Abstract
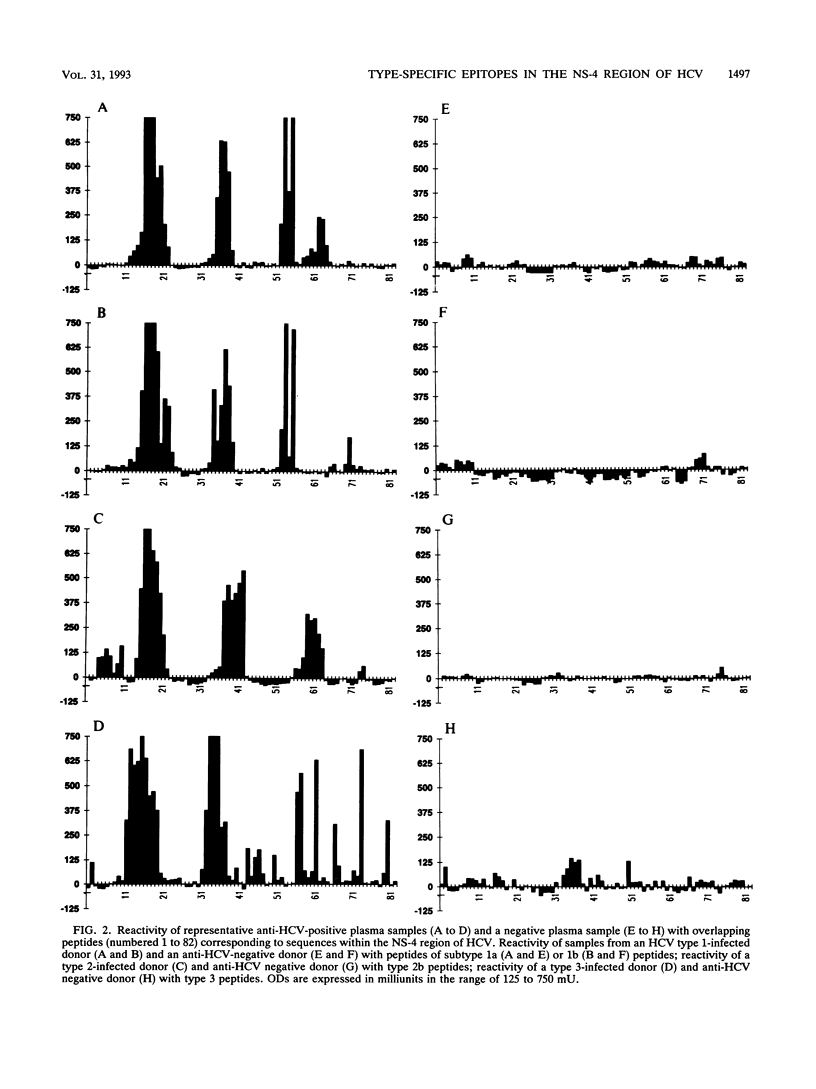
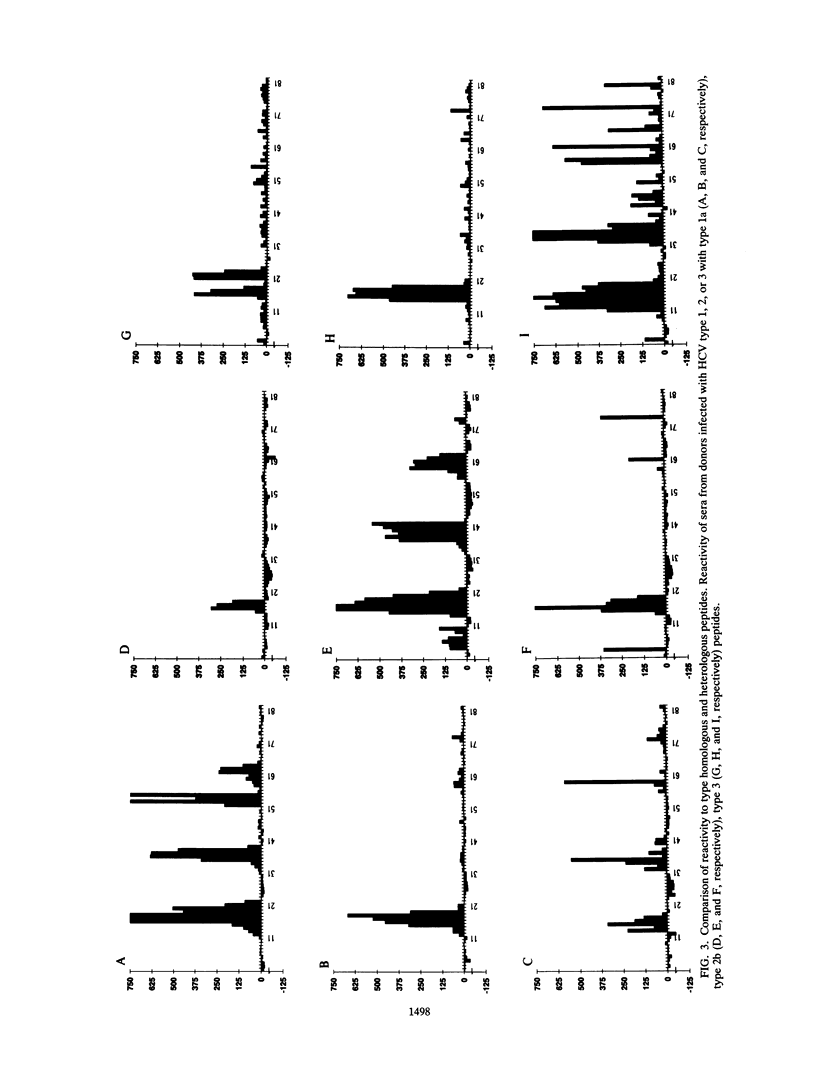
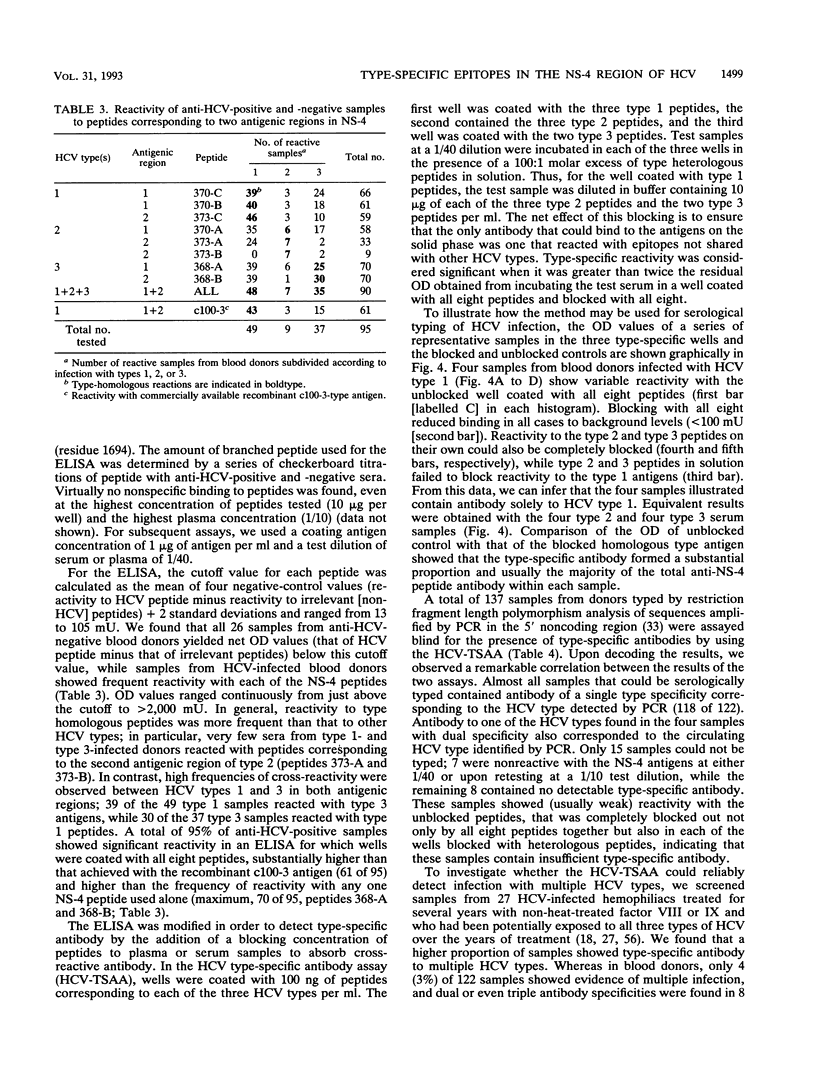
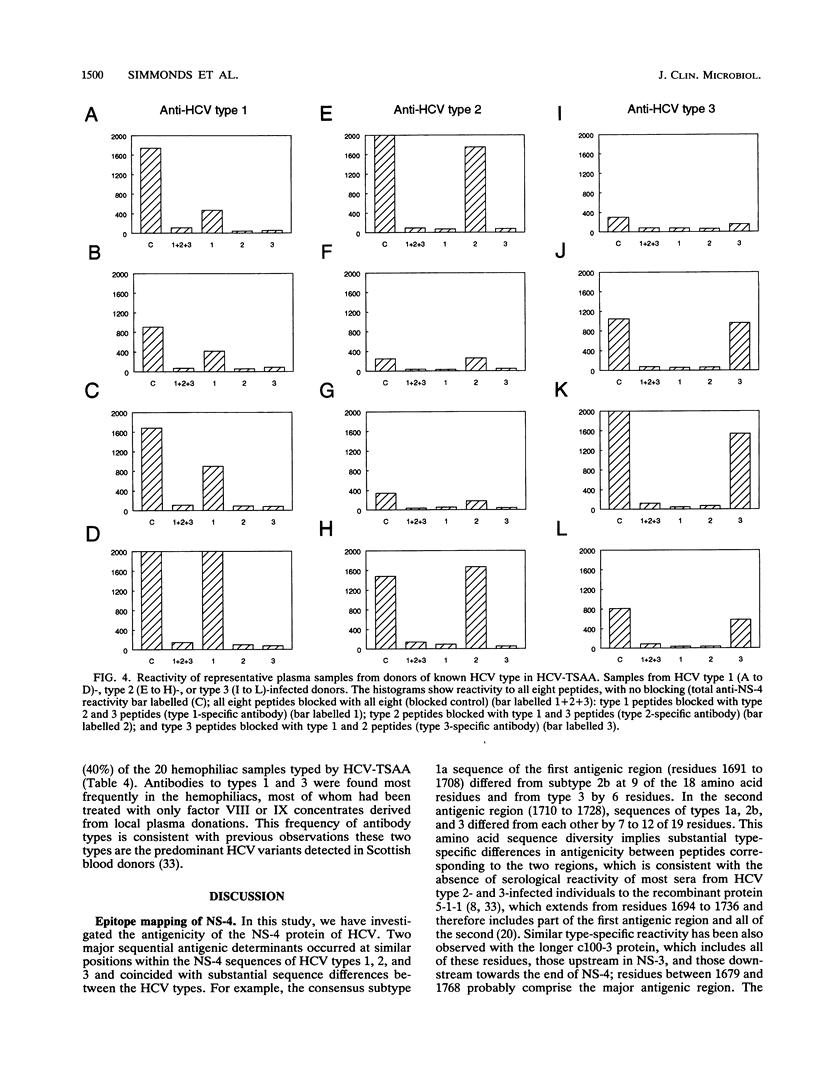
The effect of sequence variability between different types of hepatitis C virus (HCV) on the antigenicity of the NS-4 protein was investigated by epitope mapping and by enzyme-linked immunosorbent assay with branched oligopeptides. Epitope mapping of the region between amino acid residues 1679 and 1768 in the HCV polyprotein revealed two major antigenic regions (1961 to 1708 and 1710 to 1728) that were recognized by antibody elicited upon natural infection of HCV. The antigenic regions were highly variable between variants of HCV, with only 50 to 60% amino acid sequence similarity between types 1, 2, and 3. Although limited serological cross-reactivity between HCV types was detected between peptides, particularly in the first antigenic region of NS-4, type-specific reactivity formed the principal component of the natural humoral immune response to NS-4. Type-specific antibody to particular HCV types was detected in 89% of the samples from anti-HCV-positive blood donors and correlated almost exactly with genotypic analysis of HCV sequences amplified from the samples by polymerase chain reaction. Whereas almost all blood donors appeared to be infected with a single virus type (97%), a higher proportion of samples (40%) from hemophiliacs infected from transfusion of non-heat-inactivated clotting factor contained antibody to two or even all three HCV types, providing evidence that long-term exposure may lead to multiple infection with different variants of HCV.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aach R. D., Stevens C. E., Hollinger F. B., Mosley J. W., Peterson D. A., Taylor P. E., Johnson R. G., Barbosa L. H., Nemo G. J. Hepatitis C virus infection in post-transfusion hepatitis. An analysis with first- and second-generation assays. N Engl J Med. 1991 Nov 7;325(19):1325–1329. doi: 10.1056/NEJM199111073251901. [DOI] [PubMed] [Google Scholar]
- Alter H. J., Purcell R. H., Shih J. W., Melpolder J. C., Houghton M., Choo Q. L., Kuo G. Detection of antibody to hepatitis C virus in prospectively followed transfusion recipients with acute and chronic non-A, non-B hepatitis. N Engl J Med. 1989 Nov 30;321(22):1494–1500. doi: 10.1056/NEJM198911303212202. [DOI] [PubMed] [Google Scholar]
- Antibodies to hepatitis C virus in haemophilia. Lancet. 1989 Sep 2;2(8662):560–561. [PubMed] [Google Scholar]
- Brettler D. B., Alter H. J., Dienstag J. L., Forsberg A. D., Levine P. H. Prevalence of hepatitis C virus antibody in a cohort of hemophilia patients. Blood. 1990 Jul 1;76(1):254–256. [PubMed] [Google Scholar]
- Cerino A., Mondelli M. U. Identification of an immunodominant B cell epitope on the hepatitis C virus nonstructural region defined by human monoclonal antibodies. J Immunol. 1991 Oct 15;147(8):2692–2696. [PubMed] [Google Scholar]
- Chambers T. J., Hahn C. S., Galler R., Rice C. M. Flavivirus genome organization, expression, and replication. Annu Rev Microbiol. 1990;44:649–688. doi: 10.1146/annurev.mi.44.100190.003245. [DOI] [PubMed] [Google Scholar]
- Chan S. W., McOmish F., Holmes E. C., Dow B., Peutherer J. F., Follett E., Yap P. L., Simmonds P. Analysis of a new hepatitis C virus type and its phylogenetic relationship to existing variants. J Gen Virol. 1992 May;73(Pt 5):1131–1141. doi: 10.1099/0022-1317-73-5-1131. [DOI] [PubMed] [Google Scholar]
- Chan S. W., Simmonds P., McOmish F., Yap P. L., Mitchell R., Dow B., Follett E. Serological responses to infection with three different types of hepatitis C virus. Lancet. 1991 Nov 30;338(8779):1391–1391. doi: 10.1016/0140-6736(91)92265-4. [DOI] [PubMed] [Google Scholar]
- Choo Q. L., Kuo G., Weiner A. J., Overby L. R., Bradley D. W., Houghton M. Isolation of a cDNA clone derived from a blood-borne non-A, non-B viral hepatitis genome. Science. 1989 Apr 21;244(4902):359–362. doi: 10.1126/science.2523562. [DOI] [PubMed] [Google Scholar]
- Choo Q. L., Richman K. H., Han J. H., Berger K., Lee C., Dong C., Gallegos C., Coit D., Medina-Selby R., Barr P. J. Genetic organization and diversity of the hepatitis C virus. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2451–2455. doi: 10.1073/pnas.88.6.2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choo Q. L., Weiner A. J., Overby L. R., Kuo G., Houghton M., Bradley D. W. Hepatitis C virus: the major causative agent of viral non-A, non-B hepatitis. Br Med Bull. 1990 Apr;46(2):423–441. doi: 10.1093/oxfordjournals.bmb.a072408. [DOI] [PubMed] [Google Scholar]
- Cuypers H. T., Bresters D., Winkel I. N., Reesink H. W., Weiner A. J., Houghton M., van der Poel C. L., Lelie P. N. Storage conditions of blood samples and primer selection affect the yield of cDNA polymerase chain reaction products of hepatitis C virus. J Clin Microbiol. 1992 Dec;30(12):3220–3224. doi: 10.1128/jcm.30.12.3220-3224.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enomoto N., Takada A., Nakao T., Date T. There are two major types of hepatitis C virus in Japan. Biochem Biophys Res Commun. 1990 Aug 16;170(3):1021–1025. doi: 10.1016/0006-291x(90)90494-8. [DOI] [PubMed] [Google Scholar]
- Esteban J. I., Esteban R., Viladomiu L., López-Talavera J. C., González A., Hernández J. M., Roget M., Vargas V., Genescà J., Buti M. Hepatitis C virus antibodies among risk groups in Spain. Lancet. 1989 Aug 5;2(8658):294–297. doi: 10.1016/s0140-6736(89)90485-6. [DOI] [PubMed] [Google Scholar]
- Esteban J. I., González A., Hernández J. M., Viladomiu L., Sánchez C., López-Talavera J. C., Lucea D., Martin-Vega C., Vidal X., Esteban R. Evaluation of antibodies to hepatitis C virus in a study of transfusion-associated hepatitis. N Engl J Med. 1990 Oct 18;323(16):1107–1112. doi: 10.1056/NEJM199010183231605. [DOI] [PubMed] [Google Scholar]
- Farci P., Alter H. J., Govindarajan S., Wong D. C., Engle R., Lesniewski R. R., Mushahwar I. K., Desai S. M., Miller R. H., Ogata N. Lack of protective immunity against reinfection with hepatitis C virus. Science. 1992 Oct 2;258(5079):135–140. doi: 10.1126/science.1279801. [DOI] [PubMed] [Google Scholar]
- Fletcher M. L., Trowell J. M., Craske J., Pavier K., Rizza C. R. Non-A non-B hepatitis after transfusion of factor VIII in infrequently treated patients. Br Med J (Clin Res Ed) 1983 Dec 10;287(6407):1754–1757. doi: 10.1136/bmj.287.6407.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gretch D., Lee W., Corey L. Use of aminotransferase, hepatitis C antibody, and hepatitis C polymerase chain reaction RNA assays to establish the diagnosis of hepatitis C virus infection in a diagnostic virology laboratory. J Clin Microbiol. 1992 Aug;30(8):2145–2149. doi: 10.1128/jcm.30.8.2145-2149.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda Y., Toda G., Hashimoto N., Kurokawa K. Antibody to superoxide dismutase, autoimmune hepatitis, and antibody tests for hepatitis C virus. Lancet. 1990 Jun 2;335(8701):1345–1346. doi: 10.1016/0140-6736(90)91228-3. [DOI] [PubMed] [Google Scholar]
- Kato N., Hijikata M., Ootsuyama Y., Nakagawa M., Ohkoshi S., Sugimura T., Shimotohno K. Molecular cloning of the human hepatitis C virus genome from Japanese patients with non-A, non-B hepatitis. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9524–9528. doi: 10.1073/pnas.87.24.9524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo G., Choo Q. L., Alter H. J., Gitnick G. L., Redeker A. G., Purcell R. H., Miyamura T., Dienstag J. L., Alter M. J., Stevens C. E. An assay for circulating antibodies to a major etiologic virus of human non-A, non-B hepatitis. Science. 1989 Apr 21;244(4902):362–364. doi: 10.1126/science.2496467. [DOI] [PubMed] [Google Scholar]
- Lelie P. N., Cuypers H. T., Reesink H. W., van der Poel C. L., Winkel I., Bakker E., van Exel-Oehlers P. J., Vallari D., Allain J. P., Mimms L. Patterns of serological markers in transfusion-transmitted hepatitis C virus infection using second-generation HCV assays. J Med Virol. 1992 Jul;37(3):203–209. doi: 10.1002/jmv.1890370310. [DOI] [PubMed] [Google Scholar]
- Machida A., Ohnuma H., Tsuda F., Munekata E., Tanaka T., Akahane Y., Okamoto H., Mishiro S. Two distinct subtypes of hepatitis C virus defined by antibodies directed to the putative core protein. Hepatology. 1992 Oct;16(4):886–891. doi: 10.1002/hep.1840160406. [DOI] [PubMed] [Google Scholar]
- Makris M., Preston F. E., Triger D. R., Underwood J. C., Choo Q. L., Kuo G., Houghton M. Hepatitis C antibody and chronic liver disease in haemophilia. Lancet. 1990 May 12;335(8698):1117–1119. doi: 10.1016/0140-6736(90)91124-s. [DOI] [PubMed] [Google Scholar]
- Marsden H. S., Owsianka A. M., Graham S., McLean G. W., Robertson C. A., Subak-Sharpe J. H. Advantages of branched peptides in serodiagnosis. Detection of HIV-specific antibodies and the use of glycine spacers to increase sensitivity. J Immunol Methods. 1992 Feb 14;147(1):65–72. [PubMed] [Google Scholar]
- McFarlane I. G., Smith H. M., Johnson P. J., Bray G. P., Vergani D., Williams R. Hepatitis C virus antibodies in chronic active hepatitis: pathogenetic factor or false-positive result? Lancet. 1990 Mar 31;335(8692):754–757. doi: 10.1016/0140-6736(90)90870-b. [DOI] [PubMed] [Google Scholar]
- McLean G. W., Owsianka A. M., Subak-Sharpe J. H., Marsden H. S. Generation of anti-peptide and anti-protein sera. Effect of peptide presentation on immunogenicity. J Immunol Methods. 1991 Mar 21;137(2):149–157. doi: 10.1016/0022-1759(91)90019-c. [DOI] [PubMed] [Google Scholar]
- McOmish F., Chan S. W., Dow B. C., Gillon J., Frame W. D., Crawford R. J., Yap P. L., Follett E. A., Simmonds P. Detection of three types of hepatitis C virus in blood donors: investigation of type-specific differences in serologic reactivity and rate of alanine aminotransferase abnormalities. Transfusion. 1993 Jan;33(1):7–13. doi: 10.1046/j.1537-2995.1993.33193142314.x. [DOI] [PubMed] [Google Scholar]
- Mimms L., Vallari D., Ducharme L., Holland P., Kuramoto I. K., Zeldis J. Specificity of anti-HCV ELISA assessed by reactivity to three immunodominant HCV regions. Lancet. 1990 Dec 22;336(8730):1590–1591. doi: 10.1016/0140-6736(90)93377-2. [DOI] [PubMed] [Google Scholar]
- Mori S., Kato N., Yagyu A., Tanaka T., Ikeda Y., Petchclai B., Chiewsilp P., Kurimura T., Shimotohno K. A new type of hepatitis C virus in patients in Thailand. Biochem Biophys Res Commun. 1992 Feb 28;183(1):334–342. doi: 10.1016/0006-291x(92)91648-a. [DOI] [PubMed] [Google Scholar]
- Nakao T., Enomoto N., Takada N., Takada A., Date T. Typing of hepatitis C virus genomes by restriction fragment length polymorphism. J Gen Virol. 1991 Sep;72(Pt 9):2105–2112. doi: 10.1099/0022-1317-72-9-2105. [DOI] [PubMed] [Google Scholar]
- Okamoto H., Kurai K., Okada S., Yamamoto K., Lizuka H., Tanaka T., Fukuda S., Tsuda F., Mishiro S. Full-length sequence of a hepatitis C virus genome having poor homology to reported isolates: comparative study of four distinct genotypes. Virology. 1992 May;188(1):331–341. doi: 10.1016/0042-6822(92)90762-e. [DOI] [PubMed] [Google Scholar]
- Okamoto H., Okada S., Sugiyama Y., Kurai K., Iizuka H., Machida A., Miyakawa Y., Mayumi M. Nucleotide sequence of the genomic RNA of hepatitis C virus isolated from a human carrier: comparison with reported isolates for conserved and divergent regions. J Gen Virol. 1991 Nov;72(Pt 11):2697–2704. doi: 10.1099/0022-1317-72-11-2697. [DOI] [PubMed] [Google Scholar]
- Okamoto H., Sugiyama Y., Okada S., Kurai K., Akahane Y., Sugai Y., Tanaka T., Sato K., Tsuda F., Miyakawa Y. Typing hepatitis C virus by polymerase chain reaction with type-specific primers: application to clinical surveys and tracing infectious sources. J Gen Virol. 1992 Mar;73(Pt 3):673–679. doi: 10.1099/0022-1317-73-3-673. [DOI] [PubMed] [Google Scholar]
- Posnett D. N., McGrath H., Tam J. P. A novel method for producing anti-peptide antibodies. Production of site-specific antibodies to the T cell antigen receptor beta-chain. J Biol Chem. 1988 Feb 5;263(4):1719–1725. [PubMed] [Google Scholar]
- Prince A. M., Brotman B., Huima T., Pascual D., Jaffery M., Inchauspé G. Immunity in hepatitis C infection. J Infect Dis. 1992 Mar;165(3):438–443. doi: 10.1093/infdis/165.3.438. [DOI] [PubMed] [Google Scholar]
- Robertson C. A., Mok J. Y., Froebel K. S., Simmonds P., Burns S. M., Marsden H. S., Graham S. Maternal antibodies to gp120 V3 sequence do not correlate with protection against vertical transmission of human immunodeficiency virus. J Infect Dis. 1992 Oct;166(4):704–709. doi: 10.1093/infdis/166.4.704. [DOI] [PubMed] [Google Scholar]
- Simmonds P., Zhang L. Q., Watson H. G., Rebus S., Ferguson E. D., Balfe P., Leadbetter G. H., Yap P. L., Peutherer J. F., Ludlam C. A. Hepatitis C quantification and sequencing in blood products, haemophiliacs, and drug users. Lancet. 1990 Dec 15;336(8729):1469–1472. doi: 10.1016/0140-6736(90)93179-s. [DOI] [PubMed] [Google Scholar]
- Skidmore S. Recombinant immunoblot assay for hepatitis C antibody. Lancet. 1990 Jun 2;335(8701):1346–1346. doi: 10.1016/0140-6736(90)91230-8. [DOI] [PubMed] [Google Scholar]
- Sugitani M., Inchauspé G., Shindo M., Prince A. M. Sensitivity of serological assays to identify blood donors with hepatitis C viraemia. Lancet. 1992 Apr 25;339(8800):1018–1019. doi: 10.1016/0140-6736(92)90538-e. [DOI] [PubMed] [Google Scholar]
- Takamizawa A., Mori C., Fuke I., Manabe S., Murakami S., Fujita J., Onishi E., Andoh T., Yoshida I., Okayama H. Structure and organization of the hepatitis C virus genome isolated from human carriers. J Virol. 1991 Mar;65(3):1105–1113. doi: 10.1128/jvi.65.3.1105-1113.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam J. P. Synthetic peptide vaccine design: synthesis and properties of a high-density multiple antigenic peptide system. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5409–5413. doi: 10.1073/pnas.85.15.5409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam J. P., Zavala F. Multiple antigen peptide. A novel approach to increase detection sensitivity of synthetic peptides in solid-phase immunoassays. J Immunol Methods. 1989 Nov 13;124(1):53–61. doi: 10.1016/0022-1759(89)90185-3. [DOI] [PubMed] [Google Scholar]
- Theilmann L., Blazek M., Goeser T., Gmelin K., Kommerell B., Fiehn W. False-positive anti-HCV tests in rheumatoid arthritis. Lancet. 1990 Jun 2;335(8701):1346–1346. doi: 10.1016/0140-6736(90)91229-4. [DOI] [PubMed] [Google Scholar]
- Tsukiyama-Kohara K., Kohara M., Yamaguchi K., Maki N., Toyoshima A., Miki K., Tanaka S., Hattori N., Nomoto A. A second group of hepatitis C viruses. Virus Genes. 1991 Jul;5(3):243–254. doi: 10.1007/BF00568974. [DOI] [PubMed] [Google Scholar]
- Vallari D. S., Jett B. W., Alter H. J., Mimms L. T., Holzman R., Shih J. W. Serological markers of posttransfusion hepatitis C viral infection. J Clin Microbiol. 1992 Mar;30(3):552–556. doi: 10.1128/jcm.30.3.552-556.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. T., Wang T. H., Lin J. T., Sheu J. C., Sung J. L., Chen D. S. Hepatitis C virus in a prospective study of posttransfusion non-A, non-B hepatitis in Taiwan. J Med Virol. 1990 Oct;32(2):83–86. doi: 10.1002/jmv.1890320203. [DOI] [PubMed] [Google Scholar]
- Watson H. G., Ludlam C. A., Rebus S., Zhang L. Q., Peutherer J. F., Simmonds P. Use of several second generation serological assays to determine the true prevalence of hepatitis C virus infection in haemophiliacs treated with non-virus inactivated factor VIII and IX concentrates. Br J Haematol. 1992 Apr;80(4):514–518. doi: 10.1111/j.1365-2141.1992.tb04566.x. [DOI] [PubMed] [Google Scholar]
- Widell A., Sundström G., Hansson B. G., Moestrup T., Nordenfelt E. Antibody to hepatitis-C-virus-related proteins in sera from alanine-aminotransferase-screened blood donors and prospectively studied recipients. Vox Sang. 1991;60(1):28–33. doi: 10.1111/j.1423-0410.1991.tb00867.x. [DOI] [PubMed] [Google Scholar]
- van der Poel C. L., Bresters D., Reesink H. W., Plaisier A. A., Schaasberg W., Leentvaar-Kuypers A., Choo Q. L., Quan S., Polito A., Houghton M. Early antihepatitis C virus response with second-generation C200/C22 ELISA. Vox Sang. 1992;62(4):208–212. doi: 10.1111/j.1423-0410.1992.tb01200.x. [DOI] [PubMed] [Google Scholar]
- van der Poel C. L., Reesink H. W., Lelie P. N., Leentvaar-Kuypers A., Choo Q. L., Kuo G., Houghton M. Anti-hepatitis C antibodies and non-A, non-B post-transfusion hepatitis in The Netherlands. Lancet. 1989 Aug 5;2(8658):297–298. doi: 10.1016/s0140-6736(89)90486-8. [DOI] [PubMed] [Google Scholar]
- van der Poel C. L., Reesink H. W., Schaasberg W., Leentvaar-Kuypers A., Bakker E., Exel-Oehlers P. J., Lelie P. N. Infectivity of blood seropositive for hepatitis C virus antibodies. Lancet. 1990 Mar 10;335(8689):558–560. doi: 10.1016/0140-6736(90)90347-8. [DOI] [PubMed] [Google Scholar]


