Abstract
Natural Killer (NK) cells are powerful effectors of cytotoxicity against “stressed” cells. They also produce cytokines and chemokines to activate the adaptive immune response. Understanding NK cell development and maturation may have implications for cancer therapy and for immunity against infections. We hypothesized that Notch signaling, critical for hematopoesis, would be involved in NK cell development. The role of constitutively activated Notch1 (ICN) on NK cell maturation was studied using human umbilical cord blood (UCB) progenitors cultured on a murine embryonic liver stroma cell line (EL08-1D2) and human cytokines. UCB CD34+/ICN+ sorted cells resulted in a population of CD7+ early lymphoid precursors and subsequent NK lineage commitment independent of stroma or IL-15. Early expression of L-selectin on ICN+ precursors suggested their homing competence. These precursors further committed to the NK lineage, and were capable of producing cytokines and chemokines such as IL-13, GM-CSF, TNF-α, yet poorly acquired NK inhibitory receptors and cytotoxic effector function. In the presence of stroma, ICN+ precursors also gave rise to a population of early T lineage committed cells characterized by expression of cytoplasmic CD3 γ, ε, δ chains, RAG1/2 and production of IL-2, suggesting bona fide Th1 commitment. Importantly, signals from EL08-1D2 stroma were required for this development process. In conclusion, sustained Notch signaling can replace stroma in differentiation of a common CD7+ lymphoid precursor from UCB CD34+ progenitors and induce NK cell commitment. However, these NK cells are immature in their cytokine production profile, are hyporesponsive and poorly acquire NK cell receptors involved in self tolerance and effector function.
Keywords: NK cells, Lymphopoiesis, Notch, Lymphocyte development
INTRODUCTION
Natural killer (NK) cells defined by CD56 expression and the absence of CD3 comprise 10–15% of peripheral blood lymphocytes. They also reside in spleen, bone marrow, lymph nodes, liver, intestine and the pregnant uterus. NK cells can eliminate infected or cancer transformed cells in the absence of antigen specific receptors [1]. In order to protect healthy tissues, NK cells are under a constant inhibition delivered by engagement of inhibitory receptors, which recognize ‘self’ class 1 human leukocyte antigens (HLA) [2]. Multiple inhibitory receptors such as killer immunoglobulin receptors (KIR) and CD94/NKG2 heterodimers are stochastically expressed during NK cell development in a regulated sequential fashion until each individual NK cell is “licensed” by recognition of at least one inhibitory receptor by its cognate HLA ligand [3,4]. In this process NK cells become functionally competent. Their ability to kill is determined by net signals from their inhibitory (KIR, NKG2A, and others) and activating receptors (NKG2D, natural cytotoxicity receptors). Recently, a population of circulating CD56dim NK cells has been found in normal volunteers that lack KIR and NKG2A but are hyporesponsive or globally anergic linking expression of receptors to the acquisition of effector function [5].
In contrast to peripheral blood CD56dimCD3− cytotoxic NK cells the population of “regulatory” CD56brightKIR− NK cells resides in secondary lymphoid tissues and co-express receptors for inflammatory chemokines and homing receptors CD62L (L-selectin) and CCR7 [6]. They are highly proliferative and secrete IFN-γ, IL-10, IL-13 and GM-CSF in response to IL-12/IL-18 stimulation. Their immunoregulatory functions synergize with adoptive immune responses [7]. Lymphoid progenitors CD34+CD45RA+integrin β7+ isolated from human lymph nodes are capable of differentiation into CD56bright NK cells [8]. The process by which NK cells and their subsets differentiate and mature has important implications for reconstitution after hematopoietic cell transplantation and cancer therapy [5,9,10,11].
In humans, NK cell development from hematopoetic stem cells has been studied using stromal and non-stroma based cultures [12,13,14]. NK cells derived from CD34+/Lin− primitive progenitors can develop in the bone marrow microenvironment [15,16]. Surface expression of CD7 is an early event in lymphoid commitment and NK cell differentiation [13] and all mature T and NK lymphocytes continue to express CD7. We have shown that human bone marrow and cord blood CD34+ progenitors effectively differentiate into NK cells when cultured with the murine stroma cell lines AFT024 or EL08-1D2. These cultures not only induce NK cell development but also support the acquisition of KIR and NKG2A, vital steps preceding “licensing” [17 18]. In addition to direct contact between hematopoetic cell and their microenviroment, NK cell commitment requires the presence of human cytokines [14].
One conserved mechanism to regulate cell-to-cell interactions, proliferation and differentiation is through Notch signaling [19,20]. Notch 1 triggers CD7 expression on hematopoetic progenitors in vitro and has been shown to regulate early lymphoid development and commitment towards the T and NK lineage [21]. Recent mouse and human data suggest that transient Notch signaling can be permissive to NK commitment [22,23], yet the effect of Notch signaling on NK cell maturation and function is still not well understood.
The Notch ligands Delta and Jagged are transmembrane proteins expressed in tissues such as bone marrow stroma, thymus and lymphoid organs which trigger Notch receptors (1,2,3,4) on the surface of hematopoetic cells [24,25,26]. After binding to ligand, intracellular Notch (ICN) is released in a series of proteolytic cleavages and migrates into the nucleus to displace co-repressors from the CSL (CBF1/Suppressor of Hairless/Lag-1) protein. This further recruits a co-activator from the master-mind family (MAML) and converts the complex into a DNA binding transcription factor to target genes such as HES (Hairy Enhancer of Split) family members [27]. Given the dynamic changes which occur in models to recapitulate NK cell maturation, we chose to overexpress constitutively activated Notch1 in hematopoietic progenitors as a “constant on signal” to better understand its function in NK cell development. Our results show that activated Notch alone is sufficient for development of early lymphoid precursors, which are capable of differentiation into KIR−, poorly cytotoxic, cytokine producing CD56+CD3− NK cells that are not fully mature.
METHODS
Cell Isolation
Umbilical cord blood (UCB) was obtained from full-term deliveries from the Memorial Blood Bank (Minneapolis, MN) or the New York Blood Bank. The committee on the Use of Human Subjects in Research at the University of Minnesota approved the use of all tissue. Mononuclear cells were separated using Ficoll-Hypaque (sp.grav.1.077 Sigma Diagnostics, St. Louis, MO) density gradient centrifugation. CD34+ progenitor cells were isolated by double positive selection with magnetic bead (Miltenyi Biotech, Oberlin, CA) column separation (MACS) resulting in a purity of >95%. CD34+ cells were plated in 6-well plates and cultured in media (Iscove’s modified Dulbecco’s medium) supplemented with 20% bovine calf serum, 100 U/mL penicillin, 100 U/mL streptomycin (Gibco), and exogenous cytokines c-kit ligand (c-kit-L), Flt3-ligand (Flt3-L), IL-7 and thrombopoietin (TPO) (all at 20 ng/mL) for 48 hours. Following pre-activation of these cytokines, retroviral transduction was performed (described below). Transduced cells were harvested and stained with anti CD34-APC monoclonal antibody (BD Biosciences) and sorted for CD34+/GFP+ cells to >98 % purity using a fluorescence activated cell sorter (FACS) DiVa or Aria (Becton Dickinson, San Jose, CA).
Retroviral transduction
Constructs were transiently transfected into 293kj cells (a kind gift from Dr. M. Haas, University of California, San Diego) with PCL plasmid and Mig-R–based retroviral vectors encoding the intracellular domain of human Notch1 (ICN) tagged with green fluorescent protein from a bicistronic transcript (gift from Dr. W. Pear, University of Philadelphia, Pennsylvania), [28] and eGFP empty vector control (eGFP). We confirmed positive fluorescence and obtained viral supernatants after 48 and 72 hours as described [29]. Following preactivation UCB CD34+ progenitors were transduced twice, 24 hours apart, with 3 mL of supernatant containing eGFP and ICN retrovirus in Fibronectin (Retronectin, Tarka) coated transwells at a density of 300,000 cells/well [30].
Culture of hematopoietic progenitors
Post-double column purified non-manipulated CD34+ cells, CD34+ICN+ and CD34+eGFP+ transduced and sorted cells were suspended at density of 50–100 cells/well on 96W plates (Costar, Cambridge, MA) without stroma or on plates pre-established with a murine embryonic liver cell line EL08-1D2 irradiated (3,000 cGy) prior to culture. CD34+ progenitors were cultured in media containing 2:1 mix of Dulbecco modified Eagle medium (DMEM high glucose without sodium pyruvate)/Ham F12-based medium (Gibco Laboratories, Grand Island, NY) and supplemented with 24 μM 2-mercaptoethanol, 50μM ethanolamine, 20mg/L ascorbic acid, 50μg/L sodium selenite, 100 U/mL penicillin, 100 U/mL streptomycin (Gibco), 20% heat inactivated human AB serum (Valley Biomedical, Inc., Winchester, VA). Human cytokines (10 ng/mL Flt3-L, 20 ng/mL IL-7, 20 ng/mL c-kit-L, 5 ng/mL IL-3 with or without 10 ng/mL IL-15 [all from R&D Systems, Minneapolis]) were supplemented weekly except for IL-3 which was used once at culture initiation. Cultures were re-fed weekly with 50% volume changes. Plates were maintained in a humidified atmosphere of 5% CO2 at 37°C.
Antibodies, proliferation
Cultured cells were stained with: APC-conjugated monoclonal antibodies against CD34 (8G12), CD56 (NCAM 16.2), CD8 (SK1), CD44 (G44-26), CD3 (SK7), CD94; PE-conjugated monoclonal antibodies against CD7 (M-T701), CD4 (SK3), CD45RA (HI100), CD62L (Dreq56), CD117(104D2), CD25 (M-A251), NKG2A (Z199), CD158a (EB6), CD158b (GL183), CD158e (NKB1, DX9), CD158i (KARp50.3, FES172), NKG2D (ON72), NKp30 (Z25), NKp44 (Z231), NKp46 (BAB281), TCR α/β (T10B9.IA-31), TCR γ/δ (B1); and PerCP conjugated antibodies against CD3 (SK7) and CD34 (8G12). Intracellular proteins were analyzed using monoclonal antibodies against CD3 (SK7), interferon-γ (B27), IL-2 (5344.111), and perforin (δG9) (all PE). Appropriate fluorochrome-conjugated, isotype matched control Igs were used in all experiments. All antibodies were from Beckman Coulter, BD Pharmigen, or BD Biosciences. Flow cytometry data was acquired using a FACS Calibur (Becton Dickinson, San Jose, CA) and analyzed using Flow-Jo software (Tree Star Inc, Ashland, OR). The absolute number of NK cells were determined by adding 30,000 polystyrene microspheres (Polysciences, Warrington, PA) to each aliquot and calculated as previously described [11].
Cytokine production, cytotoxicity assays and statistical analysis
Cultured progeny of UCB CD34+ICN+ and CD34+eGFP+ cells were harvested at day 28 and suspended at a concentration of 1×106 cells/mL. Cytokine production was tested after stimulating cells with IL-12 (10 ng/mL) and IL-18 (100 ng/mL) for 18 hours or in the presence of phorbol ester and calcium ionophore (PMA/ionomycin) for 5 hours. Cell-free supernatants were analyzed by a 22-plex Luminex assay for cytokine and chemokine protein concentrations as recommended by the manufacturer. Before staining for intracellular perforin, IFNγ and IL-2, Brefeldin A was added to cultures in the last 5 hours of the incubation and cells were permeabilized using cytofix/cytoperm (BD Biosciences). Day 28 cells were tested in 4-hour 51Cr release cytotoxicity assay against the NK sensitive target K562. Effector to target ratios ranged from 0.082:1 to 20:1. All assays were performed in triplicate and percentage lysis was calculated as previously described [31].
All results were reported from multiple independent experiments (n) as the mean ± SEM. Significance levels were determined by two-sided Student t-test.
Quantitative reverse transcriptase-polymerase chain reaction (Q-RT-PCR) analysis and PCR
Total RNA isolated from cultured unmanipulated cells at day 28 using RNAeasy Mini Kit (Qiagen, Santa Clarita, CA) was converted to cDNA using Superscript II kit (Invitrogen, Carlsbad, CA). The cDNA product was mixed with forward and reverse primers and SYBRGreen PCR Master Mix (Applied Biosystems, Foster City, CA) and Q-RT- PCR was performed and analyzed on an ABI Prism 7900. Relative Quantities were normalized for Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) expression and compared to the mRNA ratio in eGFP+ control cells. We designed all primer sequences to span 2 exons to eliminate interference from genomic DNA using Primer3 software (http://frodo.wi.mit.edu/cgibin/primer3/primer3_www.cgi).
Primer sequences for ICN: AGG ATC TCT CGA GGT TAA CGA A (forward [fw]), GAC GGC AAT ATG GTG GAA A (reverse [rs]); Hes1: GGA AAT GAC AGT GAA GCA CCT (fw), GTC ACC TCG TCC ATG CAC TC (rs); Hes5: CAG CAT CGA GCA GCA GCT GAA (fw), TAG TCC TGG TGC TGC AGG CTC TT (rs); GATA-3: GTC CTG TGC GAA CTG TCA GA (fw), TTT TTC GGT TTC TGG TCT GG (rs); preTα: AGA GGC TTC TAC AGC CAG GAC (fw), AAA CAG CAG CAG CTT GAA GAG (rs); RAG1: AAT ATC AAC CAA ATT GCA GAC ATC(fw) GCA GAA CTG AGT CCC AAG GT (rs); RAG2: ATT CAG AGA GGC GTG AGC A (fw), CCT GGC TGA ATT AAG GCT ATG T (rs). CD3 chains and CD127 reactions used previously published primers.
RESULTS
Notch1 activation in UCB CD34+ progenitors generates a population of CD7+ early lymphoid precursors
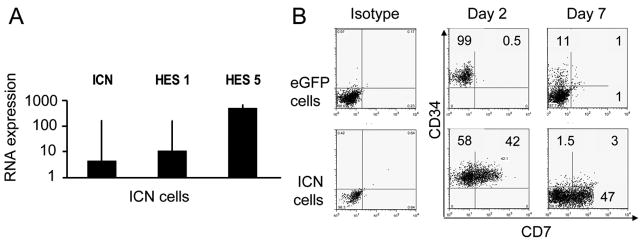
To investigate the effect of cell-to-cell interactions on differentiation, growth and function of NK cells, human UCB CD34+ progenitors were cultured in direct contact with the murine embryonic liver cell line EL08-1D2. In the presence of exogenous human cytokines (IL-7, IL-15, Flt3-L, c-kit-L, IL-3) this model powerfully recapitulates lymphoid differentiation toward the NK cell lineage [11,32]. In the absence of EL08-1D2 stroma virtually no NK cells (62±27) developed from 10 starting progenitors compared to 58510±4887 NK cells (P< .0001) when stroma was present, showing the requirement for contact between hematopoietic progenitors and their supportive microenvironment. To study whether the Notch pathway plays a role in NK differentiation, we overexpressed intracellular Notch1 (ICN) and an eGFP-empty vector (eGFP) in UCB CD34+ progenitors and compared them in NK differentiation culture. There were no phenotype or functional differences between NK cell progeny transduced with the eGFP control vector and untransduced CD34+ cells (data not shown). To ensure the integrity of transduced genes, we demonstrated a 4.4-fold increase in ICN mRNA levels and increased expression of downstream Notch transcripts HES1 (15-fold) and HES5 (515-fold) in CD34+ICN+ cells compared to CD34+eGFP+ cells (Fig. 1A).
Figure 1. Notch1 induces CD7 on CD34+ UCB progenitors.
UCB CD34+ progenitors were transduced in Fibronectin coated transwells in the presence of c-kit-L, Flt3-L, IL-7 and TPO with MSCV-based eGFP and ICN vectors, FACS sorted for CD34+eGFP+ cells, plated in contact with murine embryonic liver stroma cell line EL08-1D2 with IL-3, c-kit-L, Flt3-L, IL-7 and IL-15 and cultured for 28 days. (A) The induction of specific mRNA from cultured ICN+ cells (■) and eGFP+ cells was analyzed by Q-RT- PCR. Relative transcripts of Notch1 (ICN) and downstream ICN targets Hes1 and Hes5 are relative to the level of GAPDH as a control for RNA integrity and reported as a ratio to eGFP controls. Bars represent mean expression ± SEM of 3 independent experiments (B) ICN+ and eGFP+ control cells were analyzed 2 days after transduction or after culture with EL08-1D2 stroma and cytokines (IL-3, c-kit-L, Flt3-L, IL-7, IL-15) for 7 additional days. All flow cytometry plots were gated on eGFP+ lymphocyte gate. Only ICN cell gave rise to a population of CD34+CD7+cells, which subsequently differentiated into CD34−CD7+ (and predominantly CD56−) lymphoid precursors.
CD34+ICN+ and CD34+eGFP+ progenitors were tested for CD7 expression immediately following the transduction prior to any contact with EL08-1D2 stroma or exposure to IL-15. There was rapid acquisition of CD7 in ICN+ cells compared to essentially no expression in the eGFP+ vector control (Fig. 1B, P = .05). The rapid and marked effect of ICN on CD7 expression prior to culture with stroma suggests that Notch1 signals are strong enough that they obviate the need for and can replace the requirement for stroma to induce early NK cell development. In contrast to CD34+ eGFP control cells, all CD34+ICN+ cells lost the CD34 marker early in culture to become CD34−CD7+ precursors (Fig. 1B).
Activated Notch1 increased expression of CD62L (L-selectin) and CD45RA on UCB CD34+ lymphoid precursors
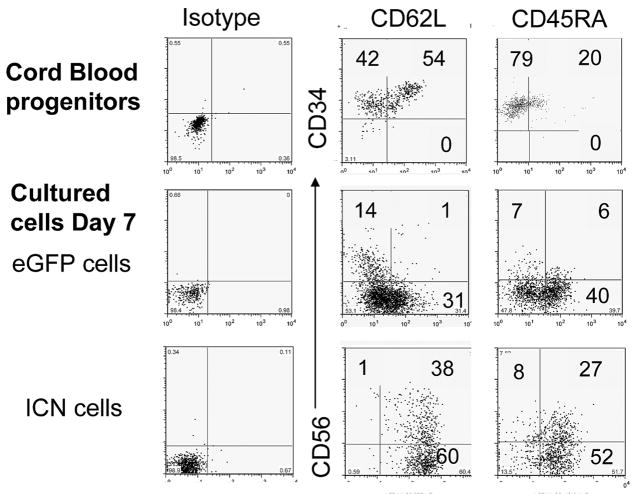
Fresh UCB derived CD34+ progenitors express a low level of L-selectin and CD45RA (Fig. 2 – top row). After seven days of culture with EL08-1D2 stroma, a significant population of CD34+ICN+ derived cells expressed both L-selectin (60±9%) and CD45RA (45±8%), respectively, a level significantly higher than the eGFP empty vector control derived cells. The expression of L-selectin and CD45RA was dependent on the presence of EL08-1D2 stroma, and was reduced in its absence (ICN+CD45RA+ 9.9%; ICN+CD62L+ 4.4%, data not shown), suggesting that stroma mediates additional signals which are required in this developmental process. We further tested these NK cell intermediates for important developmental antigens [33]. After one week, the progeny of CD34+ICN+ cultured cells expressed higher levels of CD25 compared to CD34+eGFP+ cells. ICN+ and eGFP+ precursors from NK cell differentiation cultures expressed high levels of CD117 and were negative for surface expression of CD10, CD1a, IL-7Rα, CD122, CD16, CD94 or CD161, while expression of LFA-1 and CD44 was heterogeneous and not significantly different between ICN+ and eGFP+ control starting populations (data not shown).
Figure 2. Activated Notch1 induced early expression of CD45RA and adhesion marker L-selectin (CD62L).
Expression of CD62L and CD45RA on fresh CD34+ UCB progenitors is shown in the top row. CD34+eGFP+ and CD34+ICN+ progenitors cultured with EL08-1D2 stroma and cytokines (Flt3-L, IL-7, c-kit-L, IL-15, IL-3) for 7 days exhibited higher L-selectin and CD45RA expression compared to CD34+eGFP+ (representative of 4 independent experiments).
CD34+ICN+ derived cells have a capacity to differentiate into CD7+CD56+CD3− NK cells
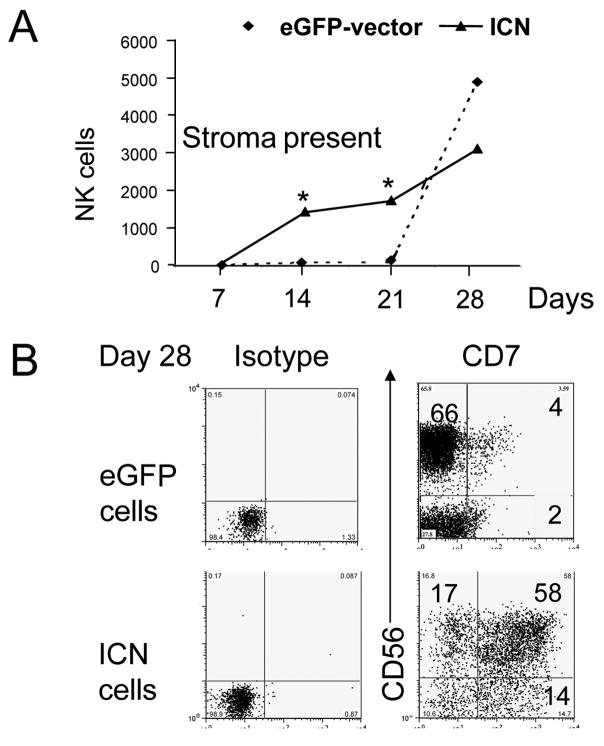
In the next set of experiments, we followed cells for longer culture intervals on stroma to study NK cell commitment and further maturation. Notably, CD34+ICN+ derived CD34−CD7+ precursors rapidly developed into CD56+CD3− NK cells, compared to weak CD56 acquisition on CD34+eGFP+ cells at day 7, 14 or 21 (Fig. 3A). By day 28, CD56+CD3−NK cell development was present in both conditions, although CD7 expression was homogeneously high only on ICN+ cells (Fig. 3B). The kinetics of CD34+ICN+ derived NK cells were significantly different from eGFP control cells (P< .0005 at day 21, Fig. 3A).
Figure 3. Notch1 enhances early NK cell commitment from CD34+ UCB precursors.
EL08-1D2 stroma and cytokine (Flt3-L, IL-7, c-kit-L, IL-15, IL-3) cultured cells were harvested, counted and analyzed by flow cytometry. (A) Absolute numbers of cultured eGFP+(◆) and ICN+(▲) derived CD56+CD3− NK cells calculated per 100 plated cells were compared at weekly intervals. In the presence of stroma, activated Notch1 conferred an early growth advantage to cultured cells compared to eGFP+ control cells (○) at days 14 and 21 (*P=0.005; mean of 3 independent experiments shown). (B) Stroma cultured eGFP+ cells and ICN+ cells were harvested at day 28 and compared for expression of CD56 and CD7 (gated on CD56+CD3− cells). CD7 was highly expressed only on ICN+ cells (representative of 6 experiments shown).
Activated Notch1 renders NK cells functionally immature and inhibits KIR acquisition
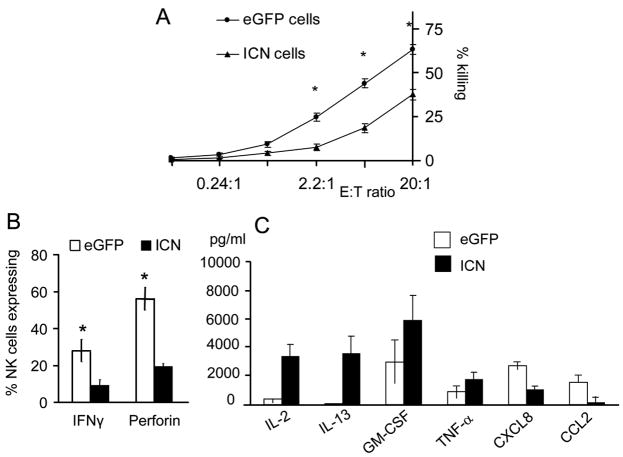
Given that UCB CD34+ progenitors overexpressing Notch1 can efficiently commit to the NK cell lineage, we next asked whether they could acquire KIR, cytotoxicity and produce cytokines, all characteristics of mature NK cells. In cytotoxicity assays against K562 cells ICN+ NK cells exhibited a significant reduction in killing, while eGFP+ control derived NK cells effectively lysed targets (eGFP+ 63±2.9% vs ICN+ 44±18%; n=7, P= .04; Fig. 4A). The mechanism of NK cell killing involves exocytosis of perforin and granzyme containing cytoplasmic granules [34]. Staining for intracellular perforin expression revealed a 2.5 fold reduction in ICN+ NK cells compared to eGFP+ derived NK cells (eGFP+ 56.2±6% vs ICN+ 19±1.5%, n=4, P= .01; Fig. 4B). In addition to target lysis, NK cells exert regulatory functions through cytokines and chemokines [35]. We stimulated ICN+ cells with exogenous IL-12 and IL-18 and demonstrated two-fold lower IFNγ production (eGFP+ 28.6±6% vs ICN+ 11±5%, n=3, P= .05; Fig. 4B). Supernatants collected form ICN+ cells at the end of culture showed 10-fold lower concentrations of monocyte chemo-attractant protein 1 (CCL2 [MCP-1], n=5; p=0.01) and CXCL8 (IL-8, n=5, P=.004) (Fig. 4C), suggesting a pattern of immaturity. In contrast, following PMA/ionomycin stimulation, CD34+ICN+ derived NK cells secreted 90 times higher levels of IL-13 (P=.04) and modestly increased concentrations of TNFα and GM-CSF, all characteristic of immature NK cells [36,37].
Figure 4. CD34+ICN+ derived NK cells are hyporesponsive and exhibit immature function.
(A) ICN+ and eGFP+ derived cells were cultured with EL08-1D2 stroma and cytokines (Flt3-L, IL-7, c-kit-L, IL-15, IL-3). Stroma cultured CD34+ derived cells were harvested at day 28 and compared in 51Cr release cytotoxicity assay against K562 targets at various E:T ratio as shown. ICN+ derived cells (▲) showed a significant reduction in cytotoxicity compared to eGFP+ cells ( ) (mean of 7 independent experiment ± SEM shown; *P< .05). (B) Cultured CD34+ICN+ and CD34+eGFP+ cells were harvested at day 28 and suspended in concentration 1×106 cells/mL. Interferon-γ production was analyzed by intracellular stain following stimulation with IL-12 (10 ng/mL) and IL-18 (100 ng/mL) for 18 hours, followed by Brefeldin A in the last 5 hours of the incubation and permeabilization using cytofix/cytoperm (4 independent experiments ± SEM; *P< .05). (C) Chemokines and cytokines were measured by Luminex from eGFP+ and ICN+ cell free supernatants prepared after stimulation of 106 cells/well with PMA/ionomycin. Significant differences between eGFP+ (□) and ICN+ (■) cell-free supernatants were seen for IL-13, GM-CSF, and CCL2 (MCP-1) and IL-2 (mean of 5 independent experiments with SEM is shown; *P< .05).
) (mean of 7 independent experiment ± SEM shown; *P< .05). (B) Cultured CD34+ICN+ and CD34+eGFP+ cells were harvested at day 28 and suspended in concentration 1×106 cells/mL. Interferon-γ production was analyzed by intracellular stain following stimulation with IL-12 (10 ng/mL) and IL-18 (100 ng/mL) for 18 hours, followed by Brefeldin A in the last 5 hours of the incubation and permeabilization using cytofix/cytoperm (4 independent experiments ± SEM; *P< .05). (C) Chemokines and cytokines were measured by Luminex from eGFP+ and ICN+ cell free supernatants prepared after stimulation of 106 cells/well with PMA/ionomycin. Significant differences between eGFP+ (□) and ICN+ (■) cell-free supernatants were seen for IL-13, GM-CSF, and CCL2 (MCP-1) and IL-2 (mean of 5 independent experiments with SEM is shown; *P< .05).
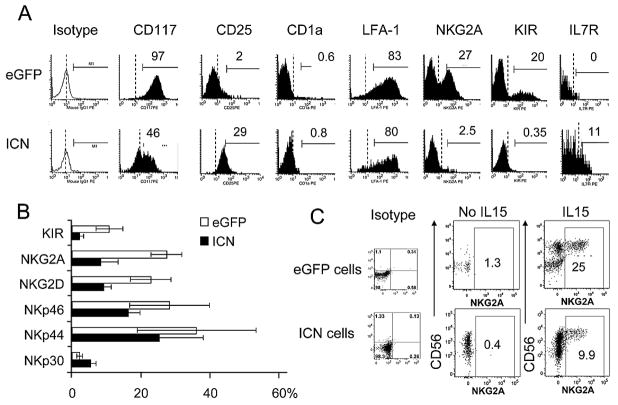
NK cell maturation is dependent upon coordinated acquisition of both activating and inhibitory cell surface receptors that equip NK cells with the capability to recognize and lyse targets based upon HLA class I mismatch [38]. C-lectin receptors (NKG2A/CD94, NKG2C/CD94, NKG2D, CD161), natural cytotoxicity receptors (NKp30, NKp44, NKp46) and killer immunoglobulin receptors (KIR) have heterogeneous expression on individual NK cells. We have previously shown that NK cells differentiation on murine EL08-1D2 stroma supports the acquisition of KIR on NK cells only after CD56 expression [14]. The expression of a full array of inhibitory receptors such as KIR and NKG2A receptors was virtually absent from CD34+ICN+ derived NK cells compared to control conditions even when cultures were extended out to 5 weeks (Figure 5A, B). The proportion of NK cells positive for the activating receptors NKG2D and NKp46 was slightly reduced while there was no change in NKp30 and NKp44 expression (Figure 5B). Expression of the IL7-Rα receptor (CD127) was low and not different between populations, which was confirmed by Q-RT-PCR. CD16, responsible for antibody-dependent cytotoxicity, was not expressed by NK cells derived from ICN+ nor eGFP+ progenitors (data not shown).
Figure 5. Expression of NK cell receptors is altered by activated Notch.
(A) Representative histograms of NK cell receptors on NK cells derived from CD34+eGFP+ and CD34+ICN+ cells co-culured with EL08-1D2 stroma and cytokines (Flt3-L, IL-7, c-kit-L, IL-15, IL-3) are shown (gated on CD56+CD3− cells). ICN+ NK cells demonstrated less NKG2A and KIR expression. (B) Bars represent the average expression of receptors on CD56+CD3− NK cells derived from stroma cultured CD34+eGFP+ (□) and CD34+ICN+ (■) at day 28 (mean of 4 independent experiments±SEM). (C) CD34+eGFP+ and CD34+ICN+ cells were cultured for 28 days on EL08-1D2 with Flt3-L, IL-7, c-kit-L, IL-3 with and without IL-15. In the absence of IL-15, only CD34+ICN+ population gave rise to NK cells. NKG2A expression on CD34+ICN+ derived NK cells is compared to eGFP derived NK cells with and without IL-15.
Activated Notch abrogates the requirement for IL-15 in stromal-based NK cell development cultures
To understand the role of IL-15 on NK cell commitment and receptor acquisition, we compared NK cells derived from stroma cultures supplemented with and without IL-15. In the absence of IL-15, virtually no NK cells developed after 28 days from CD34+eGFP+ progenitors compared to 53±12% (n=3) NK cells derived from CD34+ICN+ cells (Fig 5C), showing the lack of dependence on IL-15 for NK cell development when signals from both EL08-1D2 stroma and activated Notch1 are present. These NK cells did not express NKG2A (Fig. 5C) or KIR. Cultures with IL-15 not only allowed NK cell commitment from CD34+eGFP+ progenitors, but also stimulated the expression of inhibitory receptors such as NKG2A. However, NKG2A was expressed to a lesser degree on ICN+ derived NK cells (Fig 5C).
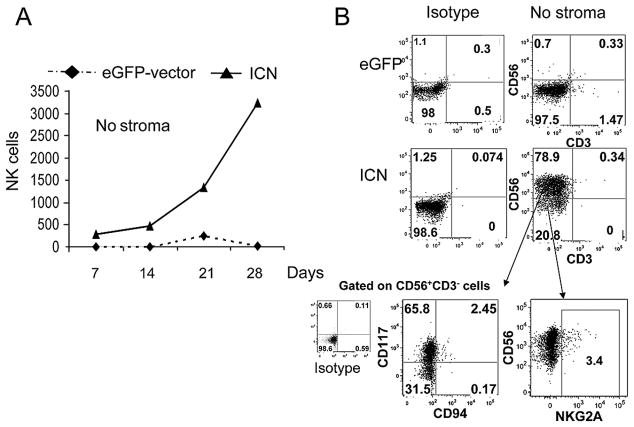
Notch1 and IL-15 can replace stroma in supporting NK cell commitment
Under stroma-free conditions supplemented with human cytokines including IL-15, CD34+eGFP+ cells were unable to develop into NK cells (Fig 6A,B). In contrast, CD34+ICN+ progenitors proliferated and differentiated along the NK lineage (Fig. 6A,B). These NK cells showed low to absent expression of inhibitory receptors NKG2A (Fig. 6B) and KIR but did exhibit a low capacity to kill K562 targets (not shown). Functionally, stroma-free ICN+ derived NK cells produced high levels of IL-13, MCP-1 and secreted low levels of IL-8, characteristics similar to NK cells derived from EL08-1D2 cultured CD34+ICN+ progenitors. In the absence of stromas, these ICN+ derived CD56+ NK cells were CD117high and CD94− cells (Fig. 6B) suggesting a block in NK cell differentiation.
Figure 6. Notch 1 can replace stroma in supporting NK cell commitment from CD34+ UCB progenitors.
(A) NK cells derived from CD34+eGFP+ and CD34+ICN+ progenitors were cultured without stroma but with cytokines (Flt3-L, IL-7, c-kit-L, IL-15, IL-3). Absolute numbers of cultured eGFP+(●) and ICN+(▲) derived CD56+CD3− NK cells are shown. Only CD34+ICN+ cells were able to expand without stroma (mean of 3 independent experiments shown). (B) The phenotype of CD34+ICN+ derived stroma free cultured NK cells at day 28 is shown and shows the accumulation of CD56+CD117high CD94lo NKG2Alocells.
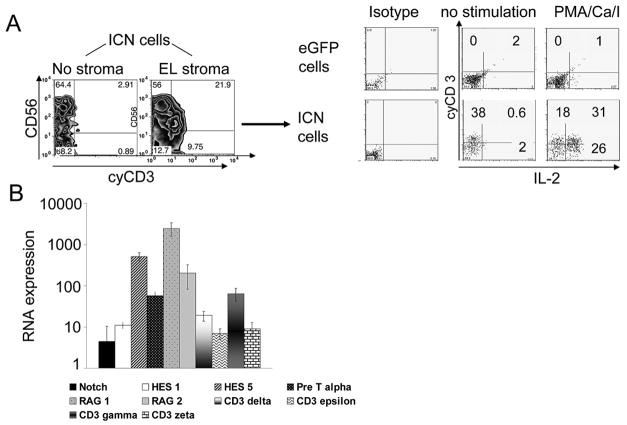
Generation of IL2 producing cyCD3+ cells from human CD34+ICN+ progenitors
CD3 expression was next examined on the progeny of CD34+ICN+ cells cocultured with and without EL08-1D2 stroma. In presence of stroma signals and activated Notch, we observed a distinct population of cells expressing cytoplasmic CD3, while essentially no CD3 was seen in stroma-free conditions and eGFP controls derived NK cells (cyCD3+cells: eGFP+ 1.6±0.26% vs ICN+ 21±3.9%, n=5, P<.01) . Expression of CD3 did not change with prolonged culture and cells did not express surface CD3, TCR-αβ or TCR-γδ, and were double negative for CD4, CD8 and positive for high-affinity IL-2 receptor (CD25). Following stimulation with PMA/Ionomycin, 62±4% of ICN+CD3+ cells expressed intracellular IL-2 compared to 1.6±1% in eGFP+ control cells (Fig. 6A). We confirmed these results by showing a 100-fold increase in IL-2 in supernatants of Notch1 transduced cells (Fig. 4C). Pre-T-alpha, RAG-1, RAG-2, GATA-3 and particularly CD3 gamma chain, CD3 delta chain, and CD3 epsilon chain transcripts were upregulated by Q-RT-PCR in CD34+ICN+ derived cells as compared to controls (Fig. 6B), all consistent with the presence of a population capable of T-lineage commitment.
DISCUSSION
NK cell development proceeds through an orderly sequence of maturational stages governed by signals provided by a supportive microenvironment [30,31,34]. Using an approach where Notch is constitutively activated, we found that Notch1 signaling results in brisk acquisition of the CD7 antigen resulting in a bipotent CD7+ lymphoid precursor, brightly expressing CD45RA+ and CD62L+ receptors capable of NK cell lineage commitment. Lymph node homing requires CD62L (L-selectin) expression to interact with high endothelial venules [37], and the cells we find after Notch stimulation bear some similarities to early NK cell precursors described in lymph nodes by Caligiuri’s group [8].
Results are consistent with prior studies [39,48–50] describing a link between Notch signaling and a common T/NK lymphoid precursor. Our study extends those observations by showing that Notch1 can fully replace the requirement for stroma in NK cell differentiation from cord blood CD34+ progenitors. Furthermore, Notch1 combined with signals from EL08-1D2 stroma supports NK cell differentiation even in the absence of IL-15. These findings are important as we better understand the separation of signals governing NK commitment and those important for NK activation. Once CD34 is lost, Notch1 continues to enhance NK and T-cell commitment but does not allow full maturation in our model. Sustained Notch1 activation results in a relative block in development as seen in both functional and phenotypic analyses. For example, the pattern of CD117highCD94− expression of ICN+ derived NK cells is consistent with a block in NK cell development as described by Grzywacz et al [32]. The pattern of cytokine and chemokine secretion is consistent with immature NK cells and fetal NK cells, especially the IL-13 secretion [36,37]. Immaturity is supported further by their diminished ability to kill targets and their altered phenotype, particularly reduced KIR and NKG2A expression. Lack of KIR expression is associated with functional immaturity [40] and a more primitive stage in NK cell ontogeny [43]. In accordance with our findings, the lack of mouse Ly49 receptors (KIR counterparts) was reported in NK cells that develop from Pax5−/− proB cells exposed to Notch signaling [23]. In the human system, Cooley et al has described KIR acquisition as the 6th and final stage in NK cell development, different from stage 5 CD56dim cells which do not express KIR and exhibit diminished function [5], much like the Notch1 transduced cells described here. Although the exact mechanism of KIR acquisition is poorly understood, it is possible that Notch plays a role in this process and needs to be turned off to allow NK maturation. This possibility needs further study.
We showed that expression of CD62L, CD45RA and cytoplasmic CD3 (cyCD3) was dependent on both Notch and stromal signals, which act as important cofactors in this process. The cyCD3 expression (ε, ζ, γ and δ) reported here is consistent with the finding that Wnt3a, possible stimulated by stromal signals, can modulate Notch signaling [43]. Our results are consistent with the studies by De Smedt et al who show that the addition of Wnt to the Notch ligand Delta 1 dramatically increased expression of cyCD3ε and Wnt inhibition promoted NK cell lineage differentiation. Our cyCD3+ cells are capable of IL-2 production following PMA stimulation suggesting that they already exhibit some T-cell function despite the fact that they are phenotypically immature. Since NK cells do not produce IL-2, the maturation stage induced in these IL-2 producing cells is consistent with the notion that pre-T cells are developing but are too immature to express surface CD3 and TCR [41].
Stroma derived factors provide signals in the developmental link between NK cells and T cells and there are several lines of evidence that differences in stroma dramatically modify Notch signaling [42–44]. The Notch pathway is tightly regulated by restrictive expression of Delta and Jagged ligands in different microenvironments, which provide signal timing and strength to determine cell fate. The essential and nonredundant role of Delta 1 ligand in the T cell commitment was shown in thymic cultures on OP9-DLL1 stroma [45]. In vitro cultures using Jagged1+OP9 stroma showed that the differentiation of immature thymocytes was biased toward the NK cell lineage and promoted development of rare γδ+T-cells, consistent with the known high expression of Jagged 1 in bone marrow stroma [2,3]. In addition, the use of the Notch cleavage γ-secretase inhibitor impaired development of T lymphocytes in a dose-dependent manner and favored B-cell, NK-cell, and monocytic/dendritic-cell differentiation.
We propose that local NK cell differentiation could be influenced by the close physical proximity of lymphoid precursors and the local and specific tissue expression of Notch ligands. Besides the microenvironment of bone marrow, the milieu of extramedullary tissues such as lymph nodes or fetal thymus may be permissible to NK development. Recently, Di Santo and colleagues have shown the existence of a thymic NK cell developmental pathway in the mouse, defined by high expression of CD127 and dependency on GATA-3 [48,49]. However, Notch derived NK cells described here failed to express CD127 at high levels, although Gata-3 transcripts, a direct downstream Notch target, were increased. Another example of extramedullary microenvironment specific lymphoid development is found in gastrointestinal mucosa, where extensive expression of Notch and their ligands are found [50]. The human small intestine is a site of CD34+CD7+ lymphoid precursor development, not generally present in the circulation, suggesting that the gut may be an important site of early lymphoid differentiation [51]. These findings support the notion that Notch ligand expressing tissues play a role in NK cell differentiation and ultimately in the process of NK cell education.
In summary, our data support a vital role of Notch signaling in early stages of NK cell development. The NK cells derived from Notch1 activated CD34+ progenitors resemble the phenotype of immature CD56 cells that develop in lymphoid tissues. Like these cells, Notch activated cells are functionally hyporesponsive. This is consistent with a model that Notch is physiologically regulated in extramedually lymphoid tissue by the quality and quantity of the local and specific expression of Notch ligands.
Figure 7. Activated Notch induced generation of cyCD3+ lymphocytes capable of producing IL-2.
UCB CD34+ progenitors transduced with GFP and ICN were cultured and analyzed at day 28. (A) ICN+ or eGFP+ derived cells were incubated in the presence of phorbol ester and calcium ionophore (PMA/ionomycin) for 5 hours. Brefeldin A was added to cultures and cells were permeabilized using cytofix/cytoperm. Representative flow cytometry plots for expression of CD56 and cytoplasmic CD3 (cyCD3) are shown (left panel). The cyCD3+ICN+ population was capable of IL-2 production after PMA stimulation (right panel, representative of 4 independent experiments). B) RNA transcripts of RAG1, RAG2, pre-Tα, GATA3, cyCD3 γ,δ,ε, zeta were analyzed by QRT-PCR and compared between ICN+ and eGFP derived cells harvested at day 28. The induction of specific mRNA is relative to the level of GAPDH expression and is normalized to the ratio of eGFP derived NK cells. Bars represent the average of 3 independent experiments ± SEM.
Acknowledgments
We would like to acknowledge the Cancer Center Translational Therapy Core for their outstanding assistance and Drs. Bharat Thyagarajan and Michael Berger for their assistance with molecular analysis of gene re-arrangement and Ryan Fremming in the University of Minnesota Cytokine Reference Lab for cytokine analysis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lanier LL, Corliss B, Phillips JH. Arousal and inhibition of human NK cells. Immunol Rev. 1997;155:145–154. doi: 10.1111/j.1600-065x.1997.tb00947.x. [DOI] [PubMed] [Google Scholar]
- 2.Blom B, Spits H. Development of human lymphoid cells. Annu Rev Immunol. 2006;24:287–320. doi: 10.1146/annurev.immunol.24.021605.090612. [DOI] [PubMed] [Google Scholar]
- 3.Miller JS, McCullar V. Human natural killer cells with polyclonal lectin and immunoglobulin-like receptors develop from single hematopoietic stem cells with preferential expression of NKG2A and KIRL2/L3/S2. Blood. 2001;98:705–713. doi: 10.1182/blood.v98.3.705. [DOI] [PubMed] [Google Scholar]
- 4.Parham P. Talking license with natural killer cell maturation and repertoire development. Immun Review. 2006;214:155–160. doi: 10.1111/j.1600-065X.2006.00462.x. [DOI] [PubMed] [Google Scholar]
- 5.Cooley S, Xiao F, Pitt M, et al. A subpopulation of human peripheral blood NK cells that lacks inhibitory receptors for self MHC is developmentally immature. Blood. 2007;110:578–586. doi: 10.1182/blood-2006-07-036228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cooper MA, Fehniger TA, Turner SC, et al. Human natural killer cells: a unique innate immunoregulatory role for the CD56bright subset. Blood. 2001;97:3146–3151. doi: 10.1182/blood.v97.10.3146. [DOI] [PubMed] [Google Scholar]
- 7.Frey M, Packianathan NB, Fehniger TA, et al. Differential expression and function of L-selectine on CD56bright and CD56dim natural killer cell subsets. J Immunol. 1998;161:400–408. [PubMed] [Google Scholar]
- 8.Freud AG, Becknell B, Roychowdhury S, et al. 2005. A human CD34(+) subset resides in lymph nodes and differentiates into CD56bright natural killer cells. Immunity. 2005;22:295–304. doi: 10.1016/j.immuni.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 9.Nguyen S, Dhedin N, Vernant JP, et al. NK-cell reconstitution after haploidentical hematopoietic stem-cell transplantations: immaturity of NK cells and inhibitory effect of NKG2A override GvL effect. Blood. 2005;105:4135–42. doi: 10.1182/blood-2004-10-4113. [DOI] [PubMed] [Google Scholar]
- 10.Shilling HG, McQueen KL, Cheng NW, et al. Reconstitution of NK cell receptor repertoire following HLA-matched hematopoietic cell transplantation. Blood. 2003;101:3730–3740. doi: 10.1182/blood-2002-08-2568. [DOI] [PubMed] [Google Scholar]
- 11.Cooley S, McCullar V, Wangen R, et al. KIR reconstitution is altered by T cells in the graft and correlates with clinical outcomes after unrelated donor transplantation. Blood. 2005;106:4370–4376. doi: 10.1182/blood-2005-04-1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miller JS, Alley KA, McGlave P. Differentiation of natural killer (NK) cells from human primitive marrow progenitors in stroma-based long-term culture system: Identification of CD34+CD7+ NK progenitor. Blood. 1994;83:2594–2601. [PubMed] [Google Scholar]
- 13.Mrozek E, Anderson P, Caligiuri MA. Role of interleukin 15 in the development of human CD56+ natural killer cells from CD34+ hematopoetic progenitor cells. Blood. 1996;87:2632–2640. [PubMed] [Google Scholar]
- 14.Lotzova E, Savary CA, Champlin RE. Genesis of human oncolytic natural killer cells from primitive CD34+CD33- bone marrow progenitors. J Immunol. 1993;150:5263–5269. [PubMed] [Google Scholar]
- 15.Miller JS, Verfaille C, McGlave P. The generation of natural killer cells from CD34+/DR- primitive progenitors in human long-term bone marrow culture. Blood. 1992;80:2182–2187. [PubMed] [Google Scholar]
- 16.Hackett J, Bennett M, Kumar V. Origin and differentiation of natural killer cells. Characteristics of transplantable NK cell precursor. J Immunol. 1985;134:3731–3738. [PubMed] [Google Scholar]
- 17.Miller JS, McCullar V, Punzel M, Lemischka IR, Moore KA. Single adult human CD34(+)/Lin-/CD38(−) progenitors give rise to natural killer cells, B-lineage cells, dendritic cells, and myeloid cells. Blood. 1999;93:96–106. [PubMed] [Google Scholar]
- 18.McCullar V, Oostendorp R, Panoskaltsis-Mortari A, et al. Mouse fetal and embryonic liver cells differentiate human umbilical cord blood progenitors into CD56-negative natural killer cell precursors in the absence of interleukin-15. Exp Hematol. 2008;36:598–608. doi: 10.1016/j.exphem.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stier S, Cheng T, Dombkowski D, Carlesso N, Scadden DT. Notch1 activation increases hematopoietic stem cell self-renewal in vivo and favors lymphoid over myeloid lineage commitment. J Exp Med. 2000;192:1775–1784. doi: 10.1182/blood.v99.7.2369. [DOI] [PubMed] [Google Scholar]
- 20.Varnum-Finney B, Brashem-Stein C, Bernstein ID. Combined effects of Notch signaling and cytokines induce a multiple log increase in precursors with lymphoid and myeloid reconstituting ability. Blood. 2003;101:1784–1789. doi: 10.1182/blood-2002-06-1862. [DOI] [PubMed] [Google Scholar]
- 21.Jaleco AC, Neves H, Hooijberg E, et al. 2001. Differential effect of Notch ligands Delta-1 and Jagged-1 in human lymphoid differentiation. J Exp Med. 2001;194:991–1001. doi: 10.1084/jem.194.7.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Smedt M, Taghon T, Van de Walle I, DeSmet G, Ieclercq G, Plum J. Notch signaling induced cytoplasmic CD3ε expression in human differentiating NK cells. Blood. 2007;110:2696–2703. doi: 10.1182/blood-2007-03-082206. [DOI] [PubMed] [Google Scholar]
- 23.Carotaa S, Brady J, Wu Li, Nutt SE. Transient Notch signaling induces NK cells potential in Pax5-deficient pro-B cells. Eur J Immunol. 2006;36:1–11. doi: 10.1002/eji.200636325. [DOI] [PubMed] [Google Scholar]
- 24.Varnum-Finney B, Purton LE, Yu M, et al. The Notch ligand, Jagged 1, influence the development of primitive hematopoietic precursor cells. Blood. 1998;91:4084–4091. [PubMed] [Google Scholar]
- 25.Radtke F, Wilson A, Ernst B, McDonald HR. The role of Notch signaling during hematopoetic lineage commitment. Immunol Review. 2002;187:65–74. doi: 10.1034/j.1600-065x.2002.18706.x. [DOI] [PubMed] [Google Scholar]
- 26.Garcia-Peydro M, de Yebenes VG, Toribio ML. Sustained Notch 1 signaling instructs the earliest human intrathymic precursors to adopt a γδ T cell fate in human fetal thymus organ culture. Blood. 2003;102:2444–2451. doi: 10.1182/blood-2002-10-3261. [DOI] [PubMed] [Google Scholar]
- 27.Bray S, Furrols M. Notch pathway: making sense of suppressor of hairless. Curr Biol. 2001;11:R217–21. doi: 10.1016/s0960-9822(01)00109-9. [DOI] [PubMed] [Google Scholar]
- 28.Aster JC, Xu L, Karnell FG, Patriub V, Pui JC, Pear WS. Essential roles for ankyrin repeat and transactivation domains in induction of T-cell leukemia by notch1. Mol Cell Biol. 2000;20:7505–15. doi: 10.1128/mcb.20.20.7505-7515.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Naviaux RK, Costanzi E, Haas M, Verma IM. The pCL vector system: rapid production of helper-free, high-titer, recombinant retroviruses. J Virol. 1996;70:5701–5705. doi: 10.1128/jvi.70.8.5701-5705.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chiorean EG, Dylla SJ, Olsen K, Lenvik T, Soignier Y, Miller JS. BCR/ABL alters the function of NK cells and the aqcuisition of killer immunoglobulin-like receptors (KIR) Blood. 2003;101:3527–3533. doi: 10.1182/blood-2002-04-1172. [DOI] [PubMed] [Google Scholar]
- 31.Pierson BA, Miller JS. CD56+bright and CD56+dim natural killer cells in patients with chronic myelogenous leukemia progressively decrease in number, respond less to stimuli that recruit clonogenic natural killer cells, and exhibit decreased proliferation on a per cell basis. Blood. 1996;88:2279–2287. [PubMed] [Google Scholar]
- 32.Grzywacz B, Kataria N, Sikora M, et al. Coordinated acquisition of inhibitory and activating receptors and functional properties by developing human natural killer cells. Blood. 2006;108:3824–33. doi: 10.1182/blood-2006-04-020198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Freud AG, Yokohama A, Becknell B, et al. Evidence for discrete stages of human natural killer cell differentiation in vivo. J Exp Med. 2006;203:1033–43. doi: 10.1084/jem.20052507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smyth MJ, Cretney E, Kelly JM, et al. Activation of NK cell cytotoxicity. Mol Immunol. 2001;42:501–510. doi: 10.1016/j.molimm.2004.07.034. [DOI] [PubMed] [Google Scholar]
- 35.Cooper MA, Fehniger TA, Caliguiri MA. The biology of human killer subset. Trends Immunol. 2001;22:633–40. doi: 10.1016/s1471-4906(01)02060-9. [DOI] [PubMed] [Google Scholar]
- 36.Loza MJ, Zamai L, Zamai L, Azzoni L, Rosati E, Perussia B. Expression of type 1 (IFNγ ) and type 2 (IL-13, IL-5) cytokines at distinct stages of NK cell differentiation from progenitor cells. Blood. 2002;99:1273–1281. doi: 10.1182/blood.v99.4.1273. [DOI] [PubMed] [Google Scholar]
- 37.Wendt K, Wild E, Buyny S, Buer J, Schmidt RE, Jacobs R. 2006. Gene and protein characteristics reflect functional diversity of CD56dim and CD56bright NK cells. J Leukoc Biol. 2006;80:1529–1541. doi: 10.1189/jlb.0306191. [DOI] [PubMed] [Google Scholar]
- 38.Ferlazzo G, Thomas D, Lin SL, et al. 2004. The abundant NK cells in human secondary lymphoid tissues require activation to express killer cell Ig-like receptors and become cytolytic. J Immunol. 2004;172:1455–1462. doi: 10.4049/jimmunol.172.3.1455. [DOI] [PubMed] [Google Scholar]
- 39.Aoyama K, Delaney C, Varnum-Finney B, Kohn AD, Moon RT, Bernstein ID. The interaction of the Wnt and Notch pathways modulates natural killer versus T cell differentiation. Stem Cells. 2007;25:2488–97. doi: 10.1634/stemcells.2007-0102. [DOI] [PubMed] [Google Scholar]
- 40.Parham P. Talking license with natural killer cell maturation and repertoire development. Immun Review. 2006;214:155–160. doi: 10.1111/j.1600-065X.2006.00462.x. [DOI] [PubMed] [Google Scholar]
- 41.Rothenberg EV, Diamond RA, Pepper KA, Yang JA. 1990. IL-2 Gene inducibility in T cells before T cell receptor expression. Changes in signaling pathways and gene expression requirements during intrathymic maturation. J Immunol. 1990;144:1614–1624. [PubMed] [Google Scholar]
- 42.Ohishi K, Varnum-Finney B, Bernstein ID. Delta-1 enhances marrow and thymus repopulating ability of human CD34+CD38- cord blood cells. J Clin Invest. 2002;110:1165–1174. doi: 10.1172/JCI16167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.De Smedt M, Hoebeke I, Reynvoet K, Leclercq G, Plum J. Different thresholds of Notch signaling bias human precursor cells toward B-, NK-, monocytic/dendritic, or T-cell lineage in thymus microenvironment. Blood. 2005;106:3498–3506. doi: 10.1182/blood-2005-02-0496. [DOI] [PubMed] [Google Scholar]
- 44.Ohishi O, Varnum-Finney B, Serda RE, Anasetti C, Bernstein ID. The Notch ligand, Delta-1, inhibits the differentiation of monocytes into macrophages but permits their differentiation into dendritic cells. Blood. 2001;98:1402–1407. doi: 10.1182/blood.v98.5.1402. [DOI] [PubMed] [Google Scholar]
- 45.La Motte-Mohs RN, Herer E, Zuniga-Pflucker JC. Induction of T cell development from human cord blood hematopoetic stem cells by Delta-like 1 in vitro. Blood. 2005;105:1432–1439. doi: 10.1182/blood-2004-04-1293. [DOI] [PubMed] [Google Scholar]
- 46.Lehar SM, Dooley J, Farr AG, Bevan MJ. Notch ligands Delta1 and Jagged1 transmit distinct signals to T-cell precursors. Blood. 2005;105:1440–1447. doi: 10.1182/blood-2004-08-3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.DeHart SL, Heikens MJ, Tsai S. Jagged2 promotes the development of natural killer cells and the establishment of functional natural killer cell lines. Blood. 2005;105:3521–3527. doi: 10.1182/blood-2004-11-4237. [DOI] [PubMed] [Google Scholar]
- 48.Vosshenrich CA, Garcia-Ojeda ME, Samson Villeger SE. A thymic pathway of mouse natural killer cell development is characterized by expression of GATA-3 and CD127. Nat Immunol. 2006;7:1217–24. doi: 10.1038/ni1395. [DOI] [PubMed] [Google Scholar]
- 49.Di Santo JP, Vosshenrich CA. Bone marrow versus thymic pathways of natural killer cell development. Immunol Rev. 2006;214:35–46. doi: 10.1111/j.1600-065X.2006.00461.x. [DOI] [PubMed] [Google Scholar]
- 50.Sander GR, Powell BC. Expression of notch receptors and ligands in the adult gut. J Histochem Cytochem. 2004;52:509–516. doi: 10.1177/002215540405200409. [DOI] [PubMed] [Google Scholar]
- 51.Lynch L, O’Donoghue D, Dean J, O’Sullivan J, O’Farrelly C, Golden-Mason L. Detection and characterization of hematopoetic stem cells in the adult human intestine. J Immunol. 2006;76:5199–5204. doi: 10.4049/jimmunol.176.9.5199. [DOI] [PubMed] [Google Scholar]