Abstract
Among the various types of DNA damage, inter-strand cross-links (ICL) represent one of the most cytotoxic lesions. Processes such as transcription and replication can be fully blocked by ICLs, as shown by the mechanism of action of some anticancer drugs. However, repair of ICLs can be a possible cause of resistance. To study the mechanisms of cross-link repair stable, site-specifically cross-linked duplexes are needed. We here report on the synthesis of site-specifically cross-linked DNA using an acyclic furan containing nucleoside. Selective in situ oxidation of the incorporated furan moiety generates a highly reactive oxo-enal that instantly reacts with the complementary base in a non-modified strand, yielding one specific stable cross-linked duplex species. Varying sequence context showed that a strong selectivity for cross-linking to either complementary A or complementary C is operating, without formation of cross-links to neighboring or distant bases. Reaction times are very short and high isolated yields are obtained using only one equivalent of modified strand. The formed covalent link is stable and the isolated cross-linked duplexes can be stored for several months without degradation. Structural characterization of the obtained ICL was possible by comparison to the natural mutagenic adducts of cis-2-butene-1,4-dial, a metabolite of furan primarily responsible for furan carcinogenicity.
INTRODUCTION
Interstrand cross-link (ICL) forming agents are frequently used in cancer treatment but it has been shown that resistance can arise by the action of repair enzymes that can repair the induced lesions in tumor cells (1,2). The mechanism by which ICLs are repaired in mammalian cells is still unknown, and only hypothetical models exist to date (3). Knowledge about formation and repair of ICLs should help to understand the clinical effectiveness and side-effects of these agents and possibly help to improve cytostatic therapy (4). The study of ICL repair is hampered by the difficulty of producing suitable substrates for the mechanistic studies: as the ICLs only represent a minor fraction of all lesions it is impossible to extract one specific cross-linked sequence from biological material. Therefore, considerable research effort has been invested in synthesizing short cross-linked oligonucleotide duplexes as model substrates often based on the use of reactive oligonucleotides (5,6). The most recent mechanistic investigations on cross-link repair rely on ligation of synthetically and site-specifically generated short cross-linked oligonucleotides which are incorporated into plasmids, emphasizing the need for chemical methods for high-yielding and defined oligonucleotide ICL formation (3).
ICL-forming oligonucleotides further find widespread application in other chemical biology research areas. In the antisense area, oligonucleotides that can induce covalent bond formation with the target mRNA, ensure tighter binding than traditional steric block sequences. They incorporate a functional moiety that may cause irreversible damage to the target sequences and inhibition of the elongation step has been achieved by the use of such active antisense strands (7). It has further been suggested that active oligonucleotides make RNase H activity superfluous (8,9). Not only do cross-links impose continuous double strand formation, they could also have an influence on the overall duplex structure. Double-stranded decoy-DNA can bind with high affinity to target transcription factors thereby altering gene transcription as the transcription factor is occupied by the decoy (10). Transfection of cells with this double-stranded DNA has been shown to result in modulation of gene expression (11). To enhance stability of the decoy DNA, one of the strategies used is to introduce an ICL in the double-stranded DNA (12). It has further been postulated that the cross-links have the potential of constraining the oligonucleotide in a form that is more active towards the transcription factor (13).
Construction of oligonucleotides with a functional moiety that is reactive toward the complementary strand has been achieved in various ways. Alkylating moieties have been proposed for this purpose but can suffer from lack of specificity in the reaction or tend to be unstable under physiological conditions (3,14–16). Therefore, the idea of using stable precursors with inducible reactivity was proposed as a possible solution to this problem. Next to the concept of photo cross-linking (17–20), more recently novel concepts of inducible reactivity have been proposed based on phenyl sulfide (21,22) and phenyl selenide (23,24) derivatives.
On the basis of this idea of inducible reactivity, we recently developed a new complementary methodology for DNA cross-linking based on the incorporation and subsequent in situ selective oxidation of a furan-modified nucleoside building block (25). In order to explore the full capacity and sequence selectivity of the furan-oxidation-cross-link strategy we recently synthesized a simplified, acyclic building block (26). We here describe the use of this new acyclic furan containing building block and report upon the exploration of the sequence selectivity of the proposed method. We furthermore have been able to show that a new type of adduct is formed within this series of cross-linked duplexes. Structural characterization of the formed cross-linked adducts was possible by considering the earlier described mechanisms of formation of naturally occurring nucleoside and single-stranded oligonucleotide adducts generated through reaction with frequently occurring furan or lipid peroxidation metabolites.
MATERIALS AND METHODS
All solvents and chemical reagents were purchased from Sigma-Aldrich in the highest purity available. Products for DNA synthesis were obtained from Glen Research. Exonuclease III was purchased from GE Healthcare. IR spectra were recorded on a Perkin Elmer 1600 series FTIR-spectrometer. IR intensities are reported as strong (s) or medium (m). Optical rotations were measured on a Perkin Elmer 241 polarimeter. 1H NMR and 13C NMR spectra were recorded on a Bruker Avance 300 or a Bruker DRX 500 spectrometer operating at room temperature. Chemical shifts are reported in parts per million (δ) relative to the residual solvent peak. Multiplicities are reported as singlet (s), broad singlet (bs), doublet (d), doublet of doublets (dd), triplet (t) or multiplet (m). Purities of small compounds were checked by RP–HPLC–MS (Agilent-1100 series LC/MS system) equipped with a Phenomenex Luna C18 column (250 × 4.6 mm, 5 µm at 35°C). The used solvent system was 5 mM NH4OAC in water (A) and MeCN (B). The used gradient went from 0 to 100% B in 15 min. All RP–HPLC experiments with oligonucleotides were recorded on an Agilent 1100 system equipped with a Phenomenex Jupiter 300 Å C18 column (250 × 4.6 mm, 5 µm at 50°C) with 0.1 M TEAA (with 5% MeCN) and MeCN as mobile phases (linear gradient: 0–30% MeCN in 15 min). Mass spectra of oligonucleotides were recorded on an ABI Voyager DE–STR MALDI–TOF in positive mode with 3-HPA and ammonium citrate as matrix. Samples were desalted with DOWEX as described elsewhere (27). Oligonucleotides were synthesized DMT-on on an ABI 394 DNA synthesizer. Sep-pak C18 cartridges were obtained from Waters.
(S)-Toluene-4-sulfonic acid 2-(2,2-dimethyl-[1,3]dioxolan-4-yl)-ethyl ester 5. S-(+)-3,4-o-isopropylidenedioxybutan-1-ol 4 (1.730 g, 11.849 mmol, 1 eq.) was weighed in a two-neck flask. Extra dry pyridine (1.5 ml) was added followed by paratoluenesulfonylchloride (2.589 g, 13.6 mmol, 1.15 eq.) in one portion and the flask was flushed with argon. After a couple of minutes a white suspension forms. After 2.5 h the mixture is poured into 3 ml aqueous 1 M HCl solution followed by extraction with diethylether. The organic phase was washed three times with a 1 M aqueous HCl solution and three times with saturated NaHCO3 solution. The organic layer was dried over anhydrous Na2SO4 and evaporated under reduced pressure. The obtained colourless oil (3.247 g, 10.82 mmol, 91%) can be used for further reactions without purification. ESI–MS (M + Na+): 323,0; Retention time: 17.0 min; IR (cm−1): 3448 (b), 1598 (m), 1361 (s), 1189 (s), 1177; 1H NMR (300 MHz, CDCl3): δ 7.80 (d, 2H, J = 8.27), 7.35 (d, 2H, J = 8.1), 3.85–4.10 (m, 3H), 3.51 (dd, 2H, J = 7.87, 6.94), 2.45 (s, 3H), 1.92 (m, 2H), 1.34 (s, 3H), 1.29 (s, 3H); 13C NMR (75 MHz, CDCl3): δ 144.87, 132.98, 129.81, 127.94, 109.06, 72.32, 69.10, 67.42, 33.17, 26.86, 25.56, 19.05.
(S)-4-(2-Bromo-ethyl)-2,2-dimethyl-[1,3]dioxolane 6. Tosylate 5 (1.990 g, 6.63 mmol, 1 eq.) was dissolved in extra dry DMF (10 ml) before adding anhydrous LiBr (2.540 g, 33 mmol, 5 eq.) to the mixture. The reaction mixture was slowly heated to 60°C until all the LiBr was dissolved. After 1 h the reaction mixture was poured into ice water. The aqueous phase was extracted three times with diethyl ether, the combined organic phases were collected and washed with water and saturated NaHCO3 solution. The organic layer was dried over anhydrous NaSO4 filtered and evaporated under reduced pressure. The obtained crude was purified by flash chromatography (EtOAc/pentane 1:5). Compound 6 was co-evaporated with extra dry THF before use in the next reaction. Rf (3:2 pentane: EtOAc) 0.7; IR(cm−1): 1370 (s), 1252 (s), 1214 (s), 1152 (m), 1062 (s), 847 (m), 515 (m); 1H NMR (300 MHz, CDCl3): δ 4.25 (m, 1H), 4.08 (dd, 1H, J = 6.85, 7.31), 3.57 (dd, 1H, J = 6.23, 7.98), 3.48 (m, 2H), 2.08 (m, 2H), 1.39 (s, 3H), 1.34 (s, 3H); 13C NMR (75 MHz, CDCl3): δ 109.15, 74.00, 68.92, 37.11, 29.42, 26.99, 25.58.
(S)-4-(2-Furan-2-yl-ethyl)-2,2-dimethyl-[1,3]dioxolane 7. n-Buthyllithium (7.5 ml of a 1.6 M solution in hexanes, 1.8 eq.) was added to 6 ml THF at −78°C. Upon furan addition (900 mg, 13.25 mmol, 2 eq.) a white precipitate was formed. After 15 min, the temperature was allowed to rise to 0°C. The mixture was left to stir for an additional 2 h before bromide 6 (1 eq. based on starting material of the previous reaction) was added in one portion. The mixture was stirred overnight, poured in ice water and the product was extracted from the aqueous phase with diethyl ether. The organic layer was dried over Na2SO4 before evaporation under reduced pressure. The product was purified with flash chromatography (pentane/EtOAc 10:1). The reaction resulted in 800 mg (4.08 mmol, 62% over two steps) of the desired product. ESI–MS (M-(CH3)2CO)+: 139.1; Retention time = 17.0 min; [α]D24 = +2.5 (c 0.061 M, CHCl3); 1H NMR (300 MHz, CDCl3): δ 7.26 (dd, 1H, J = 0.61, 1.44), 6.23 (dd, 1H, J = 3.1, 1.9), 5.97 (dd, 1H, J = 0.7, 3.1), 3.97 (dd, 1H, J = 6.0, 7.8), 3.74 (m, 1H), 3.48 (dd, 1H, J = 7.4, 7.7), 2.69 (m, 2H), 1.86 (m, 2H), 1.38 (s, 3H), 1.31 (s, 3H); 13C NMR (75 MHz, CDCl3): δ 155.20, 141.00, 110.15, 108.81, 105.08, 75.25, 69.19, 32.08, 26.98, 25.89, 24.34.
(S)-4-Furan-2-yl-butane-1,2-diol 8. Protected diol 7 (309 mg, 1.57 mmol, 1 eq.) was dissolved in THF (3 ml). A 4 M solution of HCl (1.5 ml) was added under stirring and external cooling with an ice bath. The reaction mixture was allowed to stir at 0°C for 3 h. The product was extracted from the reaction mixture with EtOAc and the organic layer was concentrated under reduced pressure. Traces of starting material could be removed by flash chromatography using a 1:1 mixture of pentane:EtOAc. (S)-4-furan-2-yl-butane-1,2-diol was obtained in 89% yield (220 mg). ESI–MS(M+H+): 157.1; retention time: 11.0 min; [α]D24 = −11.5 (c 0.025 M,CHCl3), IR (cm−1): 3385 (b), 1596 (m), 1508 (m); 1H NMR (300 MHz, CDCl3): δ 7.26 (d, 1H, J = 0.61), 6.24 (dd, 1H, J = 1.9, 3.0), 5.98 (d, 1H, J = 3.0), 3.67 (m, 1H), 3.59 (m, 1H), 3.41 (m, 1H), 3.21 (bs), 3.14 (bs), 2.73 (m, 2H), 1.73 (m, 2H); 13C NMR (75 MHz, CDCl3): δ 155.41, 141.02, 110.20, 105.14, 71.46, 66.60, 31.35, 24.10.
(S)-1-[bis-(4-Methoxy-phenyl)-phenyl-methoxy]-4-furan-2-yl-butan-2-ol 9. The diol (212 mg, 1.36 mmol, 1 eq.) was co-evaporated three times with extra dry pyridine and dissolved in extra dry pyridine (2 ml). Dimethoxytritylchloride (DMTCl) (460 mg, 1.36 mmol, 1 eq.) was added at 0°C. After 1.25 h MeOH (1 ml) was added and stirring continued for 10 min. EtOAc was added and the organic layer was washed three times with saturated NaHCO3 solution. The organic layer was dried over Na2SO4 and evaporated under reduced pressure. The crude compound was purified by column chromatography using 7:1 cyclohexane:EtOAc. Total 410 mg of the mono-tritylated/compound was obtained (0.90 mmol, 66%). ESI–MS: 481.2 (M+Na+); Retention time: 19.7 min; [α]D24 = 4.7 (c 0.015 M,CHCl3); IR (cm−1): 3436 (b), 1607 (m), 1508 (s), 1249 (s), 1176 (s), 1034 (m); 1H NMR (300 MHz, CDCl3): δ 7.42 (m, 2H), 7.31 (m, 7H), 7.27 (m, 1H), 6.83 (d, 4H, J = 6.15), 6.26 (dd, 1H, J = 1.9, 3.0), 5.95 (d, 1H, J = 3.12), 3.79 (bs, 7H), 3.17 (dd, 1H, J = 3.3, 9.3), 3.05 (dd, 1H, J = 7.4, 9.3), 2.71, m, 2H), 2.34 (d, 1H, J = 3.48), 1.73 (m, 2H); 13C NMR (75 MHz, CDCl3): δ 158.37, 155.51, 144.60, 140.74, 135.82, 129.81, 127.99, 127.72, 126.70, 113.00, 109.94, 104.82, 85.98, 70.07, 67.23, 55.08, 31.15, 23.93.
Diisopropyl-phosphoramidous acid 1-[bis-(4-methoxy-phenyl)-phenyl-methoxymethyl]-3-furan-2-yl-propyl ester 2-cyano-ethyl ester 3. The DMT protected nucleoside (220 mg, 0.48 mmol, 1 eq.) was dissolved in dry CH2Cl2 (10 ml). DIPEA (0.9 ml, 0.70 g, 5.4 mmol, 11 eq.) was added followed by 2-cyanoethyl N,N-diisopropylchlorophosphoramidite (284 mg, 1.2 mmol, 2.5 eq.). After 2 h no more changes in the reaction mixture were observed by TLC and saturated NaHCO3 solution was added. The product was extracted with CH2Cl2, dried over NaSO4, filtered and evaporated under reduced pressure. The resulting oil was purified by column chromatography using 7:1 cyclohexane:EtOAc. The phosphoramidite (250 mg, 0.38 mmol, 79%) was obtained as colourless oil. Rf (cyclohexane/EtOAc 7:1) 0.38; mass: 681.3 (M + Na+); 31P NMR (121 MHz): δ 148.47, 149.26.
DNA synthesis and purification
All oligonucleotides were synthesized DMT-on on an ABI 394 DNA/RNA synthesizer in 1 µmol scales. A standard synthesis protocol was used except for the modified residues. These were coupled manually for 10 min with dicyano-imidazole (DCI) as activator instead of tetrazole. All couplings were estimated to be over 99%. Oligonucleotides were cleaved from solid support and deprotected by incubation at 55°C overnight in concentrated aqueous ammonia. The trityl groups were removed with 1.5% aqueous TFA on sep-pak and the resulting oligonucleotides were purified by RP–HPLC. Oligonucleotides 10–13 were obtained in this way. The complements, oligonucleotides 14–29 were purchased were purchased from Eurogentec Liège (see Table 1 for specific sequences).
Table 1.
Synthesized modified sequences and purchased complementary oligonucleotides
| Modified sequences | ||||
| 10 5′-CTGACG A2ATGC-3′ | ||||
| 11 5′-CTGACG G2GTGC-3′ | ||||
| 12 5′-CTGACG C2C TGC-3′ | ||||
| 13 5′-CTGACG T2T TGC-3′ | ||||
| Complements,N= | ||||
| 3′-GAC TGC TNT ACG-5′ | A | G | C | T |
| 3′-GAC TGC CNC ACG-5′ | 14 | 15 | 16 | 17 |
| 3′-GAC TGC GNG ACG-5′ | 18 | 19 | 20 | 21 |
| 3′-GAC TGC ANA ACG-5′ | 22 | 23 | 24 | 25 |
Thermal denaturation experiments
All UV experiments were recorded on a Varian Cary 300 Bio equipped with a six-cell thermostatted cell holder. Melting curves were monitored at 260 nm with a heating rate of 0.3°C/min. The buffer contained 100 mM NaCl and 10 mM phosphate buffer (pH 7). Oligonucleotide concentration was 2 μM for each strand. Melting temperatures were calculated from the first derivative of the heating curves using the Cary 300 Bio software.
Cross-linking reactions
The modified strands were mixed with their complements in equimolar amounts in 10 mM phosphate buffer (pH 7) and 100 mM NaCl before annealing from 95°C to room temperature. Temperatures during the whole reaction were kept constant in an Eppendorf thermomixer comfort at 20°C. A stock solution of NBS (1 eq./5 µl) was freshly prepared and to start the reaction, 1 eq. NBS was added. This was repeated every 15 min until complete disappearance of the modified oligonucleotide. The reaction was monitored by RP–HPLC. Reaction mixtures were concentrated in vacuo before preparative RP–HPLC purification.
Enzymatic digestion
The purified cross-linked product (0.6 nmol) was dissolved in 80 µl water. Eight microlitres of the supplied 10× reaction buffer was added and the mixture was heated till 37°C. The enzyme (200 units) was added and the reaction was shaken at 37°C for 50 min and directly injected into the RP–HPLC to collect all oligonucleotide-like fractions. All collected peaks were analysed by MALDI–TOF.
Gel-electrophoresis experiments
A 20% polyacrylamide with 1× TBE buffer was used for all analyses. The temperature of the gel was stabilized with a Julabo F12 at 60°C. The power supply used for gel-electrophoresis was a consort EV202. Gels were stained with GelRed (VWR) and pictures were taken with an Autochemi imaging system (UVP).
RESULTS AND DISCUSSION
The furan-oxidation strategy as a new method for cross-linking
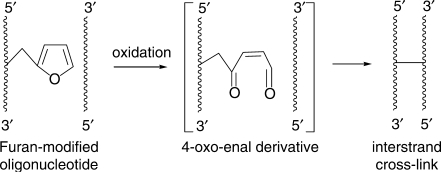
Furan is an industrially important chemical found in food and cigarette smoke (28). Studies have demonstrated that the DNA damage that is responsible for the mutagenicity and possibly the carcinogenicity of furan is caused by an oxidation product, the cis-2-butene-1,4-dial. This principle of converting a stable aromatic moiety into a reactive aldehyde formed the basis of our new approach. Selective oxidation of the incorporated furan moiety generates a reactive 4-oxo-enal derivative that reacts instantaneously with the complementary strand, yielding a cross-linked duplex species as characterized by ESI–MS (25). Preliminary studies with the first generation of furan-modified oligonucleotides indicated the formation of an imine derivative generated by attack of an exocyclic aminogroup onto the aldehyde functionality, however no firm proof as to the nature of the covalent link was obtained (Figure 1).
Figure 1.
The furan-oxidation cross-linking strategy.
Design of a simplified furan-containing building block
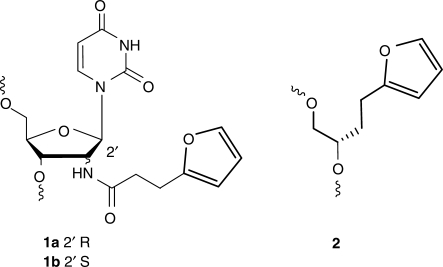
Our first generation furan-modified building blocks 1 involved conjugation of the furan moiety to 2′-amino nucleosides via an amide bond (Figure 2). Synthesis required the 2′-amino uridine derivatives which served to attach the desired furan moiety via an amide bond. In order to fully explore the sequence selectivity of the furan-oxidation cross-link methodology in terms of influence of complementary and neighbouring bases and to structurally identify the type of covalent bond formed, we needed easy access to larger quantities of modified nucleoside building block. A new acyclic building block 2 was developed featuring a simple acyclic backbone where the base was replaced by the desired furan moiety.
Figure 2.
First- and second-generation furan-modified nucleoside building blocks.
The synthetic route towards the desired phosphoramidite 3 for incorporation into oligonucleotides is short and easily scalable (Figure 3). Starting from the commercially available S-(+)-3,4-O-isopropylidenedioxybutan-1-ol, tosylation in pyridine was immediately followed by conversion to the bromide without intermediate purification. Treatment with lithium bromide in DMF yields bromide 6 which needs to be purified by chromatography. This bromide can be easily substituted by lithiated furan to generate the skeleton of the target nucleoside 2. Using a 4 M HCl solution in THF at low temperature led to deprotection without side reactions at the furan level. In the subsequent treatment with DMTCl the selectivity for monotritylation can be increased by working at low temperature and the small amount of ditritylated compound can be easily separated by chromatography. Conversion into the desired phosphoramidite 3 was performed under standard conditions.
Figure 3.
Synthesis of acyclic furan containing building block 3. Reagents and conditions: (a) pTsCl (1.15 eq.), pyridine, 0°C, 2.5 h, 91%; (b) LiBr (5 eq.), DMF, 60°C, 1 h; (c) Furan (2 eq.), BuLi (1.8 eq.), THF,−78 to 0°C, 16 h, 62% over two steps; (d) 4 M HCl, THF, 3 h, 0°C, 89%; (e) DMTCl (1 eq.), pyridine, 0°C, 1.25 h, 66%; (f) (iPr2N)(NCCH2CH2O)PCl (2.5 eq.), DIPEA, CH2Cl2, 1.5 h, rt, 79%.
Preparation of furan-containing oligonucleotides
Exploration of the cross-link selectivity being one of our goals, 3 was incorporated into four different sequences varying the neighbouring bases (10, 11, 12 and 13, Table 2). Oligonucleotide masses were confirmed using MALDI–TOF MS (Supplementary Data).
Table 2.
Melting temperatures in °C for modified versus non-modified duplexes
| Complementary base | 2*A | 2*C | 2*T | 2*G |
|---|---|---|---|---|
| 10 5′-CTGACG A2ATGC-3′ | 30.9 (−17.2) | 24.0 (−28.0) | 30.0 (–19.7) | 29.3 (−24.1) |
| 11 5′-CTGACG G2GTGC-3′ | 40.1 (−16.6) | 39.6 (−19.7) | 36.1 (−17.0) | 38.9 (−23.5) |
| 12 5′-CTGACG C2C TGC-3′ | 30.5 (−23.2) | 24.9 (−36.7) | 32.5 (−23.8) | 32.4 (−26.3) |
| 13 5′-CTGACG T2T TGC-3′ | 29.2 (−20.5) | 23.1 (−30.4) | 27.3 (−19.9) | 26.5 (−25.2) |
Melting curves were recorded at 260 nm with a heating rate of 0.3°C/min. Oligonucleotide concentrations were 2 μM of each strand in 100 mM NaCl and 10 mM phosphate buffer at pH. Values between parentheses refer to destabilization with respect to unmodified duplexes.
The influence of incorporated 2 on duplex stability was evaluated. Varying the opposing base in the complementary strand leads to 16 possible duplexes that were studied. As expected a severe drop in stability can be observed (Table 2). Reported losses in stability upon incorporation of acyclic nucleoside analogues range from 3°C (29) to 15°C (30). Since these values refer to cases where the natural bases are still present, it is not surprising to see that destabilization caused by 2 is higher, reflecting the additional loss of hydrogen bonding and stacking interactions.
Cross-linking experiments: determination of cross-linking complementary bases
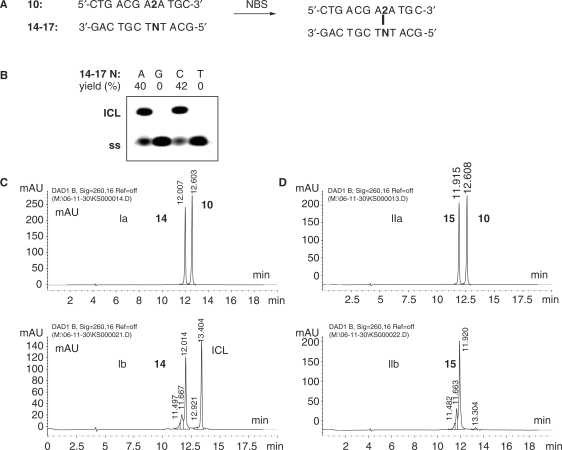
Assessment of the capacity of different opposing bases included in the complementary strand to cross-link to the oxidized furan moiety was conducted using oligonucleotide 10. Since in this case the furan-containing 2 is flanked by two A residues, the complementary strand will contain two T residues flanking the complementary base N (Figure 4A). In view of the non-reactivity of T towards oxidation products of furan (28), duplexes with 10 as modified strand allow evaluating which bases situated exactly opposite 2 will lead to cross-linked duplex.
Figure 4.
(A) Schematic representation of the cross-link reaction; furan-containing oligonucleotide 10 was annealed to complementary oligonucleotides 14–17 (14: N = A; 15: N = G; 16: N = C; 17: N = T) after which NBS was gradually added. (B) ICL formation was analysed by 20% denaturing PAGE. Yields of isolated cross-linked duplexes are indicated above the gel. (C) RP–HPLC chromatograms (λ = 260 nm) before (Ia) and after (Ib) cross-link reaction with NBS for duplex 10 (CTGACGA2ATGC):14 (GACTGCTATACG). (D) RP–HPLC chromatograms (λ = 260 nm) before (IIa) and after (IIb) cross-link reaction with NBS for duplex 10 (CTGACGA2ATGC):15 (GACTGCTGTACG).
Cross-linking experiments were conducted after annealing 10 to complementary oligonucleotides 14–17 using 1 eq. of both modified and complementary strand. A freshly prepared N-bromosuccinimide (NBS) solution was added for the in situ oxidation of the furan unit and the resulting reaction mixture was analysed by 20% denaturing PAGE and RP–HPLC (Figure 4B, C and D). As in the case of the first generation furan-modified oligonucleotides, cross-linking takes place immediately upon NBS addition. In this case, after addition of maximum 3 eq. of NBS, consumption of the modified strand was completed and a new species was formed. The new slower running species on PAGE and RP–HPLC was shown by MALDI–TOF MS to have a mass consistent with the sum of the masses of the oxidized and complementary strand. Both A and C complementary bases give rise to efficient cross-link formation while T and G do not. RP–HPLC spectral analysis shows that in case of a complementary G (Figure 4D), the modified strand is completely consumed but no clear peak corresponding to a cross-linked product can be observed, consistent with the PAGE data. This observed selectivity for A and C contrasts with the earlier observation that aldehydes such as acrolein and crotonaldehyde, which have been shown by others to arise endogenously in mammalian cells, form preferably guanine adducts in the DNA of these cells (31,32). Furthermore, inter-chain cross-linking with these reagents has been investigated and shown to give rise to G-G cross-links (33,34). On the other hand, cis-2-butene-1,4-dial has been shown to form mutagenic adducts with three out of the four nucleosides 2′-deoxycytidine, 2′-deoxyadenosine and 2′-deoxyguanosine but no cross-linked duplexes directly resulting from treatment with this furan metabolite have been described (28). In the currently studied cross-linking context, where the furan has been fixed to one of the oligonucleotide strands in the duplex, the observed selectivity for cross-linking to A and C can be possibly explained in terms of a major groove location of the furan moiety and/or its oxidized form (a molecular model, showing the proximity of the base functionalities to the oxidized furan moiety is included in the Supplementary Data).
Site-specific ICL formation
Whereas in an A2A:TNT context ICL to N-flanking bases can be completely excluded, this is not the case in sequences containing either A, C or G as N-flanking residues. Analysis of the complete series of duplexes however, indicated formation of mainly one single cross-linked species in all cases as derived from PAGE (Figure 5) and RP–HPLC analysis (Supplementary Data). With all four modified strands fast and efficient cross-linking is generally observed to A and C complementary bases (odd numbered lanes) and not to G and T (even numbered lanes). One exception can be noticed in the C2C:GTG context (lane 8) where a small spot can be observed at the ICL level. The isolated species was shown to have a mass consistent with a cross-linked duplex (Supplementary Data). Surprisingly, in a C2C:GGG context (lane 6) cross-linking was not observed. The minor groove located exocyclic amine of G has been previously shown to react with cis-2-butene-1,4-dial. This might seem to contrast with the assumed major groove orientation of the furan moiety (as derived from the A/C selectivity). In the specific case of GTG, duplex conformation might allow for the oxo-enal to be temporarily exposed to the only available nucleophile being the minor groove located N2 of one of the flanking G residues. The bulkiness of the complementary G in the latter case presumably prevents the furan moiety from reaching the minor groove. Moreover, it has been recently shown that crosslinking to abasic sites (with generation of an aldehyde in the major groove) also occurs via reaction of the exocyclic N2-amino group of an opposing guanine (35).
Figure 5.
PAGE analysis of cross-linking experiments with all duplexes. Duplex combinations are depicted above. The specific modified strand used (10–13) is indicated above the gel. Just below, indication is given of the specific opposing base in each complementary strand (14–29) and the last line gives yields of cross-linked duplexes after isolation and purification.
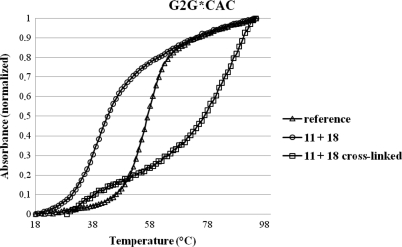
Whereas 2′-deoxyguanosine- and 2′-deoxyadenosine-cis-2-butene-1,4-dial adducts are substantially unstable under physiological conditions and only the corresponding 2-deoxycytidine adducts have a prolonged half-life, all our cross-linked duplexes have shown to be stable showing no degradation when stored for several months. Thermal stability of the different cross-linked duplexes was further analysed by UV-melting experiments. A representative graph concerning duplex 11:18 is shown in Figure 6.
Figure 6.
Comparison of melting curves for reference non-modified duplex (GTG/CAC), modified duplex (G2G/CAC) and cross-linked (G2G/CAC XL) duplex containing strands 11 and 18. Melting curves were recorded at 260 nm with a heating rate of 0.3°C/min. Duplex concentration was 2 μM in 100 mM NaCl and 10 mM phosphate buffer at pH 7.
Previous analysis showed that the modified duplex (left curve in Figure 6, open circle) is quite strongly destabilized compared to the non-modified duplex (middle curve in Figure 6, open triangle). The melting profile of the cross-linked duplex, purified from the reaction mixture by RP–HPLC, is represented by the curve on the right in Figure 6 (open square). Whereas the introduction of the modification leads to a destabilization of 16°C, the cross-linking reaction increases duplex stability with more than 28°C compared to the unmodified reference duplex or 44°C when comparing to modified duplex. This steep increase is seen for all cross-linked duplexes (Table 3).
Table 3.
Melting temperatures of the cross-linked duplexes as derived from the corresponding melting curves
| Cross-linking strands | Cross-linked to A | Cross-linked to C |
|---|---|---|
| 10: 5′-CTG ACG A2A TGC-3′ | >84°C | 71.5°C |
| 11: 5′-CTG ACG G2G TGC-3′ | >84°C | 80.0°C |
| 12: 5′-CTG ACG C2C TGC-3′ | >84°C | >84°C |
| 13: 5′-CTG ACG T2T TGC-3′ | 79.5°C | 79.0°C |
Melting temperatures for half of the samples cannot accurately be determined since at 95°C the plateau representing the single strands is not reached for these duplexes and calculation of the first derivative is not possible. These melting temperatures are estimated to be higher than 84°C.
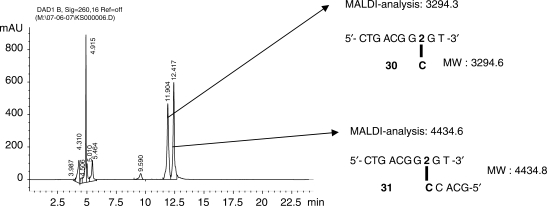
In order to confirm site-specific formation of only one cross-linked species and to further exclude cross-linking to neighbouring bases, enzymatic degradation studies were performed on all isolated cross-linked duplexes. Figure 7 shows the RP–HPLC spectrum obtained after enzymatic digestion of the cross-linked duplex 11:20 containing a G2G/CCC context, using Exonuclease III. This specific example was chosen in order to illustrate that also in this case we were able to unambiguously determine the complementary C to be the site of cross-linking and not one of the neighbouring C residues.
Figure 7.
RP–HPLC chromatogram after enzymatic degradation of duplex 11:20 CTGACGG2GTGC:GACTGCCCCACG. Enzymatic degradation was performed using 200 units of Exo III for 0.6 nmol of purified cross-linked duplex. Digestions were carried out at 37°C for 50 min. Collected fractions were analysed by MALDI–TOF MS. Products eluting at retention times below 10 min correspond to short single-stranded fragments or single nucleosides. See Supplementary Data for remaining degradation studies.
As evidenced by the analysis in Figure 7 the enzyme digests the modified strand up to two bases before the location of the modified residue. As for the non-modified strand, after 50 min, next to digestion resulting in product 30 containing the extra C-residue from the complementary strand, fragment 31 is isolated with a mass consistent with the only partially digested modified strand cross-linked to a partially digested complementary strand. The presence of this specific 5′-tail unambiguously indicates the exact position of the cross-link.
Structural characterization of the formed covalent adduct
The basic idea behind the current cross-linking strategy is the use of furan as masked reactive enal functionality. In preliminary studies where we reported on the results of this type of cross-linking reaction using 2′-amide modified furan derivatives 1, no exact structure determination was possible (Stevens,K., Petersen,M. and Madder,A., unpublished results).
Oxidation of the furan moiety in our modified oligonucleotides leads to the generation of a 4-oxo-enal. This species structurally resembles C4′-oxidized abasic sites (C4-AP), lesions that are commonly formed in high yields by the antitumor agent, bleomycin. As was very recently shown (36,37), ICLs can be attributed to this type of lesion, involving attack of a 5′ neighbouring adenine in the complementary strand onto the free aldehyde. Moreover, it was suggested that the αβ-unsaturated aldehyde, generated by endonuclease III catalysed treatment of C4-AP containing duplexes, could be an intermediate in the ICL formation (Figure 8).
Figure 8.
C4-AP lesion generated by the action of bleomycin and conversion into an oxo-enal.
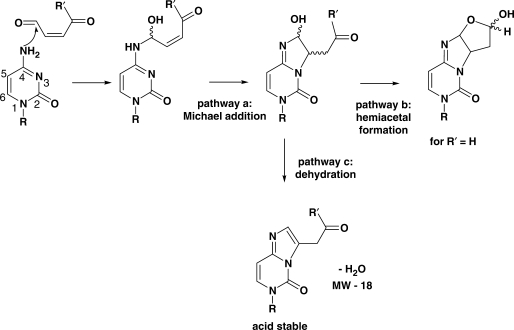
In the current work, cross-linked duplexes formed through use of the new acyclic building block 2 led to adducts that survived the acidic MALDI–TOF conditions well. The observed mass via MALDI is consistent with mere addition of both oligonucleotides without loss of H2O. In earlier studies by Dedon (38) and Peterson (28) the adducts formed by dC, dA and dG with furan metabolite cis-2-butene-1,4-dial have been completely characterized. A general reaction mechanism was proposed involving initial reaction of the C1 atom of cis-2-butene-1,4-dial with the exocyclic nitrogen atom of the nucleosides (N4 of dC, N2 of dG and N6 of dA). This event is followed by the 1,4-addition of the adjacent endocyclic nitrogen atom (N3 of dC, N1 of dG and dA) to the double bond of the remaining αβ-unsaturated aldehyde and subsequent attack of the alcohol on the second aldehyde group to form the final product (Figure 9, pathways a + b).
Figure 9.
Possible mechanisms for formation of a covalent bond in the cross-link reaction.
As for the exact structure of the formed adducts, we propose that after initial attack of the exocylic nitrogen on the 4-oxo-enal, the current duplex geometry allows for subsequent Michael addition with formation of a bi- (in case of C) or tricyclic (in case of A) structures, in a similar process as described for cis-2-butene-1,4-dial,. This would explain why stable adducts were observed in MS without loss of H2O.
In some cases MALDI–TOF and RPHPLC spectra of the isolated duplexes contained a second signal corresponding to stable cross-linked duplexes that had lost one molecule of H2O. We therefore propose that under the acidic MALDI conditions, dehydration with concurrent aromatization of the structure (pathway c) is the most probable pathway that explains the loss of water in this second generation cross-linked duplexes. Adducts of this last type have been isolated from reactions of the lipid peroxidation product 4-oxo-2(E)-nonenal with free nucleosides as well as those in dsDNA (39). Moreover acid-catalysed rearrangement of the cis-2-utene-1,4-dial adducts of 2′-deoxyadenosine has been proven to give rise to analogous tricyclic derivatives (40).
Remarkable in our case is the high stability of the duplexes containing cross-links of the oxidized furan moiety to adenine bases. In view of the much lower reported stability of adducts of cis-2-butene-1,4-dial with dA than with dC this is somewhat surprising (reported half-lifes at pH 7.4 and 37°C are 275 h for adducts with dC and only 6.1 h for adducts with dA; see 41). Presumably, the stability of the formed adducts is enhanced in this intra-molecular duplex context.
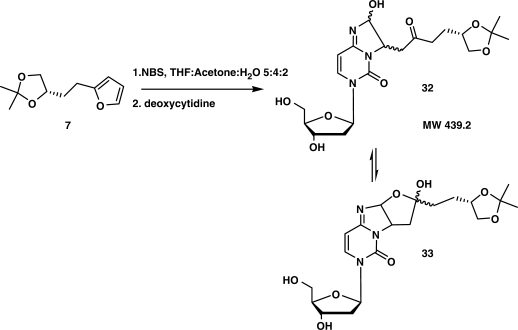
As additional proof we decided to try and confirm the proposed cross-link structure by reacting protected intermediary building block 7 with deoxycytidine under similar conditions used for the duplex cross-linking. Protected diol 7 was therefore treated with 1 eq. of NBS in a THF–acetone–H2O mixture (Figure 10) after which 1 eq. of deoxycytidine was added.
Figure 10.
Reaction of protected furan-modified diol with deoxycytidine under oxidation conditions.
Purification of the crude residue allowed isolation of a product with a mass consistent with 32/33. 1H NMR spectra allowed to completely structurally characterize the obtained dimer. The product turned out to be a mixture of 32 and a small amount of cyclized 33. It has been shown that 3, N4-ethenocytidine derivatives are fluorescent in their protonated state (42). Therefore the mixture consisting of 32/33 was subjected to a 0.1 M HCl treatment. In this way an ethenocytidine derivative is generated (see acid stable structure in Figure 9). Under these conditions a clear fluorescence emission maximum could be observed at 433 nm upon excitation at 360 nm.
The yield of the cross-link reaction between the nucleoside building blocks was very low and isolation of the desired adduct not easy which contrasts with the easy isolation and high yields obtained in the crosslink reactions with complementary oligonucleotides. Apparently the proximity effect and the pseudo-intra-molecularity have a beneficial influence on the inter-strand duplex ligation.
In the current methodology the requirement for addition of NBS might limit applications in a biological context. However, extensive research has been carried out towards photo-oxidation of furan (43). In case of monosubstituted furans, the initially generated endoperoxide has been shown to rearrange to an enal species, similar to the one generated by NBS mediated oxidation (44). The use of singlet-oxygen inducable cross-linking oligonucleotides as adjuvants in photodynamic therapy has been recently suggested (45). The singlet oxygen induced cross-linking with furan-containing oligonucleotides is currently under investigation and will be reported upon in due course.
CONCLUSIONS
We have developed a new type of acyclic furan-containing building block for incorporation in oligonucleotides and subsequent inducible cross-linking. The new furan-modified strands are capable of forming a strong and selective cross-link with complementary strands in a duplex upon addition of a selective oxidant, allowing control of the cross-link timing. The cross-link reaction is fast, high-yielding and shows selectivity towards complementary adenine and cytosine. This selectivity was confirmed by enzymatic digestion with exonuclease III. Enzymatic digestion experiments further proved that in general no cross-linking to neighbouring bases was observed.
The cross-link enhances the stability of the duplex significantly in contrast to the non cross-linked modification which causes a strong destabilization of the duplex. The obtained adducts resemble the naturally formed mutagenic reaction products of cis-2-butene-1,4-dial, a metabolite of the tobacco smoke and canned foods contaminant furan. Furthermore, the generated keto-aldehyde is structurally similar to C4′-oxidized abasic sites, commonly generated lesions by the antitumor agent bleomycin.
In view of the different selectivity results obtained with the first and the second generation building blocks, it is obvious that the modular nature of this furan-based cross-linking strategy has allowed for optimization of reactivity and selectivity of cross-link formation and shows that possibly further fine-tuning in terms of yield and selectivity can be envisaged in the future. The methodology thus lends itself ideally for the generation of well-defined and structurally characterized cross-linked duplexes as models for cross-link repair studies. Moreover the potential for singlet oxygen induced cross-link formation should give access to the use of furan-modified oligonucleotides in photodynamic therapy (46).
SUPPLEMENTARY DATA
Supplementary Data are available at NAR Online.
FUNDING
K.S. is indebted to the BOF UGent for a research fellowship. Financial support from the FWO Vlaanderen (KAN 1.5.199.07, 1.5.146.06 and 1.5.186.03) is gratefully acknowledged. Funding for open access charge: BOF UGent.
Conflict of interest statement. None declared.
Supplementary Material
ACKNOWLEDGEMENTS
Prof. A. De Picker is thanked for generous gift of a DNA synthesizer. Prof. F. Du Prez is thanked for use of the MALDI–TOF equipment. Marieke Op de Beeck is thanked for assistance.
REFERENCES
- 1.Mc Hugh PJ, Spanswick VJ, Hartley JA. Repair of DNA interstrand crosslinks: molecular mechanisms and clinical relevance. Lancet Oncol. 2001;2:483–490. doi: 10.1016/S1470-2045(01)00454-5. [DOI] [PubMed] [Google Scholar]
- 2.Panasci L, Paiement JP, Christodouplopoulos G, Belenkov A, Malapetsa A, Aloyz R. Chlorambucil drug resistance in chronic lymphocytic leukemia: The emerging role of DNA repair. Clinical Cancer Res. 2006;7:454–461. [PubMed] [Google Scholar]
- 3.Räschle M, Knipscheer P, Enoiu M, Angelov T, Sun J, Griffith JD, Ellenberger TE, Schärer OD, Walter JC. Mechanism of replication-coupled DNA interstrand cross-link repair. Cell. 2008;134:969–980. doi: 10.1016/j.cell.2008.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alzeer J, Scharer OD. A modified thymine for the synthesis of site-specific thymine-guanine DNA interstrand crosslinks. Nucleic Acids Res. 2006;34:4458–4466. doi: 10.1093/nar/gkl587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Noll DM, McGregor Mason T, Miller PS. Formation and repair of interstrand cross-links in DNA. Chem. Rev. 2006;106:277–301. doi: 10.1021/cr040478b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Noll DM, Noronha AM, Wilds CJ, Miller PS. Preparation of interstrand cross-linked DNA oligonucleotide duplexes. Frontiers Biosci. 2004;9:421–437. doi: 10.2741/1246. [DOI] [PubMed] [Google Scholar]
- 7.Kean JM, Murakami A, Blake KR, Cushman CD, Miller PS. Photochemical cross-linking of psoralen-derivatized oligonucleoside methylphosphonates to rabbit globin messenger-RNA. Biochemistry. 1988;27:9113–9121. doi: 10.1021/bi00426a008. [DOI] [PubMed] [Google Scholar]
- 8.Lee BL, Murakami A, Blake KR, Lin SB, Miller PS. Interaction of psoralen-derivatized oligodeoxyribonucleoside methylphosphonates with single-stranded DNA. Biochemistry. 1988;27:3197–3203. doi: 10.1021/bi00409a011. [DOI] [PubMed] [Google Scholar]
- 9.Sasaki S. Active oligonucleotides incorporating alkylating an agent as potential sequence- and base selective modifier of gene expression. Eur. J. Pharma. Sci. 2001;13:43–51. doi: 10.1016/s0928-0987(00)00206-2. [DOI] [PubMed] [Google Scholar]
- 10.Sullenger BA, Gallardo HF, Ungers GE, Gilboa E. Overexpression of TAR sequences renders cells resistant to human immunodeficiency-virus replication. Cell. 1990;63:601–608. doi: 10.1016/0092-8674(90)90455-n. [DOI] [PubMed] [Google Scholar]
- 11.Morischita R, Gibbons GH, Horiuchi M, Ellison KE, Nakajima M, Zhang L, Kaneda Y, Ogihara T, Dzau VJ. A gene-therapy strategy using a transcription factor decoy of the E2F binding-site inhibits smooth-muscle proliferation in-vivo. Proc. Natl Acad. Sci. USA. 1996;92:5855–5859. doi: 10.1073/pnas.92.13.5855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murakami A, Yamamoto Y, Namba M, Iwase R, Yamaoka T. Photo-cross-linked oligonucleotide duplex as a decoy-DNA for inhibition of restriction endonuclease activity. Bioorg. Chem. 2001;29:223–233. doi: 10.1006/bioo.2001.1213. [DOI] [PubMed] [Google Scholar]
- 13.Comb M, Mermod N, Hyman SE, Pearlberg J, Rose ME, Googman HM. Proteins bound at adjacent DNA elements act synergistically to regulate human proenkephalin camp inducible transcription. EMBO J. 1988;7:3793–3805. doi: 10.1002/j.1460-2075.1988.tb03264.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Webb TR, Matteucci MD. Hybridisation triggered cross-linking of deoxyoligonucleotides. Nucleic Acids Res. 1986;14:7661–7674. doi: 10.1093/nar/14.19.7661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tabone JC, Stamm MR, Meyer RB. Factors influencing the extent and regiospecificity of cross-link formation between single-stranded-DNa and reactive complement oligodeoxynucleotides. Biochemistry. 1994;33:375–383. doi: 10.1021/bi00167a048. [DOI] [PubMed] [Google Scholar]
- 16.Coleman RS, Pires RM. Covalent cross-linking of duplex DNA using 4-thio-2′-deoxyuridine as a readily modifiable platform for introduction of reactive functionality into oligonucleotides. Nucleic Acids Res. 1997;25:4771–4777. doi: 10.1093/nar/25.23.4771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Praseut D, Chassignol M, Takasugi M, Ledoan T, Thuong NT, Hélène C. Double helices with parallel strands are formed by nuclease-resistant oligo-[alpha]-deoxynucleotides and oligo-[alpha]-deoxynucleotides covalently linked to an intercalating agent with complementary oligo-[beta]-deoxynucleotides. J. Mol. Biol. 1987;196:939–942. doi: 10.1016/0022-2836(87)90416-5. [DOI] [PubMed] [Google Scholar]
- 18.Le Doan T, Perrouault L, Praseuth D, Habhoub N, Decout JL, Nguyen TT, Lhomme J, Hélène C. Sequence-specific recognition, photo-cross-linking and cleavage of the DNA double helix by an oligo-[alpha]-thymidate covalently linked to an azidoproflavine derivative. Nucleic Acids Res. 1987;15:7749–7760. doi: 10.1093/nar/15.19.7749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kobertz WR, Essigmann JM. Solid-phase synthesis of oligonucleotides containing a site-specific psoralen derivative. J. Am. Chem. 1997;119:5960–5961. [Google Scholar]
- 20.Nakatani K, Yoshida T, Saito I. Photochemistry of benzophenone immobilized in a major groove of DNA: formation of thermally reversible interstrand cross-link. J. Am. Chem. 2002;124:2118–2119. doi: 10.1021/ja017611r. [DOI] [PubMed] [Google Scholar]
- 21.Kawasaki T, Nagatsugi F, Ali MM, Maeda M, Sugiyama K, Hori K, Sasaki S. Hybridization-promoted and cytidine-selective activation for cross-linking with the use of 2-amino-6-vinylpurine derivatives. J. Org. Chem. 2005;70:14–23. doi: 10.1021/jo048298p. [DOI] [PubMed] [Google Scholar]
- 22.Ali MM, Oishi M, Nagatsugi F, Mori K, Nagasaki Y, Kataoka K, Sasaki S. Intracellular inducible alkylation system that exhibits antisense effects with greater potency and selectivity than the natural oligonucleotide. Angew. Chem. Int. Ed. 2006;45:3136–3140. doi: 10.1002/anie.200504441. [DOI] [PubMed] [Google Scholar]
- 23.Hong IS, Greenberg MM. Efficient DNA interstrand cross-link formation from a nucleotide radical. J. Am. Chem. 2005;127:3692–3693. doi: 10.1021/ja042434q. [DOI] [PubMed] [Google Scholar]
- 24.Hong IS, Ding H, Greenberg MM. Radiosensitization by a modified nucleotide that produces DNA interstrand cross-links under hypoxic conditions. J. Am. Chem. 2006;128:2230–2231. doi: 10.1021/ja058290c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Halila S, Velasco T, De Clercq P, Madder A. Fine-tuning furan toxicity: fast and quantitative DNA interchain crosslink formation upon selective oxidation of a furan containing oligonucleotide. Chem Comm. 2005;7:936–938. doi: 10.1039/b415092a. [DOI] [PubMed] [Google Scholar]
- 26.Stevens K, Madder A. Synthesis and incorporation of a simple acyclic furan containing phosphoramidite. Nucleosides, Nucleotides Nucleic Acids. 2007;26:1359–1362. doi: 10.1080/15257770701533917. [DOI] [PubMed] [Google Scholar]
- 27.Langley GJ, Herniman JM, Davies NL, Brown T. Simplified sample preparation for the analysis of oligonucleotides by matrix-assisted laser desorption/ionisation time-of-flight mass spectrometry. Rapid Commun. Mass Spectrom. 1999;13:1717–1723. doi: 10.1002/(SICI)1097-0231(19990915)13:17<1717::AID-RCM704>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 28.Chen LJ, Hecht SS, Peterson LA. Identification of cis-2-butene-1,4-dial as microsomal metabolite of furan. Chem. Res. Toxicol. 1995;8:903–906. doi: 10.1021/tx00049a001. [DOI] [PubMed] [Google Scholar]
- 29.Augustijns K, Van Aerschot A, Van Schepdael A, Urbanke C, Herdewijn P. Influence of the incorporation of (S)-9-(3,4-duhydroxy-butyl)adenine on the enzymatic stability and base-pairing properties of oligodeoxynucleotides. Nucleic Acids Res. 1991;19:2587–2593. doi: 10.1093/nar/19.10.2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schneider KC, Benner SA. Oligonucleotides containing flexible nucleoside analogs. J. Am. Chem. Soc. 1990;112:453–455. [Google Scholar]
- 31.Eder E, Hoffman C. Identification and characterization of deoxyguanosine crotonaldehyde adducts – formation of 7,8 cyclic adducts and 1,N(2),7,8 bis-cyclic adducts. Chem. Res. Toxicol. 1992;5:802–808. doi: 10.1021/tx00030a012. [DOI] [PubMed] [Google Scholar]
- 32.De los Santos C, Zaliznyak T, Johnson F. NMR characterization of a DNA duplex containing the major acrolein-derived deoxyguanosine adduct gamma-OH-1,N-2-propano-2′-deoxyguanosine. J. Biol. Chem. 2001;276:9077–9082. doi: 10.1074/jbc.M009028200. [DOI] [PubMed] [Google Scholar]
- 33.Kozekov ID, Nechev LV, Moseley MS, Harris CM, Rizzo CJ, Stone MP, Harris TM. DNA interchain cross-links formed by acrolein and crotonaldehyde. J. Am. Chem. Soc. 2003;125:50–61. doi: 10.1021/ja020778f. [DOI] [PubMed] [Google Scholar]
- 34.Dooley PA, Zhang MZ, Korbel GA, Nechev LV, Harris CM, Stone MP, Harris TM. NMR determination of the conformation of a trimethylene interstrand cross-link in an oligodeoxynucleotide duplex containing a 5′-d(GpC) motif. J. Am. Chem. Soc. 2003;125:62–72. doi: 10.1021/ja0207798. [DOI] [PubMed] [Google Scholar]
- 35.Dutta S, Chowdhury G, Gates KS. Interstrand cross-links generated by abasic sites in duplex DNA. J. Am. Chem. Soc. 2007;129:1852–1853. doi: 10.1021/ja067294u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sczepanski JT, Jacobs AC, Greenberg MM. Self-promoted DNA interstrand cross-link formation by an abasic site. J. Am. Chem. Soc. 2008;130:9646–9647. doi: 10.1021/ja8030642. [DOI] [PubMed] [Google Scholar]
- 37.Regulus P, Duroux B, Bayle P-A, Favier A, Cadet J, Ravanat J-L. Oxidation of the sugar moiety of DNA by ionizing radiation or bleomycin could induce the formation of a cluster DNA lesion. Proc. Natl Acad. Sci. 2007;104:14032–14037. doi: 10.1073/pnas.0706044104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gingipalli L, Dedon PC. Reaction of cis- and trans-2-butene-1,4-dial with 2′-deoxycytidine to form stable oxadiazabicyclooctaimine adducts. J. Am. Chem. Soc. 2001;123:2664–2665. doi: 10.1021/ja0056421. [DOI] [PubMed] [Google Scholar]
- 39.Pollack M, Yang I-Y, Kim H-YH, Blair IA, Moriya M. Translesion DNA synthesis across the heptanone-etheno-2′-deoxycytidine adducts in cells. Chem. Res. Toxicol. 2006;19:1074–1079. doi: 10.1021/tx0600503. [DOI] [PubMed] [Google Scholar]
- 40.Byrns MC, Vu CC, Peterson LA. The formation of substituted 1,N6-etheno-2′-deoxyadenosine and 1,N2-etheno-2′-deoxyguanosine adducts by cis-2-butene-1,4-dial, a reactive metabolite of furan. Chem. Res. Toxicol. 2004;17:1606–1613. doi: 10.1021/tx049866z. [DOI] [PubMed] [Google Scholar]
- 41.Byrns MC, Predecki DP, Peterson LA. Characterization of nucleoside adducts of cis-2-butene-1,4-dial, a reactive metabolite of furan. Chem. Res Toxicol. 2002;15:373–379. doi: 10.1021/tx0101402. [DOI] [PubMed] [Google Scholar]
- 42.Barrio JR, Sattsangi PD, Gruber BA, Dammann LG, Leonard NJ. Species responsible for the fluorescence of 3, N4-ethenocytidine. J. Am. Chem. Soc. 1976;98:7408–7414. doi: 10.1021/ja00439a049. [DOI] [PubMed] [Google Scholar]
- 43.Feringa BL. Photo-oxidation of furans. Rec. Trav. Chim. Pays-Bas. 1987;106:469–488. [Google Scholar]
- 44.Feringa BL, Butselaar RJ. Anomalous ozonolysis products in the addition of singlet oxygen to methoxymethylfurans. Tetrahedron Lett. 1981;22:1447–1452. [Google Scholar]
- 45.Hong IS, Greenberg MM. DNA interstrand cross-link formation initiated by reaction between singlet oxygen and a modified nucleotide. J. Am. Chem. Soc. 2005;127:10510–10511. doi: 10.1021/ja053493m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cermola F, Iesce MR, Buonerba G. Dye-sensitized photooxygenation of furanosyl furans: synthesis of a new pyridazine C-nucleoside. J. Org. Chem. 2005;70:6503–6505. doi: 10.1021/jo0504159. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.