Abstract
To address the roles of Wnts in the development of the anterior eye, we used a chicken model to perform comprehensive expression analysis of all Wnt genes during anterior eye development. In analyzing the available genomic sequences, we found that the chicken genome encodes 18 Wnt proteins that are homologous to corresponding human and mouse proteins. The mRNA sequences for 12 chicken Wnt genes are available in GenBank, and mRNAs for six other Wnt genes (Wnt2, Wnt5b, Wnt7b, Wnt8b, Wnt9b and Wnt16) were identified and cloned based on the homology to the genes from other species. In addition, we found that chicken Wnt3a and Wnt7b genes encode two alternative mRNA isoforms containing different first exons. Following in situ hybridization, we found that out of 18 Wnt genes, 11 genes were expressed in the anterior eye, exhibiting distinct temporal-spatial patterns. Several Wnts were expressed in the lens, including Wnt2 and Wnt2b in the anterior epithelium and Wnt5a, Wnt5b, Wnt7a and Wnt7b in the differentiating lens fiber cells. In the cornea, we detected Wnt3a, Wnt6 and Wnt9b in the ocular surface ectoderm, including the corneal epithelium, and Wnt9a in the corneal endothelium from the onset of its differentiation. In the optic cup, Wnt2, Wnt2b and Wnt9a were localized in the rim of the optic cup (presumptive iris), while Wnt5a and Wnt16 were detected in the ciliary epithelium/iris zone of the differentiated optic cup, and Wnt6 was expressed in the iridial mesenchyme. These data suggest that Wnt signaling might play important roles in anterior eye development.
Keywords: anterior angle, ciliary body, chicken, cornea, development, eye, in situ hybridization, iris, lens, Wnt
INTRODUCTION
The anterior part of the eye is a very complex structure that has multiple functions. It can be divided into three major parts: the lens, cornea and anterior uvea. The anterior uvea includes the ciliary body, iris, trabecular meshwork and Schlemm’s canal. These tissues of the anterior eye are derived from cells of different origins: the neural epithelium, surface ectoderm and mesenchymal cells of both the neural crest and cranial mesodermal origin (Cvekl and Tamm, 2004; Gould et al., 2004). The differentiation of the anterior part of the eye proceeds through a series of inductive interactions between different tissues. Thus, the proper functioning of all structures in the anterior segment requires perfect coordination of their development. In the recent past, the efforts of many investigators led to the discovery of the numerous genes involved in the development of the anterior part of the eye, these being mostly transcriptional factors (Cvekl and Tamm, 2004; Gould et al., 2004). However, the molecular nature of the inductive signaling controlling the precise patterning of the anterior segment has remained unknown, for the most part. Pioneering work by Jane and Chris Coulombre (Coulombre and Coulombre, 1964) showed that the first inductive signals that organize the anterior parts of the chicken eye are provided by the lens, but since then, little additional information has been accumulated to allow a full understanding of this process.
The patterning of the anterior part of the eye in a chick embryo begins as a lens vesicle invaginates and detaches from the surface ectoderm at stage 18. At this time, three signaling events occur that are probably controlled by the morphogens secreted by the adjacent tissues and establish the major tissue of the anterior eye. These events include (1) the differentiation of the epithelial cells in the posterior part of the lens vesicle into lens fiber cells, (2) the development of the corneal endothelium in front of the lens, and (3) the differentiation of the anterior part of the optic cup into the ciliary epithelium and iris. Numerous studies have been devoted to understanding lens fiber cell differentiation and elongation (McAvoy and Chamberlain, 1990; Jean et al., 1998; Sue Menko, 2002). For over 20 years, it was believed that fibroblast growth factors (FGFs) were the major regulators of lens fiber cell differentiation. However, recent data imply that the cooperative action of three different signaling pathways (FGF, BMP and WNT) can control lens fiber cell differentiation, but the exact identity of the ligands remains unknown (Belecky-Adams et al., 2002; Stump et al., 2003; de Iongh et al., 2004; Lyu and Joo, 2004; Lovicu and McAvoy, 2005).
The role of the morphogens secreted by the lens in corneal development has been clearly demonstrated in many independent experiments (Genis-Galvez et al., 1967; Zinn, 1970; Beebe and Coats, 2000). It is likely that lens-secreted signals regulate two steps during corneal development. The first is the formation of the primary corneal stroma. To date, there are no data available about the source of the signaling molecules that control this event. The second step in corneal development is the migration of the first wave of neural crest cells along the primary stroma, followed by formation of the corneal endothelium. There is compelling experimental evidence that the lens epithelium controls the formation of the corneal endothelium, and removing the lens abolishes the orderly migration of presumptive endothelial cells and subsequent mesenchymal-epithelial transition (Genis-Galvez et al., 1967; Beebe and Coats, 2000). Consequently, the anterior chamber fills with the loose mesenchymal cells. Interestingly, a 90° rotation of the lens allows the normal cornea to develop in close proximity to the lens epithelium, while the second part of the anterior chamber facing the lens fiber cells fills with mesenchymal cells (Beebe and Coats, 2000). This experiment clearly demonstrates that the lens secretes soluble signals acting only at a short distance.
Differentiation of the anterior part of the optic cup into the ciliary epithelium and iris is the least studied aspect of eye development (Coulombre and Coulombre, 1964; Thut et al., 2001). The regional specification of the optic cup into ciliary epithelium/iris (anterior part of the optic cup) and retina/RPE starts at approximately stage 18 (day 3) of chicken embryo development. Removing the lens at this stage abolishes differentiation of the ciliary epithelium and iris (Coulombre and Coulombre, 1964). The molecular nature of the inductive signal(s) that induces the ciliary epithelium differentiation remains unknown. The BMP signaling was shown to be involved at a later stage of the ciliary body development (Zhao et al., 2002).
Several recent studies suggested that Wnt signaling might be involved in the patterning of the anterior eye segment. First, analysis of TCF/Lef-LacZ transgenic mice revealed the activation of a canonical Wnt signaling pathway during early development of the lens epithelium and the anterior part of the optic cup (Liu et al., 2003). Second, mice lacking either of the Frizzled co-receptors (LRP5 or LRP6) demonstrated abnormal eye development. LRP6 knockout mice were microophthalmic and had severe defects in their retinal, lens and corneal development (Gong et al., 2001; Stump et al., 2003; Jiao et al., 2004; Toomes et al., 2004). In LRP5 knockout mice, the hyaloid vasculature did not regress and was retained throughout the lifetime of the mice (Gong et al., 2001). A mutation in human LRP5 was associated with osteoporosis-pseudoglioma syndrome, resulting in multiple eye defects, including microphthalmia, vitreoretinal abnormalities, cataract, or absence of the anterior eye chamber (Jiao et al., 2004; Toomes et al., 2004). LRP co-receptors are required for the canonical Wnt/β-catenin signaling pathway (Logan and Nusse, 2004). Two recent papers suggested that Wnt signaling plays multiple roles in lens development. Lyu et al have shown that Wnt3a stimulates lens fiber cell differentiation in vitro, but this process requires prior treatment with FGF (Lyu and Joo, 2004) and does not depend on β-catenin. Alternatively, in the absence of FGF treatment Wnt3a promotes lens epithelium proliferation through a β-catenin dependent pathway. Using a conditional transgenic approach, Smith et al have demonstrated that the β-catenin dependent pathway is essential for proper lens morphogenesis, but an activation of β-catenin prior to the lens placode formation completely abolishes lens development (Smith et al., 2005). Finally, several genes involved in Wnt signaling pathways were shown to be expressed during anterior eye development (Jasoni et al., 1999; Sumanas and Ekker, 2001; Jin et al., 2002; Fuhrmann et al., 2003; Stump et al., 2003; Ang et al., 2004; Van Raay and Vetter, 2004). However, the expression information is very fragmented and incomplete. Therefore, to identify the Wnt proteins involved in the development of the anterior part of the eye, we performed a comprehensive analysis of the expression patterns for 18 chicken Wnt genes during the key stages of the anterior eye differentiation.
RESULTS
Chicken Wnt genes
To date, 19 Wnt ligands have been identified in human and mouse genomes (Miller, 2002; Logan and Nusse, 2004). However, when we started this project, the sequence information was available only for 12 chicken Wnt genes. To identify additional chicken Wnts, we performed BLAST analysis against the Chicken Genome Trace Archive using the nucleotide sequences of the known human, mouse and Xenopus Wnt genes. Identified sequences were used to design primers for RT-PCR. The PCR fragments were cloned, sequenced and compared to available sequences in the Trace Archive to determine the consensus sequences. We identified the full or partial cDNA sequences for 3 new chicken Wnts: Wnt2, Wnt9b and Wnt16 and for 3 genes (Wnt5b, Wnt7b and Wnt8b), which were described in the literature (Hollyday et al., 1995; Theodosiou and Tabin, 2003), but for which mRNA sequences were not available in GenBank (Table 1). We have also obtained longer cDNA sequences for Wnt1, Wnt3 and Wnt10a. Recently, the full coding sequence for Wnt10a has become available (accession number AB177400). To date, we have not cloned only the chicken Wnt10b, because the part of the genome that is supposed to contain the Wnt10b gene has not been completely sequenced yet. To obtain the entire coding sequence for Wnt3a, Wnt7b and Wnt9b mRNAs, we performed 5’RACE. We found that the Wnt7b gene was transcribed as two isoforms containing alternative first exons. We also identified a second isoform containing an alternative first exon for the chicken Wnt3a gene. In addition, the cloned sequence for Wnt9a mRNA differed from the sequence available in GenBank (accession number AF031168) by a one-nucleotide insertion that led to a significant change in the N-terminal amino acid sequence. The new sequence for the Wnt9a mRNA is predicted to encode a protein that contains a classical secretory peptide and has high homology to Wnt9a genes from different species. The cDNA sequences for all new chicken Wnts were deposited into GenBank; accession numbers are listed in Table 1. All isolated chicken Wnt proteins demonstrated a high homology to known human Wnts (Fig.1 and Table 1).
Table 1.
A complete list of chicken Wnt genes and summary of Wnt expression in anterior eye.
| Gene | Accession number | Homology to human ortholog | Expression in an anterior eye |
|---|---|---|---|
| Wnt1 | AY753286* | 90.50 | no expression |
| Wnt2 | AY753287* | 83.89** | anterior lens epithelium, iris |
| Wnt2b | BX935899 | 76.21 | anterior lens epithelium, iris, corneal epithelium |
| Wnt3 | AY753288* | 97.87 | no expression |
| Wnt3a_v1 Wnt3a_v2 |
NP_990006 DQ022307* |
87.50 82.77 |
corneal epithelium |
| Wnt4 | BAA06698 | 85.19 | no expression |
| Wnt5a | AB006014 | 88.57 | equatorial lens epithelium, anterior part of the optic cup |
| Wnt5b | AY753289* | 85.52 | lens fiber cells |
| Wnt6 | NM_001007594 | 73.41 | corneal epithelium, irideal mesenchyme |
| Wnt7a | AB045629 | 93.70 | lens fiber cells |
| Wnt7b_v1 Wnt7b_v2 |
AY753290* AY753291* |
95.13 89.11 |
lens fiber cells |
| Wnt8b | AY753292* | 82.02 | no expression |
| Wnt8c (Wnt8a) | U02097 | 70.14 | no expression |
| Wnt9a | AY753293* | 82.11 | corneal endothelium, iris |
| Wnt9b | AY753294* | 76.82 | corneal epithelium |
| Wnt10a | AY753295* | 74.83 | no expression |
| Wnt11 | D31901 | 84.75 | no expression |
| Wnt16 | AY753296* | 85.89 | anterior part of the optic cup |
marks the Wnt genes identified in this study
We used a full-length Wnt2 sequence that was predicted from the Chicken genomic sequence but has not been yet confirmed by cloning.
The sequences that are not full-length are in italic.
Figure 1.
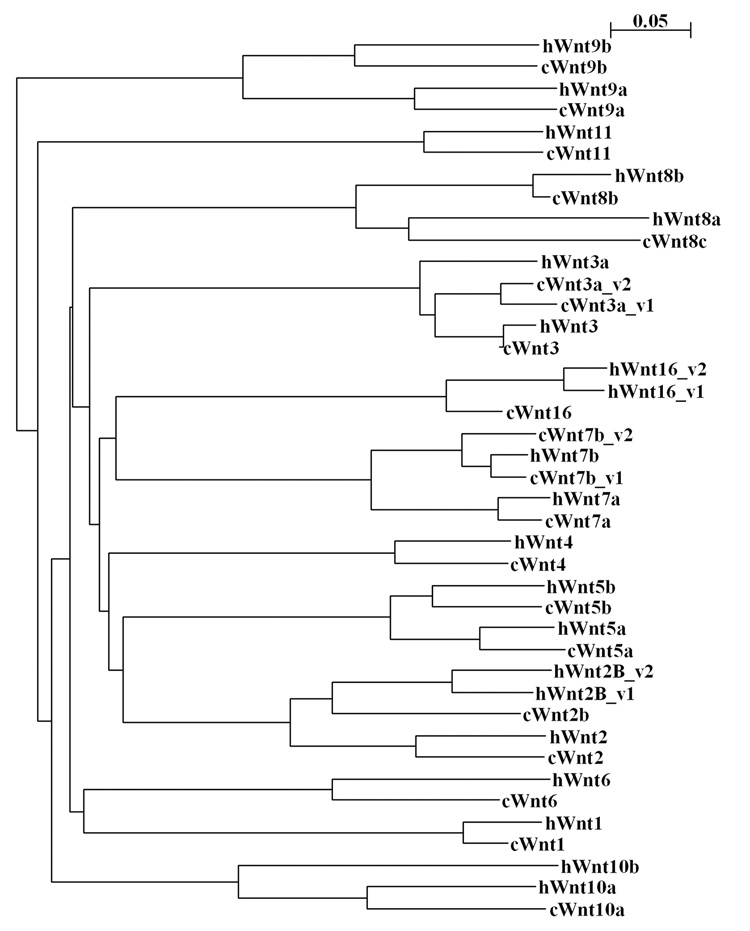
The phylogenetic tree shows the sequence relationships among chicken and human Wnt proteins. The tree was built using a ClustalW program at EMBL-EBI server (http://www.ebi.ac.uk/clustalw/). The branch lengths are proportional to the amount of inferred evolutionary change.
Summary of expression patterns
As a first step toward understanding the role of Wnt signaling in the development of the anterior segment of the eye, we have analyzed the expression of all chicken Wnt genes during anterior eye development. Since the anterior structures of the eye are very complex, it is not feasible to precisely dissect specific tissues for PCR-based analysis. Therefore, we used in situ hybridization to determine the localization of different messenger RNAs. First, we examined the expression of all Wnt genes at three different stages representing transitional phases during development of the anterior segment of the eye: stages 23–24 (E4), 26–27 (E5) and 30–31 (E7). At stages 23–24, the primary lens fiber cells and ciliary epithelium continue to differentiate; the neural crest cells accumulated at the optic cup margins start to differentiate into the corneal endothelium and migrate along primary stroma. At stages 26–27, the secondary lens fiber cells appear, the corneal endothelium are formed, and the second wave of the neural crest cells migrate into the corneal stroma. At stages 30–31, the neural crest cell migration is completed, and those cells start to differentiate into corneal keratocytes; the differentiation of other structures of the anterior uvea (trabecular meshwork, ciliary body, iris muscles, etc) begin. To ensure that the hybridization probes were gene-specific and did not cross-hybridize to other Wnt genes, we confirmed that all probes had distinct expression patterns throughout the embryo at stages 19–24. Of 18 Wnt genes analyzed, 11 genes were expressed in the anterior part of the eye in distinct spatial and temporal patterns (Table 1). For these Wnt genes, we additionally analyzed expression early in the development process to determine the stage at which they began to be expressed in the anterior eye. We will describe the expression patterns for each gene below.
Wnt2 and Wnt2b
We first detected Wnt2 expression in the dorsal tip of the optic cup (presumptive iris) and anterior lens epithelium at stage 23 (Fig. 2A). As the embryo developed, the Wnt2 signal became more distinct in both layers of the presumptive iris (pigmented and non-pigmented) and in the anterior lens epithelium (Fig. 2B, stage 26 and Fig. 2C, stage 29). However, the Wnt2-specific signal was weaker at all stages than that of its closest homolog, Wnt2b. The expression profile for Wnt2b, previously named Wnt13, was identical to that described by another research group (Jasoni et al., 1999) and is not included in this report.
Figure 2.
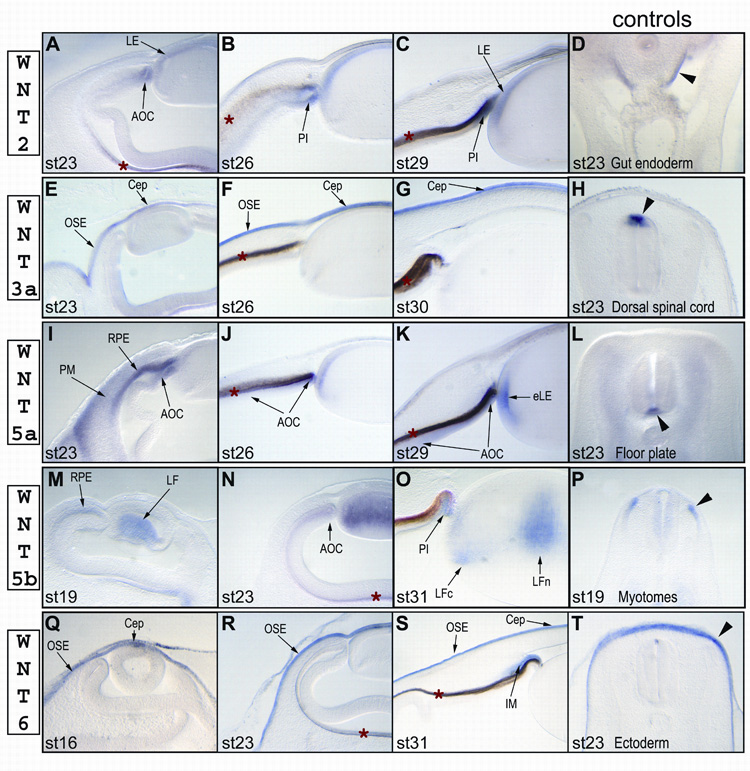
Expression profiles of chicken Wnt2 (A–D), Wnt3a (E–H), Wnt5a (I–L), Wnt5b (M–P), and Wnt6 (Q–T) in the anterior eye at different stages of development. The last panel in each row (Sections D, H, L, P and T) shows the expression in the control tissues (indicated by black arrowheads) for that row: section D shows Wnt 2 expression in the gut endoderm (Theodosiou and Tabin, 2003); section H shows Wnt3a expression in the dorsal spinal cord (Hollyday et al., 1995); section L shows Wnt5a expression in the floor plate of the spinal cord (Hollyday et al., 1995); section P shows Wnt5b expression in the dorsomedial lip of the dermomyotome (Linker et al., 2003); and section T shows Wnt6 expression in the surface ectoderm (Schubert et al., 2002). Abbreviations: AOC – anterior part of the optic cup, Cep – corneal epithelium, eLE – equatorial lens epithelium, IM – irideal mesenchyme, LE – lens epithelium, LF – lens fiber cells, LFc – cortical lens fiber cells, LFn – nuclear lens fiber cells, OSE – ocular surface epithelium, PI – presumptive iris epithelium, PM – periocular mesenchyme, RPE - retinal pigmented epithelium. A red asterisk (*) marks the retinal pigmented epithelium, which has a light or dark brown color due to natural pigmentation.
Wnt3a
In the eye, we first observed Wnt3a expression at stage 23 in the ocular surface epithelium ventral to the corneal epithelium (Fig. 2E). By stage 26, Wnt3a was strongly expressed in the entire ocular surface, including the corneal epithelium (Fig. 2F). The signal remained strong at stage 30 (Fig. 2G), the last stage analyzed. The Wnt3a-specific probe was not designed to distinguish between different isoforms. It should be noted that Jin et al reported the expression of Wnt3, but not of Wnt3a, in the corneal endothelium at stage 27 (Jin et al., 2002). However, we could not detect Wnt3 in the corneal epithelium at any stages, even through the Wnt3-specific probe has demonstrated a strong signal in the brain (data not shown). We could not exclude the possibility that Fast-Red substrate used by Jin et al is more sensitive than NBT/BCIP reagent used in our study and detects very low concentrations of Wnt3 transcripts in the corneal epithelium. Another explanation could be that Fast-Red substrate produces a high background which will be more intense in dense tissue, such as the corneal epithelium, versus that of the adjacent corneal mesenchyme. We have also repeated in situ hybridization with two different Wnt3a probes to confirm the specificity of the Wnt3a signal in the cornea.
Wnt5a
At stage 17, we found that Wnt5a was expressed in the outer layer of the optic cup and periocular mesenchyme (data not shown). At stage 23, we first detected Wnt5a in the equatorial region of the lens, where lens epithelial cells exit the cell cycle and start to elongate (Fig. 2I–K), and the signal gradually becomes stronger by stage 29. From stage 23, Wnt5a was also expressed in the both layers (pigmented and non-pigmented) of the anterior optic cup (Fig. 2I–K). By stage 30, we could clearly detected Wnt5a in the equatorial lens epithelial cells and in the ciliary epithelium/iris region of the optic cup (Fig. 2K). Although Wnt5a was expressed in the periocular mesenchyme, it was excluded from the anterior ocular mesenchyme (Fig. 2I).
Wnt5b
Wnt5b expression started in the lens fiber cells just before they began to elongate at stage 17 (Fig. 2M, shows stage 19). At stage 23, all primary fiber cells were expressing Wnt5b (Fig. 2N). From stage 31, Wnt5b was present in two distinct areas in the lens: a weak signal was detected in newly differentiated cortical fiber cells, and a stronger signal was seen in the central mature fiber cells that had already detached from the lens capsule (Fig. 2O). It is puzzling that Jin et al did not detected Wnt5b or Wnt7b (described below) in the lens (Jin et al., 2002), and, thus, we have also confirmed the presence of Wnt5b and Wnt7b in the lens by PCR (data not shown).
Wnt6
We first detected Wnt6 in the head ectoderm, including the ocular surface, at stage 16 (Fig. 2Q). However, Wnt6 was clearly excluded from the invaginated lens vesicle. After the lens vesicle had closed at stage 18, Wnt6 expression was detected in entire ocular surface epithelium, including the corneal epithelium (Fig. 2R, shows stage 23). Wnt6 expression remained strong in the ocular surface epithelium at least till stage 31 (Fig. 2S). Beginning from stage 26, we also found Wnt6 in the iridial mesenchyme (Fig. 2S, stage 31).
Wnt7a
In the eye, we found Wnt7a expression only in the cortical lens fiber cells (Fig. 3A–C). The signal was detected first at stage 23, and become gradually stronger by stage 31. Wnt7a transcripts were clearly excluded from the equatorial lens epithelium (Fig. 3C).
Figure 3.
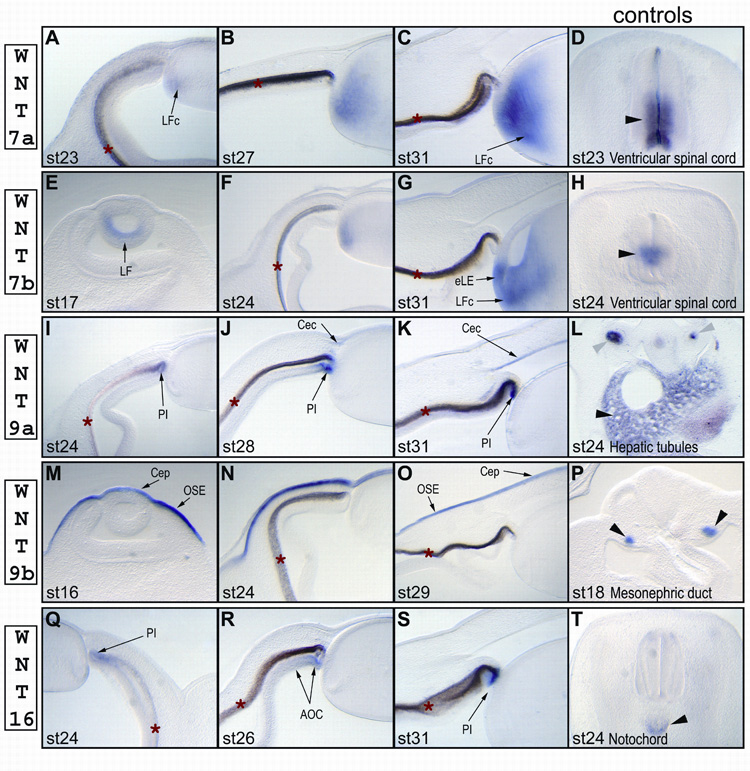
Expression profiles of chicken Wnt7a (A–D), Wnt7b (E–H), Wnt9a (I–L), Wnt9b (M–P) and Wnt16 (Q–T) in the anterior eye at different stages of development. The last panel in each row (Sections D, H, L, P and T) shows the expression in the control tissues (indicated by black arrowheads) for that row: section D shows Wnt7a expression in the ventricular epithelium of the spinal cord (Riddle et al., 1995); section H shows Wnt7b expression in the ventricular epithelium of the spinal cord (Hollyday et al., 1995); section L shows Wnt9a expression in the hepatic tubules (indicated by black arrowhead) and lung buds (indicated by gray arrowheads); section P shows Wnt9b in the mesonephric duct (Carroll et al., 2005); and section T shows Wnt16 expression in the notochord. Abbreviations: AOC – anterior part of the optic cup, Cep – corneal epithelium, Cec – corneal ectoderm, eLE – equatorial lens epithelium, LE – lens epithelium, LF – lens fiber cells, LFc – cortical lens fiber cells, LFn – nuclear lens fiber cells, OSE – ocular surface epithelium, PI – presumptive iris epithelium. A red asterisk (*) marks the retinal pigmented epithelium, which has a light or dark brown color due to natural pigmentation.
Wnt7b
We first found Wnt7b expression in the posterior lens epithelium at stage 17, just before lens fiber cells began to elongate (Fig. 3E). By stage 24, the signal gradually faded from the lens nuclear fiber cells (Fig. 3F). Starting from this stage, Wnt7b was expressed in newly differentiated fiber cells, but in a broader region than that found for Wnt7a; a Wnt7b-specific signal was also present in the equatorial lens epithelial cells that had just begun to differentiate (Fig. 3G). Our Wnt7b-probe was not aimed at distinguishing between the alternative isoforms of Wnt7b. However, PCR analysis revealed that both isoforms are present in the chick lenses at stage 18 (data not shown).
Wnt9a
Wnt9a was expressed in the anterior rim of the optic cup and the corneal epithelium. Wnt9a signal first appeared at stage 24 (Fig. 3I) in the tip of the optic cup (presumptive iris) and persisted there up to stage 31 (Fig. 3K). As the corneal endothelium started to differentiate at stage 25, the cells migrating along the primary corneal stroma to form corneal endothelium began to express Wnt9a (Fig. 3J, shows stage 28). Wnt9a expression remained in the corneal endothelium up to stage 31 (Fig. 3K).
Wnt9b
Wnt9b was detected in the ocular surface, including the corneal epithelium. Wnt9b expression started just before stage 16 in the brain ectoderm, and extended to the ectoderm of the branchial arches (Fig. 3M and data not shown). By stage 24, Wnt9b signal gradually disappeared from the ectoderm of the branchial arches and remained only in the ocular surface ectoderm, including the corneal epithelium (Fig. 3N and data not shown). Wnt9b transcript persisted in the ocular ectoderm up to stage 29 (Fig. 3O).
Wnt16
In the eye, Wnt16 was first detected at stage 24 in the non-pigmented layer of the anterior optic cup (Fig. 3Q), and its expression was initiated in the ventral part of the optic cup. By stage 26, Wnt16 transcripts were localized in the non-pigmented layer of the ciliary epithelium and iris (Fig. 3R). By stage 31, Wnt16 expression gradually disappeared from the ciliary epithelium, and the signal became stronger in the tip of the optic cup (presumptive iris, Fig. 3S).
DISCUSSION
Wnts are a group of secreted glycoproteins that play important roles in embryogenesis (Miller, 2002; Veeman et al., 2003; Yanfeng et al., 2003; Yang, 2003; Logan and Nusse, 2004; Van Raay and Vetter, 2004; Wang and Wynshaw-Boris, 2004). Currently, there are 19 known Wnt protein-coding genes in the human and mouse genomes. The Wnt proteins signal through 10 different Frizzled receptors, which are seven-pass transmembrane proteins located on the external membrane. Upon binding to the receptor, a Wnt ligand can induce three different signaling cascades: 1) the canonical Wnt pathway that acts through β-catenin and TCF transcription factors, 2) the Wnt/Ca2+ pathway, and 3) the planar cell polarity (PCP) pathway. The combination of distinct Wnt and Frizzled proteins determines the activation of a particular pathway. Wnt signaling is also complicated by the existence of many modulators that interact with Wnt ligands, Frizzled receptors, TCF transcriptional factors, or β-catenin. The large number of Wnts and Wnt receptors and their ability to signal through alternative pathways complicates the analysis of the role of Wnts during development.
Different Wnt signaling pathways have been shown to regulate the development and maintenance of major organs. However, the role of Wnt signaling in anterior eye development has been poorly analyzed. As a first step to understanding the roles of Wnt signaling in anterior eye development, we analyzed the temporal and spatial expression of 18 chicken Wnt genes during the first week of the chicken embryonic development.
Analysis of chicken Wnt genes
To perform a comprehensive analysis of Wnts expression during anterior eye development, we first isolated several additional chicken Wnt genes. We determined that the chicken genome encodes a total of 18 Wnt genes missing only Wnt10b. All chicken Wnt proteins are orthologues of corresponding human Wnts (Fig. 1) having homology of over 80%, with the exception of the Wnt2b, Wnt6, Wnt8a, Wnt9a and Wnt10a proteins that have homology of between 70 and 80 percent (Table 1).
The present data and previous studies reveal that several Wnt genes contain alternative first exons which, in all cases, encode different signaling peptides. The most striking example is the chicken Wnt3a gene. The previously published isoform 1 (Wnt3a_v1, accession number NP_990006) encodes a protein that lacks a signaling peptide and is not secreted under standard tissue culture conditions (EF, unpublished data). This isoform is probably secreted through alternative pathways similar to those described for other leaderless proteins, such as, IL-1, FGF, etc (Prudovsky et al., 2003). In contrast, isoform 2 (Wnt3a_v2, accession number DQ022307) contains a classical signaling peptide, as was predicted using SignalIP 3.0 (http://www.cbs.dtu.dk/services/SignalP); this form is secreted when overexpressed in tissue culture (EF, unpublished data). Alternative transcripts are not unique to chicken Wnt3a, and it was reported that human Wnt2b (Katoh et al., 2000) and Wnt16 (Fear et al., 2000) genes also have two alternative isoforms, of which one does not have a classical signaling peptide. Interestingly, the secreted isoforms for Wnt3a, Wnt2b and Wnt16 are present in all species, and the leaderless isoforms seem to be unique to particular species. To date, the presence of the second leaderless isoform for Wnt3a, Wnt2a and Wnt16 has not been shown in other species. Thus, it will be important to determine if these leaderless isoforms have distinctive species-specific functions.
We have also found that the chicken Wnt7b gene has two isoforms containing different first exons (Wnt7b_v1 and Wnt7b_v2; accession numbers AY753290 and AY753291). However, in this case, both protein variants were predicted to have different signaling peptides. Computational analysis revealed that at least two other species also have two isoforms of Wnt7b. The second alternative transcript of the mouse Wnt7b was recently submitted in GenBank (Accession number AAH66003). The existence of a second mouse isoform is supported by the presence of several expression sequence tags (ESTs) sequenced from different cDNA libraries. Interestingly, two different Wnt7b knock-out mice have different phenotypes (Parr et al., 2001; Shu et al., 2002). The transgenic mice lacking the first exon had a milder phenotype than mice lacking the third exon. In view of our findings, this difference could be explained by the presence of a transcript with an alternative first exon. In addition, we found that two Wnt7b isoforms can be predicted from the dog genomic sequence. These findings suggest the existence of an additional layer of regulation in Wnt signaling. Thus, a comprehensive analysis of the expression of the two isoforms will be needed to understand the significance of the alternative mRNAs.
Wnt signaling in lens development
In this study, we found that six Wnt genes are expressed in the embryonic chick lens. Their expression patterns strongly correlate with the specific steps in the lens and anterior eye differentiation. We have shown that the expression of first two Wnts, Wnt 5b and Wnt7b, begins in the posterior lens epithelium just prior to the initiation of lens fiber cell elongation at stage 17, and, thus, suggests that these two Wnts are involved in lens fiber cell differentiation. Recently, Lyu et al have shown that Wnt3a is expressed in rat fiber cells and can induce lens fiber cell differentiation in epithelial explants pretreated with FGF in vitro (Lyu and Joo, 2004). However, Wnt3a is not expressed in the chick or mouse lenses, but Wnt5b and Wnt7b are expressed in the lens fiber cells in all analyzed species, including the mouse, rat and chick (Liu et al., 2003; Ang et al., 2004; Lyu and Joo, 2004). Taken together, these results imply that Wnt5b and/or Wnt7b are more likely to regulate lens fiber cell differentiation in vivo in most of the species, but we could not exclude that some species-specific differences exists. Interestingly, Wnt5b expression is downregulated in the cortical lens fiber cells by E7, and upregulated in the nuclear fiber cells when they have just detached from the posterior lens capsule. These fiber cells start to form unique cell-cell connections (syncytium) that allow large molecules, including proteins, to diffuse between the cells (Shestopalov and Bassnett, 2000). This expression pattern suggests that Wnt5b might, in addition, regulate the formation of the lens syncytium. The expression of third, fiber cell-specific Wnt, Wnt7a, starts later at stage 23 and coincides with the differentiation of the secondary fiber cells, and, accordingly, Wnt7a might be involved in regulation of secondary fiber morphogenesis.
Lyu et al have also shown that Wnt3a also increases the proliferation rate in the rat lens epithelial explants in vitro and suggested that Wnt signaling regulates anterior lens epithelial proliferation (Lyu and Joo, 2004). We have shown that Wnt2 and Wnt2b, but not Wnt3a, are expressed in chick lens epithelium, and therefore, these two Wnts might regulate lens epithelium proliferation in a chick embryo. Interestingly, we found that Wnt5a, which has been shown to signal through a non-canonical pathway and antagonize canonical Wnts (Wallingford et al., 2001), is expressed in the equatorial epithelial cells, and, thus, might control the withdrawal of the equatorial cells from the cell-cycle and the activation of lens fiber cell differentiation.
Wnt signaling in corneal development
We found that three Wnt genes (Wnt2b, Wnt6 and Wnt9b) are expressed in the ocular surface ectoderm, including the corneal epithelium, starting from the onset of the lens vesicle closing at stage 16. Recently, Smith et al have shown that forced activation of Wnt/β-catening signaling in ocular surface ectoderm abolishes lens placode development, and the suppression of Wnt signaling induces the formation of ectopic lenses in the head surface (Smith et al., 2005). Therefore, one of the functions of Wnts in the ocular ectoderm could be to set boundaries between the lens placode and presumptive corneal epithelium. Interestingly, a different set of Wnt genes (Wnt2, Wnt3, Wnt4, and Wnt6) is expressed in the embryonic mouse surface ectoderm at equivalent stages of development (Liu et al., 2003). These data imply that Wnt signaling is important in corneal epithelium morphogenesis, but identities of Wnts vary between species. The expression of the fourth Wnt gene, Wnt3a, in the ocular ectoderm starts later at stage 23 and coincides with the onset of the secondary corneal stroma development.
The signaling molecules regulating corneal endothelial development are largely unknown, except that those molecules are secreted by the lens (Beebe and Coats, 2000). Our expression study suggests that three Wnts, Wnt2, Wnt2b and Wnt9a, might regulate corneal endothelium differentiation. Wnt2 and Wnt2b expression starts in the lens epithelium just before initiation of corneal endothelial morphogenesis, and, thus, suggests that these Wnts might be the lens-secreted morphogens that control corneal endothelial differentiation. As the mesenchymal cells, committed to form corneal endothelium, start to migrate between the lens epithelium and the primary corneal stroma, they begin to express Wnt9a, which might be involved in control of the mesenchymal-epithelial transformation.
Wnt signaling in anterior optic cup patterning
Only Wnt2b expression was described in the anterior part of the optic cup in chick and mouse eyes (Jasoni et al., 1999; Liu et al., 2003). We now report the expression of five more Wnt genes (Wnt2, Wnt5a, Wnt5b, Wnt9a and Wnt16). Wnt2, Wnt2b and Wnt9a transcripts are detected in the rim of the optic cup (presumptive iris) starting from stage 23. The role of Wnt2b in the optic cup morphogenesis was extensively studied. Overexpression of Wnt2b has been shown to inhibit differentiation and promote proliferation of the retinal progenitor cells and also to inhibit ciliary epithelium differentiation (Kubo et al., 2003; Kubo et al., 2005). The expression of Wnt2b in the presumptive iris correlates with an inhibitory role of Wnt2b in ciliary epithelium development. However, the expression of 5 more Wnt genes in the anterior optic cup suggests that a different Wnt, than Wnt2b, might regulate the fate of neural progenitor cells in vivo.
Only two Wnt genes, Wnt5a and Wnt16, are expressed in both parts of the anterior optic cup, the ciliary epithelium and iris, but Wnt16 transcripts become gradually restricted to the presumptive iris by stage 31. At the same time (stage 31), Wnt5b expression is initiated in the presumptive iris. Interestingly, these changes in the expression of Wnt16 and Wnt5b genes correlate with the loss of expression of the early marker of the ciliary epithelium, the long-isoform of collagen IX α1 chain (EF, unpublished data) and the formation of the ciliary folds. In the mouse model, Liu et al demonstrated that the ciliary epithelium/iris region of the optic cup has active Wnt/β-catenin signaling (Liu et al., 2003). Thus, Wnt5a and/or Wnt16 might act through a β-catenin-dependent pathway. Moreover, the expression of Wnt5a and Wnt16 in the ciliary margin zone next to the developing retina indicates that one of these Wnts might maintain retinal progenitor cells in the undifferentiated state in vivo. Interestingly, a Wnt5a-specific signal is also present in the RPE and gradually decreases with the progression of retinal differentiation.
In summary, we have shown that the chicken genome encodes at least 18 Wnts. The expression of all chicken Wnt genes was extensively analyzed during anterior eye development. Our data demonstrate that 11 Wnts are expressed during the development of the anterior segment of the eye in dynamic patterns; 6 genes are expressed in the lens, 5 in the cornea and 6 in the anterior optic cup. These data suggest that different Wnt signaling pathways, canonical and non-canonical, are involved in the patterning of the anterior segment of the eye. Moreover, we predict that a precise balance between Wnt ligands and their modulators at different stages of development determines the anterior segment differentiation. The comprehensive expression analysis of Wnt modulators and Wnt receptors is underway in our research group. Our knowledge of expression patterns of genes involved in Wnt signaling pathways will provide a basis for further functional studies of Wnt signaling in anterior eye development
EXPERIMENTAL PROCEDURES
Embryos
Fertilized White Leghorn chick eggs were purchased from SPAFAS Avian Products (North Franklin, CT). Eggs were incubated at 38°C until desired stages. Embryos were staged according to the Hamburger-Hamilton series (Hamburger and Hamilton, 1992).
Cloning of chicken Wnt cDNAs
Total RNA was purified from several chick embryonic tissues at different stages using TRIzol reagent (Invitrogen Corp., Carlsbad, CA) according to the manufacturer's protocol. The cDNAs were synthesized using Superscript II or Superscript III reverse transcriptase (Invitrogen) and random decamers. For known chicken Wnt genes (Wnt2b, 3a, 4, 5a, 6, 7a, 8c, 9a and 11), primers were selected based on published sequences. We identified the new chicken Wnt genes (Wnt2, 3, 5b, 7b, 8b and 16) or extended sequences for Wnt1, 3 and 10a by BLAST analysis against the Chicken Trace Archive, using nucleotide sequences of known human, mouse or Xenopus genes. To clone cDNAs, primers were designed based on assembled sequences; primer information can be provided upon request. PCR reactions were performed under standard conditions using Platinum Taq polymerase (Invitrogen). The PCR fragments were cloned into pGEM3z(f+) plasmid (Promega). At least two clones were sequenced for each cDNA; consensus sequences of two or more clones were also compared with sequences in the Chicken Trace Archive. To obtain the full-length coding sequences for Wnt3a, Wnt7b and Wnt9b, 5’ RACE was performed using FirstChoice™ RLM-RACE Kit (Ambion). The cDNA sequences for Wnt1, Wnt2, Wnt3, Wnt3a_v2, Wnt5b, Wnt7b_v1, Wnt7b_v2, Wnt8b, Wnt9a, Wnt10a and Wnt16 were submitted to GenBank. The accession numbers are provided in Table 1.
In situ hybridization in thick slices
We developed a new in situ hybridization protocol that is particularly useful for fragile embryonic eyes. The commonly used protocols require using frozen sections. Despite the fact that the use of frozen sections is believed to better preserve mRNAs, the tissue morphology, particularly in the anterior segment of the eye, is usually severely damaged. The whole-mount in situ hybridization often gives false optic cup staining due to the trapping of reagents in the vitreous cavity. We adapted a procedure for embedding the samples in a mixture of acrylamide gel with agarose, previously described by Dr. David Beebe (Beebe and Coats, 2000), that generates very reproducible results.
Embryos were collected at stages from E2 to E7 and fixed overnight in 4% paraformaldehyde. After being washed in PBS for 1 h, embryos were incubated in 1.5 ml of acrylamide solution (10% 30:1 acrylamide in PBS containing 3 µl/ml of TEMED) for 15 min at room temperature, and then for 5–10 min in a 48°C water bath. Then 4.5 ml of 4% agarose (SeaKem LE, Cambrex) in PBS was added and the solution was mixed thoroughly. Then the samples were incubated 1–2 min at 48°C. To polymerize the acrylamide, 180 µl of freshly prepared 4% ammonium persulfate was mixed with this solution. Samples were returned to a water bath at 45°C for 45 min for complete polymerization of the acrylamide, and then placed on ice for at least 1 hour to solidify the agarose. Next, the samples were sectioned at 70–200 µm using a vibratome (Leica VT1000S).
To synthesize digoxigenin-labeled antisense RNAs, we used a DIG RNA labeling kit (SP6/T7) (Roche Applied Science). The reactions were performed according to the manufacturer’s recommendations. The probes were purified using NucAway Spin Columns (Ambion) and precipitated with LiCl. The resulting probes were dissolved in 200 µl of formamide, tested for RNA integrity by agarose gel electrophoresis, and stored at −80°C.
In situ hybridization was performed on floating sections in 6- or 12-well Costar plates. For E5 and E7 embryos, sections were pretreated with 10–20% hydrogen peroxide in PBS for 1–2 h to reduce RPE pigmentation. After a brief wash in PBS, sections were incubated in proteinase K solution in PBS/0.5% Triton X-100 for 30 min, for which concentration was adjusted according to the ages of the embryos. After two washes in PBS for 10 min, sections were additionally fixed in 1% paraformaldehyde for 15 min and washed in PBS/0.5% Triton X-100 3 times for 15 min. The washing solution was replaced with HYB mix (50% formamide, 5xSSC, pH7.0, 0.1% Tween-20, 0.1% SDS, 100 µg/ml heparin, 1mM EDTA) and the sections were incubated for 14 min. Then new HYB mix supplemented with tRNA (200 µg/ml) was added, and the sections were placed in a hybridization oven for 3–4 hours at 63°C. After pre-hybridization, the solution was replaced with HYB mix containing tRNA and an antisense probe (2–3 µl/ml). Hybridization was carried out for 16–20 hours at 63°C. The washing steps were performed in the following order at 63°C: (1) SSC-FT (50% formamide, 2xSSC, pH7.0, 0.1% Tween-20, 0.5% SDS) twice for 30 min; (2) 1:1 mixture of SSC-FT and SOL2 (10 mM Tris·HCl, pH 7.5, 0.5 M NaCl, 0.1% Tween-20) for 30 min; (3) SOL2 for 30 min. Finally, the sections were washed in KTBT (50 mM Tris-HCl, pH7.5, 150 mM NaCl, 10 mM KCl, 0.1% Tween-20) twice for 30 min at room temperature. Next, the sections were incubated in a blocking solution containing 1% blocking reagent (Roche Applied Science) and 10% sheep serum in PBS/0.1% Tween-20 for 3 hours, incubated with anti-DIG Fab antibodies (Roche Applied Science) in blocking solution at 4°C overnight. After four hour-long washes with KTBT, the sections were equilibrated in detection buffer NTMT (100 mM Tris-HCl, pH9.5, 100 mM NaCl, 5 mM MgCl2, 0.1% Tween-20). Detection was carried out with NBT/ BCIP reagent (4.5 µl/3.5 µl per ml of NTMT) or Blue reagent (Roche Applied Science) at room temperature. When the color had developed to the desired extent, the sections were washed with PBS and mounted in 50% glycerol in PBS.
ACKNOWLEDGEMENTS
We thank Mrs. Mardelle Susman for technical assistance with preparation of the manuscript. We also thank Drs. Nancy Wills and David Konkel for critical reading of the manuscript. This research was supported by NIH grant R01 EY012973 to E.F.
REFERENCES
- Ang SJ, Stump RJ, Lovicu FJ, McAvoy JW. Spatial and temporal expression of Wnt and Dickkopf genes during murine lens development. Gene Expr Patterns. 2004;4:289–295. doi: 10.1016/j.modgep.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Beebe DC, Coats JM. The lens organizes the anterior segment: specification of neural crest cell differentiation in the avian eye. Dev Biol. 2000;220:424–431. doi: 10.1006/dbio.2000.9638. [DOI] [PubMed] [Google Scholar]
- Belecky-Adams TL, Adler R, Beebe DC. Bone morphogenetic protein signaling and the initiation of lens fiber cell differentiation. Development. 2002;129:3795–3802. doi: 10.1242/dev.129.16.3795. [DOI] [PubMed] [Google Scholar]
- Carroll TJ, Park JS, Hayashi S, Majumdar A, McMahon AP. Wnt9b plays a central role in the regulation of mesenchymal to epithelial transitions underlying organogenesis of the mammalian urogenital system. Dev Cell. 2005;9:283–292. doi: 10.1016/j.devcel.2005.05.016. [DOI] [PubMed] [Google Scholar]
- Coulombre AJ, Coulombre JL. Lens Development. I. Role of the Lens in Eye Growth. J Exp Zool. 1964;156:39–47. doi: 10.1002/jez.1401560104. [DOI] [PubMed] [Google Scholar]
- Cvekl A, Tamm ER. Anterior eye development and ocular mesenchyme: new insights from mouse models and human diseases. Bioessays. 2004;26:374–386. doi: 10.1002/bies.20009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Iongh RU, Chen Y, Kokkinos MI, McAvoy JW. BMP and activin receptor expression in lens development. Mol Vis. 2004;10:566–576. [PubMed] [Google Scholar]
- Fear MW, Kelsell DP, Spurr NK, Barnes MR. Wnt-16a, a novel Wnt-16 isoform, which shows differential expression in adult human tissues. Biochem Biophys Res Commun. 2000;278:814–820. doi: 10.1006/bbrc.2000.3852. [DOI] [PubMed] [Google Scholar]
- Fuhrmann S, Stark MR, Heller S. Expression of Frizzled genes in the developing chick eye. Gene Expr Patterns. 2003;3:659–662. doi: 10.1016/s1567-133x(03)00107-8. [DOI] [PubMed] [Google Scholar]
- Genis-Galvez JM, Santos-Gutierrez L, Rios-Gonzalez A. Causal factors in corneal development: an experimental analysis in the chick embryo. Exp Eye Res. 1967;6:48–56. doi: 10.1016/s0014-4835(67)80053-8. [DOI] [PubMed] [Google Scholar]
- Gong Y, Slee RB, Fukai N, Rawadi G, Roman-Roman S, Reginato AM, Wang H, Cundy T, Glorieux FH, Lev D, Zacharin M, Oexle K, Marcelino J, Suwairi W, Heeger S, Sabatakos G, Apte S, Adkins WN, Allgrove J, Arslan-Kirchner M, Batch JA, Beighton P, Black GC, Boles RG, Boon LM, Borrone C, Brunner HG, Carle GF, Dallapiccola B, De Paepe A, Floege B, Halfhide ML, Hall B, Hennekam RC, Hirose T, Jans A, Juppner H, Kim CA, Keppler-Noreuil K, Kohlschuetter A, LaCombe D, Lambert M, Lemyre E, Letteboer T, Peltonen L, Ramesar RS, Romanengo M, Somer H, Steichen-Gersdorf E, Steinmann B, Sullivan B, Superti-Furga A, Swoboda W, van den Boogaard MJ, Van Hul W, Vikkula M, Votruba M, Zabel B, Garcia T, Baron R, Olsen BR, Warman ML. LDL receptor-related protein 5 (LRP5) affects bone accrual and eye development. Cell. 2001;107:513–523. doi: 10.1016/s0092-8674(01)00571-2. [DOI] [PubMed] [Google Scholar]
- Gould DB, Smith RS, John SW. Anterior segment development relevant to glaucoma. Int J Dev Biol. 2004;48:1015–1029. doi: 10.1387/ijdb.041865dg. [DOI] [PubMed] [Google Scholar]
- Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo 1951. Dev Dyn. 1992;195:231–272. doi: 10.1002/aja.1001950404. [DOI] [PubMed] [Google Scholar]
- Hollyday M, McMahon JA, McMahon AP. Wnt expression patterns in chick embryo nervous system. Mech Dev. 1995;52:9–25. doi: 10.1016/0925-4773(95)00385-e. [DOI] [PubMed] [Google Scholar]
- Jasoni C, Hendrickson A, Roelink H. Analysis of chicken Wnt-13 expression demonstrates coincidence with cell division in the developing eye and is consistent with a role in induction. Dev Dyn. 1999;215:215–224. doi: 10.1002/(SICI)1097-0177(199907)215:3<215::AID-AJA4>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Jean D, Ewan K, Gruss P. Molecular regulators involved in vertebrate eye development. Mech Dev. 1998;76:3–18. doi: 10.1016/s0925-4773(98)00117-8. [DOI] [PubMed] [Google Scholar]
- Jiao X, Ventruto V, Trese MT, Shastry BS, Hejtmancik JF. Autosomal recessive familial exudative vitreoretinopathy is associated with mutations in LRP5. Am J Hum Genet. 2004;75:878–884. doi: 10.1086/425080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin EJ, Burrus LW, Erickson CA. The expression patterns of Wnts and their antagonists during avian eye development. Mech Dev. 2002;116:173–176. doi: 10.1016/s0925-4773(02)00128-4. [DOI] [PubMed] [Google Scholar]
- Katoh M, Kirikoshi H, Saitoh T, Sagara N, Koike J. Alternative splicing of the WNT-2B/WNT-13 gene. Biochem Biophys Res Commun. 2000;275:209–216. doi: 10.1006/bbrc.2000.3252. [DOI] [PubMed] [Google Scholar]
- Kubo F, Takeichi M, Nakagawa S. Wnt2b controls retinal cell differentiation at the ciliary marginal zone. Development. 2003;130:587–598. doi: 10.1242/dev.00244. [DOI] [PubMed] [Google Scholar]
- Kubo F, Takeichi M, Nakagawa S. Wnt2b inhibits differentiation of retinal progenitor cells in the absence of Notch activity by downregulating the expression of proneural genes. Development. 2005;132:2759–2770. doi: 10.1242/dev.01856. [DOI] [PubMed] [Google Scholar]
- Linker C, Lesbros C, Stark MR, Marcelle C. Intrinsic signals regulate the initial steps of myogenesis in vertebrates. Development. 2003;130:4797–4807. doi: 10.1242/dev.00688. [DOI] [PubMed] [Google Scholar]
- Liu H, Mohamed O, Dufort D, Wallace VA. Characterization of Wnt signaling components and activation of the Wnt canonical pathway in the murine retina. Dev Dyn. 2003;227:323–334. doi: 10.1002/dvdy.10315. [DOI] [PubMed] [Google Scholar]
- Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- Lovicu FJ, McAvoy JW. Growth factor regulation of lens development. Dev Biol. 2005;280:1–14. doi: 10.1016/j.ydbio.2005.01.020. [DOI] [PubMed] [Google Scholar]
- Lyu J, Joo CK. Wnt signaling enhances FGF2-triggered lens fiber cell differentiation. Development. 2004;131:1813–1824. doi: 10.1242/dev.01060. [DOI] [PubMed] [Google Scholar]
- McAvoy JW, Chamberlain CG. Growth factors in the eye. Prog Growth Factor Res. 1990;2:29–43. doi: 10.1016/0955-2235(90)90008-8. [DOI] [PubMed] [Google Scholar]
- Miller JR. The Wnts. Genome Biol. 2002;3 doi: 10.1186/gb-2001-3-1-reviews3001. REVIEWS3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parr BA, Cornish VA, Cybulsky MI, McMahon AP. Wnt7b regulates placental development in mice. Dev Biol. 2001;237:324–332. doi: 10.1006/dbio.2001.0373. [DOI] [PubMed] [Google Scholar]
- Prudovsky I, Mandinova A, Soldi R, Bagala C, Graziani I, Landriscina M, Tarantini F, Duarte M, Bellum S, Doherty H, Maciag T. The non-classical export routes: FGF1 and IL-1alpha point the way. J Cell Sci. 2003;116:4871–4881. doi: 10.1242/jcs.00872. [DOI] [PubMed] [Google Scholar]
- Riddle RD, Ensini M, Nelson C, Tsuchida T, Jessell TM, Tabin C. Induction of the LIM homeobox gene Lmx1 by WNT7a establishes dorsoventral pattern in the vertebrate limb. Cell. 1995;83:631–640. doi: 10.1016/0092-8674(95)90103-5. [DOI] [PubMed] [Google Scholar]
- Schubert FR, Mootoosamy RC, Walters EH, Graham A, Tumiotto L, Munsterberg AE, Lumsden A, Dietrich S. Wnt6 marks sites of epithelial transformations in the chick embryo. Mech Dev. 2002;114:143–148. doi: 10.1016/s0925-4773(02)00039-4. [DOI] [PubMed] [Google Scholar]
- Shestopalov VI, Bassnett S. Expression of autofluorescent proteins reveals a novel protein permeable pathway between cells in the lens core. J Cell Sci. 2000;113(Pt 11):1913–1921. doi: 10.1242/jcs.113.11.1913. [DOI] [PubMed] [Google Scholar]
- Shu W, Jiang YQ, Lu MM, Morrisey EE. Wnt7b regulates mesenchymal proliferation and vascular development in the lung. Development. 2002;129:4831–4842. doi: 10.1242/dev.129.20.4831. [DOI] [PubMed] [Google Scholar]
- Smith AN, Miller LA, Song N, Taketo MM, Lang RA. The duality of beta-catenin function: A requirement in lens morphogenesis and signaling suppression of lens fate in periocular ectoderm. Dev Biol. 2005 doi: 10.1016/j.ydbio.2005.07.019. [DOI] [PubMed] [Google Scholar]
- Stump RJ, Ang S, Chen Y, von Bahr T, Lovicu FJ, Pinson K, de Iongh RU, Yamaguchi TP, Sassoon DA, McAvoy JW. A role for Wnt/beta-catenin signaling in lens epithelial differentiation. Dev Biol. 2003;259:48–61. doi: 10.1016/s0012-1606(03)00179-9. [DOI] [PubMed] [Google Scholar]
- Sue Menko A. Lens epithelial cell differentiation. Exp Eye Res. 2002;75:485–490. doi: 10.1006/exer.2002.2057. [DOI] [PubMed] [Google Scholar]
- Sumanas S, Ekker SC. Xenopus frizzled-5: a frizzled family member expressed exclusively in the neural retina of the developing eye. Mech Dev. 2001;103:133–136. doi: 10.1016/s0925-4773(01)00327-6. [DOI] [PubMed] [Google Scholar]
- Theodosiou NA, Tabin CJ. Wnt signaling during development of the gastrointestinal tract. Dev Biol. 2003;259:258–271. doi: 10.1016/s0012-1606(03)00185-4. [DOI] [PubMed] [Google Scholar]
- Thut CJ, Rountree RB, Hwa M, Kingsley DM. A large-scale in situ screen provides molecular evidence for the induction of eye anterior segment structures by the developing lens. Dev Biol. 2001;231:63–76. doi: 10.1006/dbio.2000.0140. [DOI] [PubMed] [Google Scholar]
- Toomes C, Bottomley HM, Jackson RM, Towns KV, Scott S, Mackey DA, Craig JE, Jiang L, Yang Z, Trembath R, Woodruff G, Gregory-Evans CY, Gregory-Evans K, Parker MJ, Black GC, Downey LM, Zhang K, Inglehearn CF. Mutations in LRP5 or FZD4 underlie the common familial exudative vitreoretinopathy locus on chromosome 11q. Am J Hum Genet. 2004;74:721–730. doi: 10.1086/383202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Raay TJ, Vetter ML. Wnt/frizzled signaling during vertebrate retinal development. Dev Neurosci. 2004;26:352–358. doi: 10.1159/000082277. [DOI] [PubMed] [Google Scholar]
- Veeman MT, Axelrod JD, Moon RT. A second canon. Functions and mechanisms of beta-cateninin-dependent Wnt signaling. Dev Cell. 2003;5:367–377. doi: 10.1016/s1534-5807(03)00266-1. [DOI] [PubMed] [Google Scholar]
- Wallingford JB, Vogeli KM, Harland RM. Regulation of convergent extension in Xenopus by Wnt5a and Frizzled-8 is independent of the canonical Wnt pathway. Int J Dev Biol. 2001;45:225–227. [PubMed] [Google Scholar]
- Wang J, Wynshaw-Boris A. The canonical Wnt pathway in early mammalian embryogenesis and stem cell maintenance/differentiation. Curr Opin Genet Dev. 2004;14:533–539. doi: 10.1016/j.gde.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Yanfeng W, Saint-Jeannet JP, Klein PS. Wnt-frizzled signaling in the induction and differentiation of the neural crest. Bioessays. 2003;25:317–325. doi: 10.1002/bies.10255. [DOI] [PubMed] [Google Scholar]
- Yang Y. Wnts and wing: Wnt signaling in vertebrate limb development and musculoskeletal morphogenesis. Birth Defects Res C Embryo Today. 2003;69:305–317. doi: 10.1002/bdrc.10026. [DOI] [PubMed] [Google Scholar]
- Zhao S, Chen Q, Hung FC, Overbeek PA. BMP signaling is required for development of the ciliary body. Development. 2002;129:4435–4442. doi: 10.1242/dev.129.19.4435. [DOI] [PubMed] [Google Scholar]
- Zinn KM. Changes in corneal ultrastructure resulting from early lens removal in the developing chick embryo. Invest Ophthalmol. 1970;9:165–182. [PubMed] [Google Scholar]


