocyte and to alter oocyte gene expression important for embryonic gene activation. Understanding how endocrine/paracrine factors and genes interact to promote oocyte developmental competence may provide new clinical strategies that target long-term correction of follicle growth and oocyte development.
Acknowledgments
This work was supported by the National Institutes of Health, as part of the NICHD National Cooperative Program on Female Health and Egg Quality under cooperative agreement U01 HD044650, and Grant P51 RR 000167 to the National Primate Research Center, University of Wisconsin, Madison (a facility constructed with support from Research Facilities Improvement Program grant numbers RR15459-01 and RR020141-01); it also was supported by Serono and Organon Pharmaceuticals. We thank Richard Tasca, Ph.D. and John Eppig Ph.D for critical review of this manuscript as well as Rebekah Herrmann R.N. for technical assistance.
Abbreviations
- PCOS
polycystic ovary syndrome
- TGFβ
transforming growth factorβ
- IVF
in vitro fertilization
- LH
luteinizing hormone
- GV
germinal vesicle
- mRNA
messenger ribonucleic acid
- FSH
follicle-stimulating hormone
- GDF9
growth differentiation factor 9
- BMP15
bone morphogenetic protein 15
- IGF
insulin-like growth factor
- E2
estradiol
- AMH
anti-mullerian hormone
- PCA
principal component analysis
References
- 1.Hutt KJ, McLaughlin EA, Holland MK. Kit ligand and c-Kit have diverse roles in mammalian oogenesis and folliculogenesis. Mol Hum Reprod. 2006;12:61–69. doi: 10.1093/molehr/gal010. [DOI] [PubMed] [Google Scholar]
- 2.McNatty KP, Smith P, Moore LG, et al. Oocyte-expressed genes affecting ovulation rate. Mol Cell Endocrinol. 2005;234:57–66. doi: 10.1016/j.mce.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 3.Yan C, Wang P, DeMayo J, et al. Synergistic roles of bone morphogenetic protein 15 and growth differentiation factor 9 in ovarian function. Mol Endocrinol. 2001;15:854–866. doi: 10.1210/mend.15.6.0662. [DOI] [PubMed] [Google Scholar]
- 4.Su YQ, Wu X, O’Brien MJ, Pendola, et al. Synergistic roles of BMP15 and GDF9 in the development and function of the oocyte-cumulus cell complex in mice: genetic evidence for an oocyte-granulosa cell regulatory loop. Dev Biol. 2004;276:64–73. doi: 10.1016/j.ydbio.2004.08.020. [DOI] [PubMed] [Google Scholar]
- 5.Sutton ML, Gilchrist RB, Thompson JG. Effects of in-vivo and in-vitro environments on the metabolism of the cumulus-oocyte complex and its influence on oocyte developmental capacity. Hum Reprod Update. 2003;9:35–48. doi: 10.1093/humupd/dmg009. [DOI] [PubMed] [Google Scholar]
- 6.Sugiura K, Eppig JJ. Society for Reproductive Biology Founders’ Lecture. Control of metabolic cooperativity between oocytes and their companion granulosa cells by mouse oocytes. Reprod Fertil Devel. 2005;17:667–67. doi: 10.1071/rd05071. [DOI] [PubMed] [Google Scholar]
- 7.Schramm RD, Bavister BD. A macaque model for studying mechanisms controlling oocyte development and maturation in human and nonhuman primates. Hum Reprod. 1999;14:2544–55. doi: 10.1093/humrep/14.10.2544. [DOI] [PubMed] [Google Scholar]
- 8.Dumesic DA, Schramm RD, Abbott DH. Early Origins of Polycystic Ovary Syndrome (PCOS) Reprod Fertil Dev. 2005;17:349–360. doi: 10.1071/rd04092. [DOI] [PubMed] [Google Scholar]
- 9.Heijnen EMEW, Eijkemans MJC, Hughes EG, et al. A meta-analysis of outcomes of conventional IVF in women with polycstic ovary syndrome. Human Reprod Update. 2006;12:13–21. doi: 10.1093/humupd/dmi036. [DOI] [PubMed] [Google Scholar]
- 10.Sengoku K, Tamate K, Takuma N, et al. The chromosomal normality of unfertilized oocytes from patients with polycystic ovarian syndrome. Hum Reprod. 1997;12:474–477. doi: 10.1093/humrep/12.3.474. [DOI] [PubMed] [Google Scholar]
- 11.Ludwig M, Finas DF, Al-Hasani S, et al. Oocyte quality and treatment outcome in intracytoplasmic sperm injection cycles of polycystic ovarian syndrome patients. Hum Reprod. 1999;14:354–358. doi: 10.1093/humrep/14.2.354. [DOI] [PubMed] [Google Scholar]
- 12.Cano F, Garcia-Velasco JA, Millet A. Oocyte quality in polycystic ovaries revisited: identification of a particular subgroup of women. J Assist Reprod Genet. 1997;14:254–60. doi: 10.1007/BF02765826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Faddy MJ, Gosden RG. Modelling the dynamics of ovarian follicle utilization throughout life. In: Trounson AO, Gosden RG, editors. Biology and Pathology of the Oocyte. Role in Fertility and Reproductive Medicine. Cambridge University Press; Cambridge: 2003. pp. 44–52. [Google Scholar]
- 14.Gougeon A. The early stages of folliclar growth. In: Trounson AO, Gosden RG, editors. Biology and Pathology of the Oocyte. Role in Fertility and Reproductive Medicine. Cambridge University Press; Cambridge: 2003. pp. 29–43. [Google Scholar]
- 15.Durlinger ALL, Gruijters MJ, Kramer P, et al. Anti-Mullerian hormone inhibits initiation of primordial follicle growth in the mouse ovary. Endocrinology. 2002;143:1076–1084. doi: 10.1210/endo.143.3.8691. [DOI] [PubMed] [Google Scholar]
- 16.Gougeon A. Regulation of ovarian follicular development in primates: facts and hypothesis. Endo Rev. 1996;17:121–155. doi: 10.1210/edrv-17-2-121. [DOI] [PubMed] [Google Scholar]
- 17.Rice S, Ojha K, Whitehead S, et al. Stage-specific expression of androgen receptor, follicle-stimulating hormone receptor, and anti-mullerian hormone type II receptor in single, isolated, human preantral follicles: relevance to polycystic ovaries. J Clin Endocrinol Metab. 2007;92:1034–1040. doi: 10.1210/jc.2006-1697. [DOI] [PubMed] [Google Scholar]
- 18.Jakimiuk AJ, Weitsman SR, Brzechffa PR, et al. Aromatase mRNA expression in individual follicles from polycystic ovaries. Mol Hum Reprod. 1998;4:1–8. doi: 10.1093/molehr/4.1.1. [DOI] [PubMed] [Google Scholar]
- 19.Hillier SG, Whitelaw PF, Smyth CD. Follicular oestrogen synthesis: the ‘two-cell, two-gonadotropin’ model revisited. Mol Cell Endocrinol. 1994;100:51–54. doi: 10.1016/0303-7207(94)90278-x. [DOI] [PubMed] [Google Scholar]
- 20.Zachow RJ, Magoffin DA. Ovarian androgen biosynthesis: paracrine/autocrine regulation. In: Azziz R, Nestler JE, Dewailly D, editors. Androgen Excess Disorders in Women. Lippincott-Raven; Philadelphia: 1997. pp. 13–22. [Google Scholar]
- 21.Willis D, Watson H, Mason H, et al. Premature response to LH of granulosa cells from anovulatory women with polycystic ovaries: relevance to mechanism of anovulation. J Clin Endocrinol Metab. 1998;83:3984–3991. doi: 10.1210/jcem.83.11.5232. [DOI] [PubMed] [Google Scholar]
- 22.Latham KE. Epigenetic modification and imprinting of the mammalian genome during development. Curr Topics Dev Biol. 1999;43:1–49. doi: 10.1016/s0070-2153(08)60377-4. [DOI] [PubMed] [Google Scholar]
- 23.Moor RM, Dai Y, Lee C, et al. Oocyte maturation and embryonic failure. Hum Reprod Update. 1998;4:223–236. doi: 10.1093/humupd/4.3.223. [DOI] [PubMed] [Google Scholar]
- 24.Albertini DF. Origins and manifestations of oocyte maturation competencies. Reprod Biomed Online. 2003;6:410–415. doi: 10.1016/s1472-6483(10)62159-1. [DOI] [PubMed] [Google Scholar]
- 25.Bachvarova R. Gene expression during oogenesis and oocyte development in mammals. In: Browder LW, editor. Developmental biology: a comprehensive synthesis. Vol. 1. Plenum; New York: 1985. pp. 453–524. [DOI] [PubMed] [Google Scholar]
- 26.Wickramasinghe D, Ebert KM, Albertini DF. Meiotic competence acquisition is associated with the appearance of M-phase characteristics in growing mouse oocytes. Dev Biol. 1991;143:162–172. doi: 10.1016/0012-1606(91)90063-9. [DOI] [PubMed] [Google Scholar]
- 27.Fair T, Hyttel P, Greve T, et al. Nucleus structure and transcriptional activity in relation to oocyte diameter in cattle. Mol Reprod Dev. 1996;43:503–512. doi: 10.1002/(SICI)1098-2795(199604)43:4<503::AID-MRD13>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 28.Pavlok A, Lucas-Hahn A, Niemann H. Fertilization and developmental competence of bovine oocytes derived from different categories of antral follicles. Mol Reprod Dev. 1992;31:63–67. doi: 10.1002/mrd.1080310111. [DOI] [PubMed] [Google Scholar]
- 29.Su YQ, Sugiura K, Woo Y, et al. Selective degradation of transcripts during meiotic maturation of mouse oocytes. Devel Biol. 2007;302:104–117. doi: 10.1016/j.ydbio.2006.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gandolfi TA, Gandolfi F. The maternal legacy to the embryo: cytoplasmic components and their effects on early development. Theriogenology. 2001;55:1255–1276. doi: 10.1016/s0093-691x(01)00481-2. [DOI] [PubMed] [Google Scholar]
- 31.Tomek W, Torner H, Kanitz W. Comparative analysis of protein synthesis, transcription and cytoplasmic polyadenylation of mRNA during maturation of bovine oocytes in vitro. Reprod Dom Anim. 2002;37:86–91. doi: 10.1046/j.1439-0531.2002.00336.x. [DOI] [PubMed] [Google Scholar]
- 32.Assey RJ, Hyttel P, Greve T, et al. Oocyte morphology in dominant and subordinate follicles. Mol Reprod. 1994;37:335–344. doi: 10.1002/mrd.1080370313. [DOI] [PubMed] [Google Scholar]
- 33.Osborn JC, Moor RM. The role of steroid signals in the maturation of mammalian oocytes. J Steroid Biochem. 1983;19:133–137. [PubMed] [Google Scholar]
- 34.Mattioli M, Galeati G, Bacci ML, et al. Follicular factors influence oocyte fertilizability by modulating the intercellular cooperation between cumulus cells and oocyte. Gamete Res. 1988;21:223–232. doi: 10.1002/mrd.1120210304. [DOI] [PubMed] [Google Scholar]
- 35.Hyttel P, Fair T, Callesen H, et al. Oocyte growth, capacitation and final maturation in cattle. Theriogenology. 1997;47:23–32. [Google Scholar]
- 36.Moor RM, Lee C, Dai YF, et al. Antral follicles confer developmental competence on oocytes. Zygote. 1996;4:289–293. doi: 10.1017/s0967199400003269. [DOI] [PubMed] [Google Scholar]
- 37.Schramm RD, Bavister BD. FSH-priming of rhesus monkeys enhances meiotic and developmental competence of oocytes matured in vitro. Biol Reprod. 1994;51:904–912. doi: 10.1095/biolreprod51.5.904. [DOI] [PubMed] [Google Scholar]
- 38.Wynn P, Picton HM, Krapez JA, et al. Pretreatment with follicle stimulating hormone promotes the numbers of human oocytes reaching metaphase II by in vitro maturation. Hum Reprod. 1998;13:3132–3138. doi: 10.1093/humrep/13.11.3132. [DOI] [PubMed] [Google Scholar]
- 39.Ectors FJ, Vanderzwalmen P, Van Hoeck JV, et al. Relationship of human follicular diameter with oocyte fertilization and development after in-vitro fertilization or intracytoplasmic sperm injection. Hum Reprod. 1997;12:2002–2005. doi: 10.1093/humrep/12.9.2002. [DOI] [PubMed] [Google Scholar]
- 40.Bergh C, Broden H, Lundin K, et al. Comparison of fertilization, cleavage and pregnancy rates of oocytes from large and small follicles. Hum Reprod. 1998;13:1912–1915. doi: 10.1093/humrep/13.7.1912. [DOI] [PubMed] [Google Scholar]
- 41.Arnot AM, Vandekerckhove P, DeBono MA, et al. Follicular volume and number during in-vitro fertilization: association with oocyte developmental capacity and pregnancy rate. Hum Reprod. 1995;10:256–261. doi: 10.1093/oxfordjournals.humrep.a135925. [DOI] [PubMed] [Google Scholar]
- 42.Stebbins-Boaz B, Richter JD. Translational control during early development. Crit Rev Eukaryot Gene Expr. 1997;7:73–94. doi: 10.1615/critreveukargeneexpr.v7.i1-2.50. [DOI] [PubMed] [Google Scholar]
- 43.Dumesic DA, Schramm RD, Bird IM, et al. Reduced intrafollicular androstenedione and estradiol levels in early-treated prenatally androgenized female rhesus monkeys receiving FSH therapy for in vitro fertilization. Biol Reprod. 2003;69:1213–1219. doi: 10.1095/biolreprod.102.015164. [DOI] [PubMed] [Google Scholar]
- 44.Vendola KA, Zhou J, Adesanya OO, et al. Androgens stimulate early stages of follicle growth in the primate ovarian. J Clin Invest. 1998;101:2622–2629. doi: 10.1172/JCI2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weil SJ, Vendola K, Zhou J, et al. Androgen receptor gene expression in the primate ovary: cellular localization, regulation, and functional correlations. J Clin Endocrinol Metab. 1998;83:2479–2485. doi: 10.1210/jcem.83.7.4917. [DOI] [PubMed] [Google Scholar]
- 46.Weil S, Vendola K, Zhou J, et al. Androgen and follicle-stimulating hormone interactions in primate ovarian follicle development. J Clin Endocrinol Metab. 1999;84:2951–2956. doi: 10.1210/jcem.84.8.5929. [DOI] [PubMed] [Google Scholar]
- 47.Vendola K, Zhou J, Wang J, et al. Androgens promote insulin-like growth factor-I and insulin-like growth factor-I receptor gene expression in the primate ovary. Hum Reprod. 1999;14:2328–2332. doi: 10.1093/humrep/14.9.2328. [DOI] [PubMed] [Google Scholar]
- 48.Vendola K, Zhou J, Wang J, et al. Androgens promote oocyte insulin-like growth factor I expression and initiation of follicle development in the primate ovary. Biol Reprod. 1999;61:353–357. doi: 10.1095/biolreprod61.2.353. [DOI] [PubMed] [Google Scholar]
- 49.Jonard S, Robert Y, Cortet-Rudelli C, et al. Ultrasound examination of polycystic ovaries: is it worth counting the follicles? Hum Reprod. 2003;18:598–603. doi: 10.1093/humrep/deg115. [DOI] [PubMed] [Google Scholar]
- 50.Dumesic DA, Damario MA, Session DR, et al. Ovarian Morphology and Serum Hormone Markers as Predictors of Ovarian Follicle Recruitment by Gonadotropins for In Vitro Fertilization. J Clin Endocrinol Metab. 2001;86:2538–2543. doi: 10.1210/jcem.86.6.7605. [DOI] [PubMed] [Google Scholar]
- 51.Nelson VL, Qin K, Rosenfield RL, et al. The biochemical basis for increased testosterone production in theca cells propagated from patients with polycystic ovary syndrome. J Clin Endocrinol Metab. 2001;86:5925–5933. doi: 10.1210/jcem.86.12.8088. [DOI] [PubMed] [Google Scholar]
- 52.Eden JA, Jones J, Carter GD, et al. Follicular fluid concentrations of insulin-like growth factor 1, epidermal growth factor, transforming growth factor-alpha and sex-steroids in volume matched normal and polycystic human follicles. Clin Endocrinol. 1990;32:395–405. doi: 10.1111/j.1365-2265.1990.tb00879.x. [DOI] [PubMed] [Google Scholar]
- 53.Webber LJ, Stubbs S, Stark J, et al. Formation and early development of follicles in the polycystic ovary. Lancet. 2003;362:1017–1021. doi: 10.1016/s0140-6736(03)14410-8. [DOI] [PubMed] [Google Scholar]
- 54.Maciel GA, Baracat EC, Benda JA, et al. Stockpiling of transitional and classic primary follicles in ovaries of women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2004;89:5321–5327. doi: 10.1210/jc.2004-0643. [DOI] [PubMed] [Google Scholar]
- 55.Tesarik J, Mendoza C. Nongenomic effects of 17B-estradiol on maturing human oocytes: relationship to oocyte developmental potential. J Clin Endocrinol Metab. 1995;80:1438–1443. doi: 10.1210/jcem.80.4.7714121. [DOI] [PubMed] [Google Scholar]
- 56.Tesarik J, Mendoza C. Direct non-genomic effects of follicular steroids on maturing human oocytes: oestrogen versus androgen antagonism. Hum Reprod Update. 1997;3:95–100. doi: 10.1093/humupd/3.2.95. [DOI] [PubMed] [Google Scholar]
- 57.Yding Andersen C. Characteristics of human follicular fluid associated with successful conception after in vitro fertilization. J Clin Endocrinol Metab. 1993;77:1227–34. doi: 10.1210/jcem.77.5.7521343. [DOI] [PubMed] [Google Scholar]
- 58.Jakimiuk AJ, Weitsman SR, Magoffin DA. 5a-Reductase activity in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 1999;84:2414–2418. doi: 10.1210/jcem.84.7.5863. [DOI] [PubMed] [Google Scholar]
- 59.Agarwal SK, Judd HL, Magoffin DA. A mechanism for the suppression of estrogen production in polycystic ovary syndrome. J Clin Endocrinol Metab. 1996;81:3686–3691. doi: 10.1210/jcem.81.10.8855823. [DOI] [PubMed] [Google Scholar]
- 60.Dumesic DA, Schramm RD, Peterson E, et al. Impaired developmental competence of oocytes in adult prenatally androgenized female rhesus monkeys undergoing gonadotropin stimulation for in vitro fertilization. J Clin Endocrinol Metab. 2002;87:1111–1119. doi: 10.1210/jcem.87.3.8287. [DOI] [PubMed] [Google Scholar]
- 61.Zheng P, Wei S, Bavister BD, et al. 17β-estradiol and progesterone improve in-vitro cytoplasmic maturation of oocytes from unstimulated prepubertal and adult rhesus monkeys. Hum Reprod. 2003;18:2137–44. doi: 10.1093/humrep/deg410. [DOI] [PubMed] [Google Scholar]
- 62.Dumesic DA, Schramm RD, Abbott DH. Steroid and oocyte development. In: Filicori M, editor. Updates in Infertility Treatment 2004. Medimond, Bologna, Italy: 2005. pp. 457–475. [Google Scholar]
- 63.Foong SC, Abbott DH, Zschunke MA, et al. Follicle luteinization in hyperandrogenic follicles of polycystic ovary syndrome (PCOS) patients undergoing gonadotropin therapy for in vitro fertilization. J Clin Endocrinol Metab. 2006;91:2327–2333. doi: 10.1210/jc.2005-2142. [DOI] [PubMed] [Google Scholar]
- 64.Wood JR, Dumesic DA, Abbott DH, et al. Molecular abnormalities in oocytes from women with polycystic ovary syndrome revealed by microarray analysis. J Clin Endocrinol Metab. 2007;92:705–713. doi: 10.1210/jc.2006-2123. [DOI] [PubMed] [Google Scholar]
- 65.Hickey T. Ph.D. thesis. University of Adelaide; Adelaide, Australia: 2006. Androgen Receptor Mediated Activity in the Ovary: Implications for Polycystic Ovary Syndrome. [Google Scholar]
- 66.Phy JL, Conover CA, Abbott DH, et al. Insulin and messenger ribonucleic acid expression of insulin receptor isoforms in ovarian follicles from nonhirsute ovulatory women and polycystic ovary syndrome patients. J Clin Endocrinol Metab. 2004;89:3561–3566. doi: 10.1210/jc.2003-031888. [DOI] [PubMed] [Google Scholar]
- 67.Samoto T, Maruo T, Ladines-llave C, et al. Insulin receptor expression in the follicular and stroma compartments of the human ovary over the course of follicular growth, regression, and atresia. Endocr J. 1993;40:715–726. doi: 10.1507/endocrj.40.715. [DOI] [PubMed] [Google Scholar]
- 68.Kezele PR, Nilsson EE, Skinner MK. Insulin but not insulin-like growth factor-I promotes the primordial to primary follicle transition. Mol Cell Endocrinol. 2002;192:37–43. doi: 10.1016/s0303-7207(02)00114-4. [DOI] [PubMed] [Google Scholar]
- 69.Balen AH, Conway GS, Homburg R, Legro RS. Polycystic Ovary Syndrome. A Guide to Clinical Management. Taylor and Francis; London: 2005. pp. 47–67. [Google Scholar]
- 70.Franks S, Gilling-Smith C, Watson H, et al. Insulin action in the normal and polycystic ovary. Endocrinol Metab Clin North Am. 1999;28:361–378. doi: 10.1016/s0889-8529(05)70074-8. [DOI] [PubMed] [Google Scholar]
- 71.Franks S, Mason H, Willis D. Follicular dynamics in the polycystic ovary syndrome. Mol Cell Endocrinol. 2000;163:49–52. doi: 10.1016/s0303-7207(99)00239-7. [DOI] [PubMed] [Google Scholar]
- 72.Eppig JJ, O’Brien MJ, Pendola FL, et al. Factors affecting the developmental competence of mouse oocytes grown in vitro: follicle stimulating hormone and insulin. Biol Reprod. 1998;59:1445–53. doi: 10.1095/biolreprod59.6.1445. [DOI] [PubMed] [Google Scholar]
- 73.Jakimiuk AJ, Weitsman SR, Navab A, et al. Luteinizing hormone receptor, steroidogenesis acute regulatory protein, and steroidogenic enzyme messenger ribonucleic acids are overproduced in thecal and granulosa cells from polycystic ovaries. J Clin Metab Endocrinol. 2001;86:1318–1323. doi: 10.1210/jcem.86.3.7318. [DOI] [PubMed] [Google Scholar]
- 74.Kjotrod SB, During VV, Carlsen SM. Metformin treatment before IVF/ICSI in women with polycystic ovary syndrome; a prospective, randomized, double blind study. Hum Reprod. 2004;19:1315–1322. doi: 10.1093/humrep/deh248. [DOI] [PubMed] [Google Scholar]
- 75.Tang T, Glanville J, Orsi N, et al. The use of metformin for women with PCOS undergoing IVF treatment. Hum Reprod. 2006;21:1416–1425. doi: 10.1093/humrep/del025. [DOI] [PubMed] [Google Scholar]
- 76.Legro RS, Barnhart HX, Schlaff WD, et al. Cooperative Multicenter Reproductive Medicine Network. Clomiphene, metformin, or both for infertility in the polycystic ovary syndrome. N Engl J Med. 2007;356:551–566. doi: 10.1056/NEJMoa063971. [DOI] [PubMed] [Google Scholar]
- 77.Knight PG, Glister C. Local roles of TGF- β superfamily members in the control of ovarian follicle development. Anim Reprod Sci. 2003;78:165–183. doi: 10.1016/s0378-4320(03)00089-7. [DOI] [PubMed] [Google Scholar]
- 78.Elvin JA, Yan C, Matzuk MM. Oocyte-expressed TGF-beta superfamily members in female fertility. Mol Cell Endocrinol. 2000;159:1–5. doi: 10.1016/s0303-7207(99)00185-9. [DOI] [PubMed] [Google Scholar]
- 79.Hayashi M, McGee EA, Min G, et al. Recombinant growth differentiation factor-9 (GDF-9) enhances growth and differentiation of cultured early ovarian follicles. Endocrinology. 1999;140:1236–1244. doi: 10.1210/endo.140.3.6548. [DOI] [PubMed] [Google Scholar]
- 80.Vitt UA, Hayashi M, Klein C, et al. Growth differentiation factor-9 stimulates proliferation but suppresses the follicle-stimulating hormone-induced differentiation of cultured granulosa cells from small antral and preovulatory rat follicles. Biol Reprod. 2000;62:370–377. doi: 10.1095/biolreprod62.2.370. [DOI] [PubMed] [Google Scholar]
- 81.Hreinsson JG, Scott JE, Rasmussen C, et al. Growth differentiation factor-9 promotes the growth, development, and survival of human ovarian follicles in organ culture. J Clin Endocrinol Metab. 2002;87:316–321. doi: 10.1210/jcem.87.1.8185. [DOI] [PubMed] [Google Scholar]
- 82.Aaltonen J, Laitinen MP, Vuojolainen K, et al. Human growth differentiation factor 9 (GDF-9) and its novel homolog GDF-9B are expressed in oocytes during early folliculogenesis. J Clin Endocrinol Metab. 1999;84:2744–2750. doi: 10.1210/jcem.84.8.5921. [DOI] [PubMed] [Google Scholar]
- 83.Filho FLT, Baracat EC, Lee TH, et al. Aberrant expression of growth differentiation factor-9 in oocytes of women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2002;87:1337–1344. doi: 10.1210/jcem.87.3.8316. [DOI] [PubMed] [Google Scholar]
- 84.Stubbs SA, Hardy K, Da Silva-Buttkus P, et al. Anti-mullerian hormone protein expression is reduced during the initial stages of follicle development in human polycystic ovaries. J Clin Endocrinol Metab. 2005;90:5536–5543. doi: 10.1210/jc.2005-0907. [DOI] [PubMed] [Google Scholar]
- 85.Weenen C, Laven JS, Von Bergh AR, et al. Anti-mullerian hormone expression pattern in the human ovary: potential implications for initial and cyclic follicle recruitment. Mol Hum Reprod. 2004;10:77–83. doi: 10.1093/molehr/gah015. [DOI] [PubMed] [Google Scholar]
- 86.Fanchin R, Louafi N, Lozano DHM, et al. Per-follicle measurements indicate that anti-mullerian hormone secretion is modulated by the extent of follicular development and luteinization and may reflect qualitatively the ovarian follicular status. Fertil Steril. 2005;84:167–173. doi: 10.1016/j.fertnstert.2005.01.115. [DOI] [PubMed] [Google Scholar]
- 87.Eldar-Geva T, Margalioth EJ, Gal M, et al. Serum anti-mullerian hormone levels during controlled ovarian hyperstimulation in women with polycystic ovaries with and without hyperandrogenism. Hum Reprod. 2005;20:1814–1819. doi: 10.1093/humrep/deh873. [DOI] [PubMed] [Google Scholar]
- 88.Durlinger AL, Kramer P, Karels B, et al. Control of primordial follicle recruitment by anti-Mullerian hormone in the mouse ovary. Endocrinology. 1999;140:5789–5798. doi: 10.1210/endo.140.12.7204. [DOI] [PubMed] [Google Scholar]
- 89.Fortune JE. The early stages of follicular development: activation of primordial follicles and growth of preantral follicles. Am Reprod Sci. 2003;78:135–163. doi: 10.1016/s0378-4320(03)00088-5. [DOI] [PubMed] [Google Scholar]
- 90.Knight PG, Glister C. Potential local regulatory functions of inhibins, activins and follistatin in the ovary. Reproduction. 2001;121:503–512. doi: 10.1530/rep.0.1210503. [DOI] [PubMed] [Google Scholar]
- 91.Sadatsuki M, Tsutsumi O, Yamada R, et al. Local regulatory effects of activin A and follistatin on meiotic maturation of rat oocytes. Biochem Biophys Res Commun. 1993;196:388–395. doi: 10.1006/bbrc.1993.2261. [DOI] [PubMed] [Google Scholar]
- 92.Norman RJ, Milner CR, Groome NP, et al. Circulating follistatin concentrations are higher and activin levels are lower in polycystic ovarian syndrome. Hum Reprod. 2001;16:668–672. doi: 10.1093/humrep/16.4.668. [DOI] [PubMed] [Google Scholar]
- 93.Eldar-Geva T, Spitz IM, Groome NP, et al. Follistatin and activin A serum concentrations in obese and non-obese patients with polycystic ovary syndrome. Hum Reprod. 2001;16:2552–2556. doi: 10.1093/humrep/16.12.2552. [DOI] [PubMed] [Google Scholar]
- 94.Schneyer AL, Fujiwara T, Fox J, et al. Dynamic changes in the intrafollicular inhibin/activin/follistatin axis during human follicular development: relationship to circulating hormone levels. J Clin Endocrinol Metab. 2000;85:3319–3330. doi: 10.1210/jcem.85.9.6767. [DOI] [PubMed] [Google Scholar]
- 95.Lambert-Messerlian G, Taylor A, Leykin L, et al. Characterization of intrafollicular steroid hormones, inhibin, and follistatin in women with and without polycystic ovarian syndrome following gonadotropin stimulation. Biol Reprod. 1997;57:1211–1216. doi: 10.1095/biolreprod57.5.1211. [DOI] [PubMed] [Google Scholar]
- 96.Welt CK, Taylor AE, Fox J, et al. Follicular arrest in polycystic ovary syndrome is associated with deficient inhibin A and B biosynthesis. J Clin Endocrinol Metab. 2005;90:5582–5587. doi: 10.1210/jc.2005-0695. [DOI] [PubMed] [Google Scholar]