Abstract
The DNA replication-related element-binding factor (DREF) regulates cell proliferation-related gene expression in Drosophila. By genetic screening, taking advantage of the rough eye phenotype of transgenic flies that express DREF in the eye discs, we identified 24 genes that suppressed and 12 genes that enhanced the rough eye phenotype when heterozygous for mutations. Five genes, HP6, pigeon, lace, X box binding protein 1 and guftagu were found to carry replication-related element (DRE) sequences in their 5′-flanking regions. Of these, the HP6 gene carries two sequences that match seven out of eight nucleotides of DRE and two additional sequences that match six out of eight nucleotides of DRE in the 5′-flanking region. Band mobility shift assays using Drosophila Kc cell nuclear extracts demonstrated DREF binding to two of these sites and chromatin immunoprecipitation using anti-DREF antibodies confirmed that this occurs in vivo. Knockdown of DREF in Drosophila S2 cells decreased the HP6 mRNA level. The results, taken together, indicate that DREF directly regulates expression of the HP6 gene. HP6 mRNA was detected throughout development by RT-PCR with highest levels in adult males. In addition, immunostaining analyses revealed colocalization of HP6 and DREF in nuclei at the apical tips in the testes.
INTRODUCTION
Promoters of many DNA replication- and proliferation-related genes in Drosophila contain a common 8 bp palindromic sequence, 5′-TATCGATA, named the DNA replication-related element (DRE) (1–10). The requirement of DRE for promoter activity has been confirmed in both cultured cell and transgenic fly systems (1,11,12) and a specific DNA replication-related element-binding factor (DREF) has been identified. Molecular cloning of its cDNA has led to confirmation that DREF is a transcriptional activator of DRE-containing genes (1). It is also reported that DREF is a component of a transcription initiation complex containing TRF2 (13). In addition, the chromatin remodelling factor dMi-2 and a homeodomain protein Distal-less can bind to the DNA-binding domain of DREF to inhibit its DNA-binding activity (14,15).
Searches of the Drosophila genome database have revealed the presence of 277 genes containing DRE-like sequences within their promoter regions (16,17) and immunostaining of polytene chromosomes of salivary glands with anti-DREF monoclonal antibodies demonstrated binding of DREF to a hundred discrete interband regions of polytene chromosomes (14). In addition, serial analysis of gene expression (SAGE) showed that many genes selectively expressed in dividing cells located anterior to the morphogenetic furrow of the eye imaginal disc carry DRE in their 5′-flanking regions (18). DREF may therefore regulate the expression of many genes and play multiple roles in vivo.
Ectopic expression of the dominant-negative form of DREF using the GAL4-UAS targeted expression system causes inhibition of both endo-replication in larval salivary gland cells and mitotic DNA replication in eye imaginal disc cells (19). Ectopic expression of full length DREF in eye imaginal discs causes ectopic DNA synthesis and apoptosis in otherwise post-mitotic cells, and inhibits photoreceptor cell differentiation that results in a severe rough eye phenotype (20). RNAi mediated knockdown of DREF in growing tissues has also provided direct evidence that it is necessary for cell cycle and cell growth control (21,22).
In order to identify novel targets of DREF, we have carried out a screening, taking advantage of the rough eye phenotype of the transgenic flies that express full length DREF in the eye imaginal discs. Our previous screen identified the dE2F, brahma, moira and osa gene as suppressors and the Distal-less gene as an enhancer of the DREF-induced rough eye phenotype (20). E2F is a transcription factor regulating the genes involved in cell cycle, while Brahma, Moira and Osa are components of the chromatin-remodelling Brahma (BRM) complex (23). Suppression of the DREF-induced rough eye phenotype by reduction of dosage of the brahma, moira, or osa suggests that the genes coding for the BRM complex are targets of DREF (20). These observations combined with molecular and biochemical analyses indicate that DREF is involved in transcriptional regulation of the genes coding for the BRM complex (24). In this study, we further identified 24 suppressors and 12 enhancers of the DREF-induced rough eye phenotype. One of the strongest suppressors was a mutant for the HP6 (CG15636) gene, which carries multiple DRE-like sequences in its 5′-flanking region. The present results indicate that the HP6 gene is one of the targets of the DRE/DREF regulatory system with major physiological significance.
MATERIALS AND METHODS
Fly stocks
Fly stocks were maintained at 25°C on standard food. The Canton S fly was used as a wild type strain. dp°vlR/SM5 and dpD/SM1 were obtained from the Kyoto Institute of Technology, Drosophila Genetic Resource Center (Japan). The UAS-DREF transgenic fly line was described earlier (19) as was the transgenic fly line (line number 16) carrying pGMR-GAL4 on the X chromosome (25). All other stocks used in this study were obtained from the Bloomington, Indiana, stock centre.
Establishment of transgenic flies
P-element-mediated germ line transformation was carried out as described earlier (26). F1 transformants were selected on the basis of white-eye colour rescue (27). Two independent lines were established for the pUAS-HP6. We used line 2 carrying UAS-HP6 on the third chromosome in the present study.
Oligonucleotides
To obtain a cDNA for the HP6 (CG15636) gene, the following polymerase chain reaction (PCR) primers were chemically synthesized:
5′Bgl2P,
5′-CGATATCTAAAAGATCTCGGAAGATGCC
3′Kpn1P,
5′-CGGTGCGGTACCGTTTTATGGACTAGG
5′BamH1P,
5′-TCTGGATCCATGCCCAGCTC
3′Xho1P,
5′-GTTTCTCGAGCTAGGCATTTCG
The sequences of double-stranded oligonucleotides containing DRE (DRE-P) in the PCNA gene were as described earlier (11). The DRE-PM oligonucleotide is a two-base substitution derivative of DRE-P (11). For band mobility shift assays, the following oligonucleotides were synthesized. The DRE and DRE-like sequences are shown in bold letters and the substituted bases in the HP6 gene promoter are shown in small letters.
DRE2,
5′-CTTACACAAAAATCGATTAAATTGAAGAAC
3′-GAATGTGTTTTTAGCTAATTTAACTTCTTG
DRE2Mut,
5′-CTTACACAAAAcgCGAgTAAATTGAAGAAC
3′-GAATGTGTTTTgcGCTcATTTAACTTCTTG
DRE1,
5′-TGCCACATCGAAAGGGTTGCCAAAGCATGTCGATACCTACAGTTATCGAAACTGA
3′-ACGGTGTAGCTTTCCCAACGGTTTCGTACAGCTATGGATGTCAATAGCTTTGACT
DRE1Mut,
5′-TGCCACcgCGAAcGGGTTGCCAAAGCATGgCGAgcCCTACAGTTcgCGAAcCTGA
3′-ACGGTGgcGCTTgCCCAACGGTTTCGTACcGCTcgGGATGTCAAgcGCTTgGACT
DRE1αMutβγ,
5′-TGCCACcgCGAAcGGGTTGCCAAAGCATGTCGATACCTACAGTTATCGAAACTGA
3′-ACGGTgTAGCTtTCCCAACGGTTTCGTACAGCTATGGATGTCAATAGCTTTGACT
DRE 1βMutαγ,
5′-TGCCACATCGAAAGGGTTGCCAAAGCATGgCGAgcCCTACAGTTATCGAAACTGA
3′-ACGGTGTAGCTTTCCCAACGGTTTCGTACcGCTcgGGATGTCAATAGCTTTGACT
DRE 1γMutαβ,
5′-TGCCACATCGAAAGGGTTGCCAAAGCATGTCGATACCTACAGTTcgCGAAcCTGA
3′-ACGGTGTAGCTTTCCCAACGGTTTCGTACAGCTATGGATGTCAAgcGCTTgGACT
DRE1γ,
5′-ACAGTTATCGAAACTGAAAAATAAT
3′-TGTCAATAGCTTTGACTTTTTATTA
DRE1γMut,
5′-ACAGTTcgCGAAcCTGAAAAATAAT
3′-TGTCAAgcGCTTgGACTTTTTATTA
To carry out chromatin immunoprecipitation, the following PCR primers were chemically synthesized:
PCNAP,
5′-GATGAATGATTAACGTGGGCTG
PCNAantiP,
5′-GAAATAAATATACTCTGTAAAAAGTGTGAAC
CG15636DRE1P,
5′-ATCGAAAGGGTTGCCAAAGC
CG15636antiDRE1P,
5′-GCGTAGCCAATTGTCACGTT
CG15636DRE2P,
5′-CTGGAATACATACACACCGAG
CG15636antiDRE2P,
5′-TGGGCGCACAATTTAAAGCAG
RP49P,
5′-AGCGCACCAAGCACTTCATC
RP49antiP,
5′-CGTTCTCTTGAGAACGCAGG
To carry out RT-PCR, the following PCR primers were chemically synthesized:
CG15636P,
5′-ATGCCCAGCTCCACTTTGAC
CG15636antiP,
5′-CTAGGCATTTCGTGATCGTTTCTTC
RP49 primers used for RT-PCR were the same as used for chromatin immunoprecipitation.
For quantitative real time PCR, the following oligonucleotides were synthesized:
DREF-F, 5′-GGCAATCTCCGTTGAATGACG
DREF-R, 5′-TTCACCTCCGAGAAGCCCTT
β-tubulin-F, 5′-AGTTCACCGCTATGTTCA
β-tubulin-R, 5′-CGCAAAACATTGATCGAG
RP49-F, 5′-GCTTCTGGTTTCCGGCAAGCTTCAAG
RP49-R, 5′-GACCTCCAGCTCGCGCACGTTGTGCACCAGGAAC
CG15636 primers used for quantitative real time PCR were the same as used for RT-PCR.
Plasmid construction
To construct the pUAS-HP6 plasmid, PCR was performed using Drosophila genomic DNA as a template and primers 5′Bgl2P and 3′Kpn1P in combination. PCR products were digested with BglII and KpnI and inserted between the BglII and KpnI sites ofthe pUAST plasmid (28).
To construct the pGST-HP6 plasmid for expression of GST-HP6 fusion protein in Escherichia coli, PCR was performed using pUAS-HP6 as a template and primers 5′BamH1P and 3′Xho1P in combination. PCR products were digested with BamHI and XhoI and inserted between the BamHI and XhoI sites of pGex6p-1 (GE healthcare).
Expression of GST fusion proteins and purification of HP6 protein
Expression of GST-HP6 fusion proteins in E. coli BL21 was carried out as described elsewhere (29). Lysates of cells were prepared by sonication in PBS containing 1 mM PMSF, and 1 μM each of pepstatin and leupeptin. Lysates were cleared by centrifugation at 12 000g for 20 min at 4°C and applied to glutathione-Sepharose (GE healthcare). The columns were washed with PBS containing 0.5 M NaCl and 0.1% Triton X-100, then with a buffer containing 150 mM NaCl, 50 mM Tris–HCl pH 7.2, 1 mM EDTA and 1 mM dithiothreitol (DTT). The included GST-HP6 fusion proteins were treated with Precision protease (GE healthcare) for 16 h at 4°C (30) and then eluted with PBS.
Antibodies
The purified HP6 protein were used to elicit polyclonal antibody production in rabbit. Polyclonal antibodies reacting with HP6 were affinity-purified from anti-serum using the N-hydroxysuccinimide (NHS)-activated Sepharose HP (GE healthcare) coupled with GST-HP6 fusion protein after passage through GST-conjugated Sepharose HP. Preparation of anti-DREF monoclonal antibodies was as described previously (1,31).
Western immunoblot analysis
Adult males of Canton S, a line carrying the Act5C-GAL4 transgene and a line carrying both UAS-Flag-HP6 and Act5C-GAL4 transgenes were frozen in liquid nitrogen and homogenized in a solution containing 50 mM Tris–borate (pH 6.8), 2% SDS, 6% β-ME, 10% glycerol and 0.1% bromophenol blue. Homogenates were centrifuged at 17 800g at 4°C for 5 min, and extracts (100 μg of protein) were electrophoretically separated on SDS-15% polyacrylamide gels and transferred to polyvinylidene difluoride (PVDF) membranes (Bio-Rad) in a solution containing 25 mM Tris, 190 mM glycine and 20% methanol for 1 h at 25°C. Blotted membranes were blocked with Tris–buffered saline (TBS) solution (20 mM Tris–HCl, pH 7.4 and 150 mM NaCl) containing 0.05% Tween 20 and 5% skim milk for 1 h at 25°C and then incubated with an anti-HP6 polyclonal antibody at a 1 : 500 dilution, or the anti-FLAGM5 antibody (Sigma) at a 1 : 2000 dilution at 4°C for 16 h. After washing with TBS containing 0.05% Tween 20, the blots were incubated with horseradish peroxidase-labelled anti-mouse IgG and anti-rabbit IgG (GE healthcare) at a 1 : 20 000 dilution for 1 h at 25°C. Detection was performed with ECL Western blotting detection reagents (GE healthcare).
Scanning electron microscopy
Adult flies were anesthetized, mounted on stages and observed under a Hitachi S-3000 scanning electron microscope in the low vacuum mode.
Band mobility shift assays
Band mobility shift analysis was performed as reported previously (4), with minor modifications. Kc cell nuclear extracts were prepared as described elsewhere (4) and incubated in a reaction mixture containing 15 mM Hepes, pH 7.6, 60 mM KCl, 0.1 mM EDTA, 1 mM DTT, 12% glycerol, 0.05 mg/ml poly(dI-dC), 0.05 mg/ml Salmon sperm DNA (average size 0.2 kb) and double-stranded 32P-labelled synthetic oligonucleotides (10 000 cpm) for 15 min at 0°C. When necessary, unlabelled oligonucleotides were added as competitors at this step. DNA–protein complexes were electrophoretically resolved on 4% polyacrylamide gels in 50 mM Tris–borate, pH 8.3, 1 mM EDTA and 2.5% glycerol at 25°C. Gels were dried and autoradiographed.
Band mobility shift assays were also performed in the presence of anti-DREF monoclonal antibody 1, anti-DREF monoclonal antibody 4 (1) or anti-GST monoclonal antibody 1 as a control. Kc cell nuclear extracts were mixed with each antibody, incubated for 2 h on ice, added to mixtures containing 32P-labelled synthetic oligonucleotides (10 000 cpm) and 0.05 mg/ml poly(dI-dC), 0.05 mg/ml Salmon sperm DNA (average size 0.2 kb) and then incubated for 15 min at 0°C as described above.
Immunostaining of polytene chromosomes
Polytene chromosome spreads were prepared according to the protocol of Zink et al. from Canton S wild-type wandering third instar larvae (32) and stored in PBS-0.05% Tween 20-1% bovine serum albumin (BSA) at 4°C for 16 h before incubation with anti-DREF monoclonal antibody at a 1 : 1000 dilution at 4°C for 16 h. After extensive washing with PBS-0.05% Tween 20-1% BSA, samples were incubated at 25°C for 1 h with anti-mouse IgG conjugated with Alexa 594 (Invitrogen) at a 1 : 400 dilution. The chromosomes were then washed with PBS-0.05% Tween 20-1% BSA and mounted in Fluoroguard Antifade Reagent (Bio-Rad) for microscopic observation.
Immunostaining of testes
Preparation of testes from 1-day-old adult males for immunostaining was as described (33). After blocking with PBS containing 10% normal goat serum, the preparations were incubated with anti-DREF monoclonal antibody at a 1 : 1000 dilution or with an anti-HP6 polyclonal antibody at a 1 : 500 dilution at 4°C for 16 h. After extensive washing with PBS, samples were incubated at 25°C for 2 h with anti-rabbit IgG conjugated with Alexa 594 (Invitrogen) or anti-mouse IgG conjugated with Alexa 488 (Invitrogen) at a 1 : 400 dilution. The samples were mounted in Fluoroguard Antifade Reagent (Bio-Rad) for microscopic observation.
Chromatin immunoprecipitation
We performed chromatin immunoprecipitation using a Chip Assay kit as recommended by the manufacturer (Upstate). Approximately 1 × 107 S2 cells were fixed in 1% formaldehyde at 37°C for 10 min and then quenched in 125 mM glycine for 5 min at 25°C. Cells were washed twice in PBS containing protease inhibitors (1 mM PMSF, 1 μg/ml aprotinin and 1 μg/ml pepstatin A) and lysed in 2 ml of SDS lysis buffer. Lysates were sonicated to break DNA into fragments of less than 1 kb and centrifuged at 15 300g for 10 min at 4°C. The sonicated cell supernatants were diluted 10-fold in Chip Dilution Buffer and pre-cleared with 80 μl Salmon Sperm DNA/Protein A agarose-50% slurry for 30 min at 4°C. After brief centrifugation, each supernatant was incubated with 1 μg of the rabbit IgG or anti-DREF polyclonal antibody for 16 h at 4°C. Salmon Sperm DNA/Protein A agarose-50% slurry was added, followed by incubation for 1 h at 4°C. After washing, immunoprecipitated DNA was eluted with elution buffer containing 1% SDS and 0.1 M NaHCO3. Then the protein-DNA crosslinks were reversed by heating at 65°C for 4 h. After deproteinization with proteinase K, DNA was recovered by phenol–chloroform extraction and ethanol precipitation.
Immunoprecipitated DNA fragments were detected by quantitative real time PCR using SYBR Green I (Takara) and the Applied Biosystems 7500 Real Time PCR system (34). The ΔΔCT value for each sample was calculated by subtracting the CT value for the input sample from the CT value obtained for the immunoprecipitated samples. Fold differences relative to the controls using non-immune IgG were then calculated by raising 2 to the ΔΔCT power. The ΔΔCT was calculated by subtracting the ΔCT value for that for the sample immunoprecipitated with control IgG.
Quantitative RT-PCR
1 × 106 S2 cells were plated in 6-well dishes in 2 ml M3 medium containing 30 μg/well of DREF double stranded RNAs (DREFdsRNA) or LacZdsRNA for 1 h. After the incubation, 3 ml of 10% FBS-M3 medium was added to each well. At 5 days after the dsRNA treatment, total RNA was isolated from cells using TrizolReagent (Invitrogen) and 1 μg aliquots were reverse transcribed with oligo dT primer using a Takara high fidelity RNA PCR kit (Takara). Then, real time PCR was performed with a SYBR Green I kit (Takara) and the Applied Biosystems 7500 Real Time PCR system using one μl of reverse transcribed sample per reaction. Levels of mRNAs in the DREFdsRNA or LacZdsRNA treated cells and in no dsRNA treated cells were investigated by the CT comparative method (35). The β-tubulin gene was chosen as a negative control. Rp49 was used as an endogenous reference gene. Experiments were performed in triplicate for each of three RNA batches isolated separately.
Developmental RT-PCR
Total RNAs from Drosophila bodies at various developmental stages were purified with TRIZOL (Invitrogen). For RT-PCR, mRNAs were purified using an Oligotex-dT30 <Super> mRNA Purification kit (Takara Bio) and then were used for cDNA synthesis using an oligo d(T) primer and Bca PLUS RTase (Takara Bio) according to the manufacturer's instructions. HP6 and RP49 DNA were amplified by PCR using PyrobestTM DNA Polymerase (Takara Bio) with primer oligonucleotides CG15636P and CG15636antiP for HP6 and RP49P and RP49antiP for RP49. The PCR conditions included one cycle of 2 min at 94°C followed by 25 cycles of 94°C for 30 s, 52°C or 55°C for 30 s and 72°C for 1 min. All the PCR reactions were performed within the range of linear amplification and PCR products were separated on 2% agarose gels.
RESULTS
Genetic screening of modifiers of the DREF-induced rough eye phenotype and identification of an HP6 mutation as a dominant suppressor
As reported previously, we have established transgenic fly lines bearing GMR-GAL4 and DREF cDNAs under the control of a GAL4-binding sequence (UAS-DREF1-709) (19,28). Over-expression of DREF induced ectopic DNA synthesis and apoptosis, and inhibited the photoreceptor cell differentiation in eye imaginal discs and adult flies exhibited a severe rough eye phenotype (36). Since the eye phenotype does not impair viability or fertility (20), these flies serve as a genetic tool to screen for modifying mutations. Previous studies identified 5 and 17 deletion regions that modify the DREF-induced rough eyes phenotype in the X and the second chromosome, respectively (20). In order to identify genes in these genomic regions that are responsible for modification of the DREF-induced rough eye phenotype, various mutants mapped in and around the 22 genomic regions (5D1-2; 5E, 7D1; 7D5-6, 9B1-2; 10A1-2, 11A2; 11B9, 19A5; 19D3, 21A1; 21B7-8, 21B8-C1; 21C8-D1, 21D2-3; 21F2-22A1, 25D2-4; 26B2-5, 32F1-3; 33F1-2, 35D1; 35D4, 35D2; 35F1-2, 35D2-4; 35E2-6, 36A8-9; 36E1-2, 36E4-36F1; 38A6-7, 37B2-12; 38D2-5, 37C2-5; 38B2-C1, 37D1-2; 38C1-2, 41A, 48A-B, 55A-55F, 57B4; 58B) were collected and used to cross with transgenic flies expressing DREF (Table 1).
Table 1.
Summary of genes that genetically interact with the DREF gene
| Cytological location | gene | CG number | Allele(s) tested | Type of allele | Known function | Effect on rough eye phenotype |
|---|---|---|---|---|---|---|
| 5D1-5D2; 5E | Df(1)sqh | Deficiency | Suppression | |||
| 5E3-5E4 | Lag1 | CG3576 | Lag1G0365 | P-element insertion | Unknown | Suppression |
| 5E4 | Ubi-p5E | CG32744 | l(1)G0287G0287 | P-element insertion | Ubiquitin-dependent protein catabolic process | Suppression |
| 7D1; 7D5-7D6 | Df(1)C128 | Deficiency | Enhancement | |||
| 7B7 | Tom40 | CG12157 | Tom40G0216 | P-element insertion | Transmembrane transporter activity | Enhancement |
| 7C3 | l(1)G0155 | CG1515 | l(1)G0155G0155 | P-element insertion | Unknown | Enhancement |
| 7D3-7D5 | fs(1)h | CG2252 | fs(1)hG0093 | P-element insertion | Regulation of transcription | Enhancement |
| 7D5 | mys | CG1560 | mysKG02930 | P-element insertion | Calcium-dependent cell-cell adhesion | Enhancement |
| 7D5 | mys | CG1560 | mysG0281 | P-element insertion | Calcium-dependent cell-cell adhesion | Enhancement |
| 7E6-7E7, 7E7-7E9 | CG32711, Trf2 | CG32711, CG18009 | l(1)G0219G0219 | P-element insertion | Unknown, RNA polymerase II transcription factor activity | Enhancement |
| 7E6-7E7, 7E7-7E9 | CG32711, Trf2 | CG32711, CG18009 | l(1)G0228G0228 | P-element insertion | Unknown, RNA polymerase II transcription factor activity | Enhancement |
| 7E6-7E7, 7E7-7E9 | CG32711, Trf2 | CG32711, CG18009 | l(1)G0295G0295 | P-element insertion | Unknown, RNA polymerase II transcription factor activity | Enhancement |
| 7E6-7E7, 7E7-7E9 | CG32711, Trf2 | CG32711, CG18009 | l(1)G0332G0332 | P-element insertion | Unknown, RNA polymerase II transcription factor activity | Enhancement |
| 7E6-7E7, 7E7-7E9 | CG32711, Trf2 | CG32711, CG18009 | l(1)G0372G0372 | P-element insertion | Unknown, RNA polymerase II transcription factor activity | Enhancement |
| 7E6-7E7, 7E7-7E9 | CG32711, Trf2 | CG32711, CG18009 | l(1)G0425G0425 | P-element insertion | Unknown, RNA polymerase II transcription factor activity | Enhancement |
| 19A5; 19D3 | Df(1)16-2-19 | Deficiency | Suppression | |||
| 18D13-18E1 | dome | CG14226 | domeG0199b | Loss of function | JAK/STAT signaling pathway | Enhancement |
| 19C1 | CG9577 | CG9577 | CG9577KG09994 | P-element insertion | Unknown | Enhancement |
| 19C1 | sw | CG18000 | P{SUPor-P}KG05547 | P-element insertion | Microtubule motor activity | Suppression |
| 19C5-19C6 | l(1)G0004 | CG11738 | l(1)G0004G0004 | P-element insertion | Unknown | Suppression |
| 20B3 | l(1)G0196 | CG14616 | l(1)G0196G0196 | P-element insertion | Unknown | Enhancement |
| 21B8-C1; 21C8-21D1 | Df(2L)al | Deficiency | Suppression | |||
| 21C4-21C5 | ex | CG4114 | l(2)k06506k06506 | P-element insertion | Hippo signaling pathway | Suppression |
| 21C4-21C5 | ex | CG4114 | l(2)k07308k07308 | P-element insertion | Hippo signaling pathway | Suppression |
| 21D1 | cbt | CG4427 | l(2)k08915k08915 | P-element insertion | JNK signaling pathway | Enhancement |
| 21D2-21D3; 21F2-22A1 | Df(2L)S3 | Deficiency | Suppression | |||
| 21E2 | ds | CG17941 | l(2)0185501855 | P-element insertion | Calcium-dependent cell-cell adhesion | Enhancement |
| 21E4 | S | CG4385 | Sk09530 | P-element insertion | Effector of Egfr signalling | Enhancement |
| 21F1-2 | l(2)10685k05810 | P-element insertion | Suppression | |||
| 25D2-25D4; 26B2-26B5 | Df(2L)cl-h3 | Deficiency | Suppression | |||
| 26B2 | lid | CG9088 | lidk06801 | P-element insertion | Trithorax grop protein trimethyl H3K4 demethylase | Mild suppression |
| 26B2 | eIF-4a | CG9075 | eIF-4ak01501 | P-element insertion | Translation initiation factor activity | Suppression |
| 26B2 | eIF-4a | CG9075 | eIF-4a02439 | P-element insertion | Translation initiation factor activity | Strong suppression |
| 26D1-26D2 | l(2)k06107k06107 | P-element insertion | Enhancement | |||
| 32F1-32F3; 33F1-33F2 | Df(2L)Prl | Deficiency | Enhancement | |||
| 33A1-33A2 | crol | CG14938 | crolk05205 | P-element insertion | Transcription of a number of ecdysone-induced genes | Mild suppression |
| 33C4 | Rab6 | CG6601 | Rab6k13606 | P-element insertion | GTPase activity | Suppression |
| 33F3 | CG5776, Å@spict | CG5776, CG12292 | l(2)k05448k05448 | P-element insertion | Unknown, negative regulation of BMP signaling pathway | Suppression |
| 35D1; 35D4 | Df(2L)TW116(R)GW2 | Deficiency | Suppression | |||
| 35C5-35D1 | gft | CG11861 | gft06430 | Loss of function | Ubiquitin-protein ligase activity | Mild suppression |
| 35D2; 35F1-35F2 | Df(2L)TW116(R)GW13 | Deficiency | Suppression | |||
| 35D2-35D4; 35E2-35E6 | Df(2L)b83d29a | Deficiency | Enhancement | |||
| 35D2 | lace | CG4162 | lacek05305 | P-element insertion | Serine C-palmitoyltransferase activity | Mild suppression |
| 35E1-35E2 | P{lacW}J29 | P-element insertion | Suppression | |||
| 36A8-36A9; 36E1-36E2 | Df(2L)H20 | Deficiency | Enhancement | |||
| 36A11 | Cyt-c-d | CG13263 | Cyt-c-dbln1 | Loss of function | Cytochrome C proteins | Strong suppression |
| 36E4-36F1; 38A6-38A7 | Df(2L)TW50 | Deficiency | Enhancement | |||
| 36F4 | RpS26 | CG10305 | RpS2604553 | P-element insertion | Structural constituent of ribosome | Suppression |
| 37C7 | pigeon | CG10739 | pigeonP1 | hypomorph | unknown | Enhancement |
| 37B2-37B12; 38D2-38D5 | Df(2L)pr-A16 | Deficiency | Enhancement | |||
| 38C5 | CG16798 | CG16798 | l(2)k07219k07219 | P-element insertion | Unknown | Suppression |
| 55A-55F | Df(2R)PC4 | Deficiency | Suppression | |||
| 55B5-55B7 | stau | CG5753 | staury9 | Loss of function | RNA binding | Mild suppression |
| 55B7-55B8 | Hsf | CG5748 | Hsf03091 | Loss of function | RNA polymerase II transcription factor activity | Suppression |
| 55F3-55F4 | l(2)08717 | CG15095 | l(2)0871708717 | P-element insertion | Plasma membrane protein | Suppression |
| 57B4; 58B | Df(2R)Pu-D17 | Deficiency | Enhancement | |||
| 57B12 | CG9350 | CG9350 | l(2)0305003050 | P-element insertion | Unknown | Suppression |
| 57C3-57C4 | Xbp1 | G9415 | Xbp1k13803 | Loss of function | Regulation of transcription | Suppression |
| 57E6-57E8 | CG10496 | CG10496 | CG1049607128a | P-element insertion | Unknown | Suppression |
| 57E8-57E9 | MESK2 | CG15669 | MESK2k00119 | P-element insertion | Unknown | Suppression |
Bold characters indicate deficiency lines used in the previous study (20)
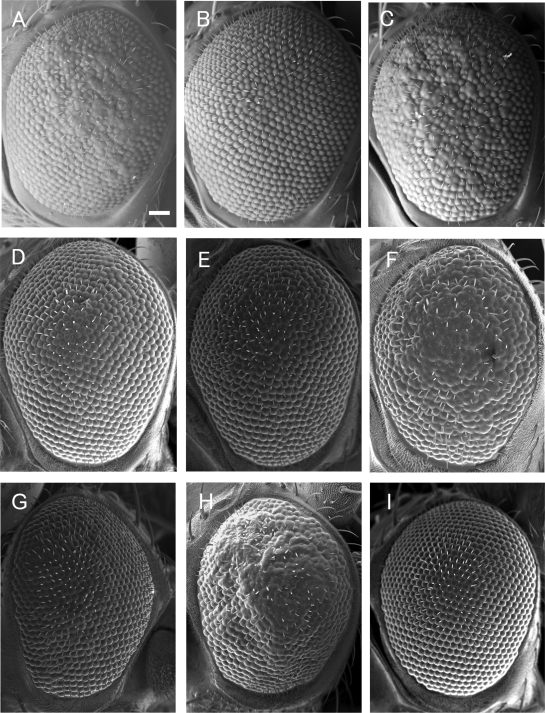
Out of 238 independent mutant lines examined, 27 lines suppressed, while 19 lines enhanced the rough eye phenotype when they were heterozygous for the mutations (Figure 1D to F, Table 1). Under the scanning electron microscope, eyes of these heterozygous mutant flies appeared normal (data not shown). The other mutant lines apparently exerted no detectable effects on the DREF-induced rough eye phenotype. Cytological locations of these negative lines are listed in Supplementary Table 1. Data base search revealed that 24 genes are responsible for the suppression and 12 genes for the enhancement. One of the strongest levels of suppression of the rough eye phenotype was observed with the P-element insertion line P{w + mGT = GT1}CG15636 (Figure 1B). The suppression could be reverted under dysgenic conditions (Figure 1C), suggesting the mutation induced by the P-element insertion to be truly responsible for the suppression. The Berkeley Drosophila genome project database (http://www.fruitfly.org/blast) revealed that the P-element is inserted 43 bp upstream of the termination codon of the HP6 (CG15636) gene (Figure 2) and Greil et al. (37) reported that the mutant is semi-lethal. In contrast coexpression of HP6 further enhanced the DREF-induced rough eye phenotype in compared with the control flies coexpressing LacZ (Figure 1G and H), despite that overexpression of HP6 alone in the eye imaginal disc exerted only a marginal effect on the adult eye morphology (Figure 1I).
Figure 1.
Scanning electron micrographs of adult eyes. (A) GMR-GAL4/+; UAS-DREF/+; +/+. (B) GMR-GAL4/+; UAS-DREF/P{w+mGT=GT1}CG15636; +/+. (C) GMR-GAL4/+; UAS-DREF/P{w+mGT=GT1}CG15636rev; +/+. (D) GMR-GAL4/+; UAS-DREF/rps26; +/+. (E) GMR-GAL4/+; UAS-DREF/pepck; +/+. (F) GMR-GAL4/+; UAS-DREF/star; +/+. (G) GMR-GAL4/+; UAS-DREF/+; UAS-nlslacZ/+. (H) GMR-GAL4/UAS-HP6; UAS-DREF/+; +/+. (I) GMR-GAL4/UAS-HP6; +/+; +/+. Bar indicates 50 μm.
Figure 2.
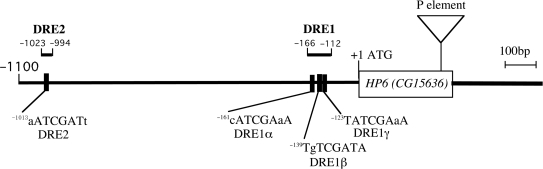
DRE and DRE-like sequences in the 5′-flanking regions of the HP6 gene. The translation initiation site is numbered as +1. DRE and DRE-like sequences are located at positions –1013 to –1006 (DRE 2), –161 to –154 (DRE 1α), –139 to –132 (DRE 1β) and –123 to –116 (DRE1γ). DRE1 comprises DRE 1α, DRE 1β and DRE 1γ. Nucleotides that do not match to DRE consensus sequences are shown in small letters. A P-element is inserted 43 bp upstream of the termination codon of the HP6 gene. The regions (DRE1 and DRE2) used as probes for band mobility shift assays are indicated.
We searched for DRE like sequences in the 5′-flanking region of the HP6 gene from the NCBI database, and found two sequences that match seven out of the eight nucleotides of DRE and two additional sequences that match six out of the eight nucleotides within the 1.4 kb upstream region (Figure 2). We named these sites as DRE1α (–161 to –154), DRE1β (–139 to –132), DRE1γ (–123 to –116) and DRE2 (–1013 to –1006) with respect to the translation initiation codon (Figure 2). It is reported that stimulatory effects of DRE can be observed at positions within at least 2.5 kb from the transcription initiation site (4) and sequences matching six out of eight nucleotides of DRE have promoter activity (11,38). Therefore, all of these DRE-like sequences of the HP6 gene likely play roles in regulation of the HP6 gene promoter activity.
DREF binds to the chromosomal region containing the HP6 gene
To examine whether DREF locates to the chromosomal region containing the HP6 gene, we carried out immunostaining of salivary gland polytene chromosomes in third instar larvae with anti-DREF monoclonal antibodies. DREF signals are detected in a number of discrete regions throughout the polytene chromosomes (14). Careful inspection allowed the mapping of signals for DREF at the HP6 gene locus, 25A1, on the 2L chromosome (Supplementary Figure 1). The Berkeley Drosophila genome project database revealed that only two genes (HP6 and dumpy) are located in this 25A1 locus. Since HP6 is located in the intron of dumpy (dp), P-element insertion in line P{w + mGT = GT1}CG15636 may affect not only HP6 but also dp gene expression. We therefore crossed DREF-overexpressing flies with two independent X ray-induced homozygous lethal dp mutant strains, dp°vlR/SM5 and dpD/SM1. However no effect on the DREF-induced rough eye phenotype by dp mutation was observed (Supplementary Figure 2). Furthermore there is no DRE like sequence within the 2 kb 5′-flanking region of the dp gene. It is therefore very likely that DREF binds to DRE-like sequences in the 5′-flanking region of the HP6 gene in the salivary glands.
DREF binding activity in vitro
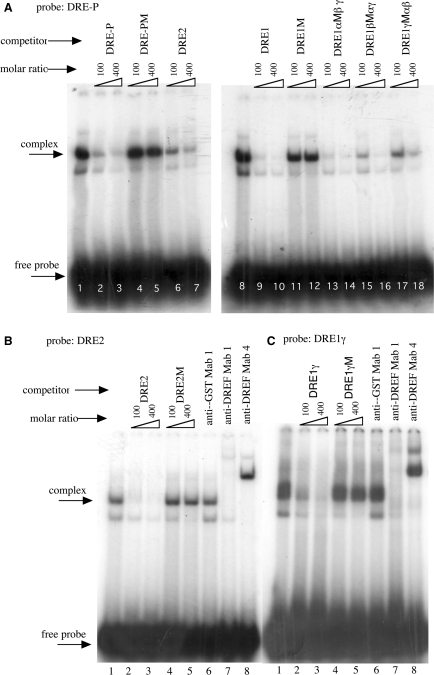
To examine this directly, oligonucleotide DRE1 containing the region from DRE1α (–161 to –154) to DRE1γ (−123 to −116), oligonucleotide DRE1γ containing the DRE1γ (−123 to −116) region and oligonucleotide DRE2 containing the DRE2 (−1013 to −1006) region (Figure 2) were chemically synthesized and used for band mobility shift assays. As previously noted (4), specific DNA protein complexes could be detected with Kc cell nuclear extracts and the oligonucleotide DRE-P carrying the DRE sequence in the PCNA gene (Figure 3A). The shifted bands were effectively diminished by adding unlabelled oligonucleotides DRE1 and DRE2. Although DRE1 carrying mutations in either DRE1α or DRE1β also effectively competed against DRE-P, oligonucleotide DRE1 carrying mutations in DRE1γ less effectively competed. These results suggest that DREF has affinity for the region containing DRE1γ and DRE2.
Figure 3.
Complex formation between DRE in the PCNA gene promoter and Kc cell nuclear extracts. 32P-labelled double stranded oligonucleotides DRE-P (A), DRE2 (B) and DRE1γ (C) were incubated with Kc cell nuclear extracts in the presence of the indicated competitor oligonucleotides or anti-DREF monoclonal antibodies. The amounts of competitors were 100- or 400-fold molar ratios. Anti-GST Mab1, anti-GST monoclonal antibody 1; anti-DREFMab1, anti-DREF monoclonal antibody 1; anti-DREFMab4, anti-DREF monoclonal antibody 4; DRE-P, oligonucleotide containing the DRE sequence of the Drosophila PCNA gene; DRE-PM, DRE-P having a mutation in the DRE sequence; DRE2, oligonucleotide containing the DRE2 sequence of the HP6 gene; DRE2M, DRE2 having mutations in the DRE-like sequence; DRE1, oligonucleotide containing the DRE1 sequence of the HP6 gene; DRE1M, DRE1 having mutations in the DRE1αβγ sequences; DRE1αM, DRE1 having mutations in the DRE1α sequence; DRE1βM, DRE1 having mutations in DRE1βsequence; DRE1γM, DRE1 having mutations in the DRE1γ sequence.
When the oligonucleotides DRE2 or DRE1γ were mixed with Kc cell nuclear extracts, specific DNA-protein complexes were detected [Figures 3B (lane 1) and 3C (lane 1)], which were diminished by addition of an excess amount of unlabelled DRE2 and DRE1γ oligonucleotides as competitors [Figures 3B (lanes 2 and 3) and 3C (lanes 2 and 3)] but not of oligonucleotides carrying mutations in the DRE-like sequences [Figures 3B (lanes 4 and 5) and 3C (lanes 4 and 5)]. Furthermore, the specific DNA-protein complexes were either diminished or super shifted by adding anti-DREF monoclonal antibodies, but not by adding the control anti-GST monoclonal antibody [Figures 3B (lanes 7 and 8) and 3C (lanes 7 and 8)]. These results indicate that DREF can bind to DRE1γ and DRE2 sequences in the HP6 gene promoter in vitro.
DREF binds to the DRE2- and DRE1-containing genomic region in vivo
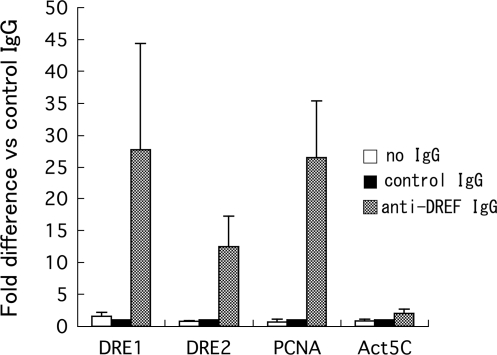
To further examine DREF-binding to the DRE1- and DRE2-containing region of the HP6 gene, primers to amplify the region from –66 to −167 and –856 to −1060 (Figure 2) were chemically synthesized and used for chromatin immunoprecipitation assays with anti-DREF polyclonal antibodies. It is well established that the Drosophila PCNA gene is regulated by the DREF pathway (1,11,12). Amplification of the PCNA gene promoter region containing the DRE in immunoprecipitates with the anti-DREF polyclonal antibody was 27-fold higher than with control rabbit IgG (Figure 4). In contrast, no amplification of the Actin 5C gene region was observed (Figure 4). Amplification of the HP6 gene promoter region containing the DRE1 in the immunoprecipitates with anti-DREF polyclonal antibody was 28-fold and that containing DRE2 was 13-fold (Figure 4). These results indicate that DREF binds to the genomic region containing DRE1 and DRE2 of the HP6 gene in S2 cells.
Figure 4.
Binding of DREF to DRE-containing genomic regions of the HP6 gene. Cross-linked chromatin of S2 cells was immunoprecipitated with anti-DREF IgG, control rabbit IgG or no IgG. The genomic regions containing DRE1 of the HP6 gene, DRE2 of the HP6 gene, DRE of the PCNA gene and Act5C gene were amplified by real time PCR and compared with the amplification products from the immunoprecipitates with the control IgG.
Effects of knockdown of the DREF gene on HP6 gene expression in cultured cells
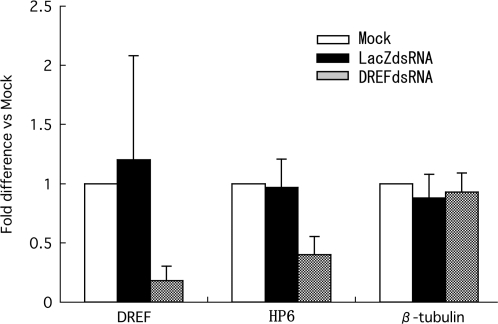
Endogenous HP6 gene expression in RNAi-mediated DREF knockdown cells was examined to further demonstrate that HP6 is a DREF target gene. Total RNAs from double-stranded RNA (dsRNA)-treated S2 cells were isolated and quantitative RT-PCR was carried out (Figure 5). The DREF mRNA level was reduced by 82% in DREFdsRNA-treated cells, but not changed with LacZdsRNA-treatment. Under these conditions, the level of endogenous HP6 mRNA was decreased to 39%, while LacZdsRNA treatment exerted no effect (Figure 5). Expression of the β-tubulin gene employed as a negative control was not affected by DREFdsRNA treatment. These results indicate that DREF is required for HP6 gene expression.
Figure 5.
Effects of dsRNA treatment on mRNA levels of HP6 in S2 cells. cDNAs were prepared from total RNA isolated from dsRNA treated S2 cells and levels of DREF, HP6 and β-tubulin mRNAs were measured by quantitative RT-PCR. Fold differences against the amplification with no treatment (Mock) are shown with standard deviations from three independent dsRNA treatments.
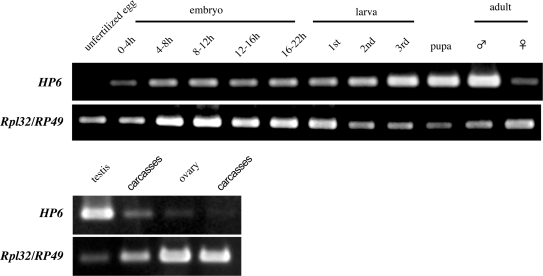
Levels of HP6 mRNA are highest in Drosophila adult testes
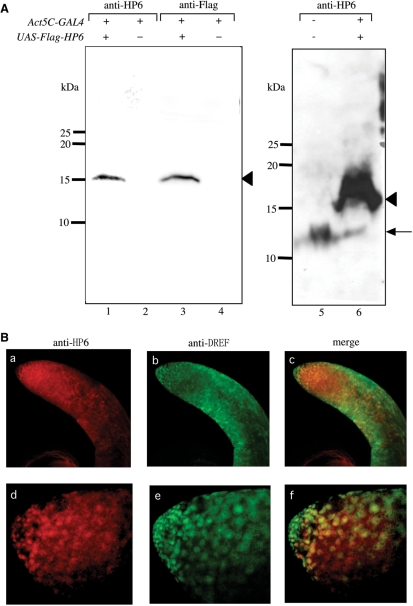
We carried out RT-PCR to determine the HP6 expression pattern during Drosophila development (Figure 6). HP6 mRNA could be detected throughout all developmental stages but with the highest expression in adult males. Furthermore, the HP6 mRNA was expressed at least 6.3-fold higher in testes than in other parts of the body (Figure 6B). The observed HP6 expression pattern is consistent with the results reported by Greil et al. (37) and FlyAtlas (http://flyatlas.org/atlas.cgi?name=FBgn0031613). Relatively high expression of both HP6 and DREF proteins in nuclei at the apical tips of testes was observed with immunostaining using anti-HP6 and anti-DREF antibodies (Figure 7B) and the specificity of anti-HP6 antibody binding was confirmed by western blot analysis with extracts from adult male flies expressing Flag-HP6 fusion protein (Figure 7A). The results suggest some specific role of HP6 during spermatogenesis.
Figure 6.
Developmental RT-PCR. Total RNA was extracted from Drosophila bodies or the indicated tissues at various developmental stages and RT-PCR was carried out. The upper panels represent the HP6 mRNA levels and lower panels the Rpl32/RP49 mRNA levels as a control.
Figure 7.
Specificity of anti-HP6 rabbit polyclonal antibody examined by western blot analysis and immunostaining of testes. (A) Extracts were: from w; +; Act5C-GAL4/+ adult male flies for immunoblotting with anti-HP6 antibody (lane 2) or anti-FlagM5 antibody (lane 4); from w; +; Act5C-GAL4/UAS-HP6 adult male flies for immunoblotting with anti-HP6 antibody (lanes 1 and 6), or anti-FlagM5 antibody (lane 3); from wild type adult male flies for immunoblotting with anti-HP6 antibody (lane 5). The arrowheads correspond to the Flag-HP6 protein and the arrow corresponds to endogenous HP6 protein. The 100 μg aliquots of protein were used for lanes 1–4, 500 μg for lane 5 and 300 μg for lane 6. (B) Immunostaining of testis with anti-HP6 antibody (a and d) or anti-DREF antibody (b and e). Merged images of HP6 and DREF signals (c and f). (d to f) Higher magnification images of a to c.
DISCUSSION
The present genetic screening of modifiers of the DREF-induced rough eye phenotype and identified 24 suppressors and 12 enhancers (Table 1 and Figure 1). Although these modifier genes are not necessarily transcriptional targets of DREF as reported previously (14), they could be critical genes in positive or negative regulation of the DREF pathway. By data base search, five genes, HP6, pigeon, lace, X box binding protein 1 (Xbp-1) and guftagu were found to carry DRE sequences in their 5′-flanking regions. These genes are therefore candidate DREF target genes. Nucleotide positions of DRE and DRE like sequences in the 5′-flanking regions of these genes are listed in the Supplementary Table 2.
The fat gene, one of the suppressors of the rough eye, encodes nonclassical cadherin (39,40) and genetically interacts with armadillo (41), a Drosophila homologue of mammalian β-catenin and downstream effecter of the Wnt signal transduction pathway (42). Interaction with fat in the eye confirms the ability of this gene to modify cytoplasmic Armadillo level (41). When sufficient Armadillo protein accumulates in the cell, it forms a complex with Pangolin, a Drosophila homologue of mammalian T-cell factor (43). Previously we demonstrated that the Armadillo/Pangolin complex activates transcription of the DREF gene (44). We therefore suggest that suppression of the DREF-induced rough eye phenotype is caused by decrease of the Armadillo protein accumulation by half reduction of the fat gene dosage. The present screen also identified the lace gene as another suppressor. The lace gene encodes a membrane protein similar to the yeast protein LCB2, a subunit of serine palmitoyltransferase (SPT), which catalyses the first step of sphingolipid biosynthesis (45). It is now well known that sphingolipids trigger elevated levels of apoptosis via the modulation of known signaling pathways (46). Previously we reported that DREF is involved in regulation of vein formation through the activation of raf, downstream of Egfr signaling in the Drosophila wing imaginal discs (21). In accordance with this, the present genetic screen identified the star gene as one enhancer of the DREF-induced rough eye (Table 1). It encodes an integral membrane protein that is expressed in cells secreting Spitz and is localized in the early endoplasmic reticulum and nuclear envelope (47). Star interacts directly with Spitz, an activating ligand for Egfr (48), and regulates its protein expression (49).
The Xbp-1 gene is also a suppressor of the DREF-induced rough eye phenotype. The Xbp-1 gene encodes a ‘bZIP’-containing transcription factor and plays a key role in the unfolded protein response, an evolutionarily conserved signalling pathway activated by an overload of misfolded proteins in the endoplasmic reticulum ER (50). The guftagu gene is an other suppressor that encodes the Drosophila Cullin-3 homologue (d-Cul3) (51) whose function impinges on the activity of many different signalling pathways and developmental events via targeted destruction or modification of specific proteins (51). Recently, we have reported that the Drosophila skpA gene is a target of DREF (52). The skpA gene encodes a component of the SCF complex that functions in combination with the ubiquitin conjugating enzyme UbcD1 and is involved in cell cycle regulation. Moreover regulation of the gene encoding the proteasome regulator REGγ by the DRE/DREF system has also been reported by others (53). The ubiquitin-proteasome pathway plays key roles in many basic cellular processes, including immune responses, development and programmed cell death (45,46). In addition to degradation of defective or misfolded proteins, a critical regulatory role has been defined in studies of the cell cycle (54–56). Some major signal transduction pathways that are of great importance during development are known to be controlled in a coordinated way, in which the DRE/DREF pathway may be intimately involved (57).
The eukaryotic initiation factor 3p40 (eIF3p40), the dribble and the ribosomal protein S26 genes were included in the other suppressors identified in the screening and they are all associated with protein synthesis (Table 1). The dribble protein encodes a novel KRR1p-like KH domain protein (58) and krr1 mutations affect biogenesis of 18S rRNA and its precursors and 40S ribosomal subunits(59). The eIF3p40 protein encodes the p40 subunit of the eIF3 complex which facilitates charging of the 40S ribosomal subunit with the ternary complex (eIF2, Met-tRNAMet, GTP) and bridging with the eIF4G subunit of the cap-binding complex, eIF4F and inhibiting the association of 40S and 60S ribosomal subunits (60,61). The ribosomal protein S26 gene encodes a Drosophila ribosomal protein (RP) with homology to rat RP S26 (62). A slow growth rate and an altered adult size are thought to be the result of a reduced capacity for protein synthesis and this phenotype has been demonstrated to disrupt genes that encode RPs (63). Recently we identified the eIF4A gene, encoding a member of the DEAD box family of ATP-dependent RNA helicases (64), as another target of DREF. eIF4A is proposed to function in cap(m7GpppN)-dependent initiation of protein synthesis by unwinding the secondary structure of 5′-untranslated regions of mRNA (65,66). Since genes responsible for degradation of defective or misfolded proteins are targets of DREF as described above, DREF apparently promotes both protein synthesis and degradation by directly or indirectly activating genes involved in these processes. This is presumably associated with the active protein metabolism typical of proliferating cells.
A number of other genes are of obvious interest given their physiological significance. Among the strongest suppressors of the DREF-induced rough eye phenotype was the mutated HP6 gene. The present studies clearly demonstrate that HP6 gene is one of the targets of DREF. Although HP6 is not a modifier of position effect variegation as are several of other Drosophila HPs, it carries chromo shadow domain (37). It has been shown that chromo shadow domain in HP1 is highly conserved across species and crucial for interaction with many proteins such as the SUV39H1 (67), SP100 (68,69), TIF1-β(KAP-1) (70), Ku70 (71), lamin B receptor, HP1 itself (72), Ki-67 (73) and HP1/origin recognition complex-associated protein (HOAP) (74). It has further been reported that HP6 directly interacts with the Caravaggio protein in a two-hybrid assay (75). The caravaggio gene is otherwise known as Drosophila HOAP. We here found the expression level of HP6 mRNA to the highest in adult males and it much higher in testes than other sites. DREF is also expressed in the testis (1). The present study revealed that both proteins at least partially co-localize in nuclei at the apical tips of testes where cell proliferation actively occurs, suggesting some roles of HP6 in regulation of cell proliferation or transcription of the meiosis-related genes in testis.
We have searched for DRE sequences in the 5′-flanking regions of other five HP family genes in Drosophila on the genome database and found that examples in promoters in all cases (Table 2). In this context it should be noted that DREF is also involved in transcriptional regulation of genes coding for the chromatin remodeling BRM complex (24). Moreover, the present genetic screen identified the little imaginal discs (lid) gene as a suppressor of the DREF-induced rough eye phenotype. The lid encodes a histone H3 trimethyl-Lys4 demethylase, a regulator of the chromatin structure (76–78). Therefore DREF may influence expression of many genes through regulation of genes involved in alteration of chromatin structures.
Table 2.
DRE or DRE-like sequences in 5′-flanking region of the Drosophila HPfamily genes
| Gene | DRE or DRE-like | Position |
|---|---|---|
| HP1 | 5′-cATCGATt | −462 to −469 |
| 5′-aATCGATt | −470 to −477 | |
| 5′-taTCGATA | −503 to −510 | |
| 5′-TcTCGATc | −979 to −986 | |
| HP2 | 5′-aATCGATt | −489 to −495 |
| HP3 | 5′-TATCGATt | −134 to −141 |
| 5′-TATCGATt | −186 to −194 | |
| 5′-gATCGAgA | −475 to −482 | |
| 5′-TATCGAcA | −920 to −927 | |
| HP4 | 5′-TATCGATA | −366 to −373 |
| 5′-atTCGATA | −536 to −543 | |
| HP5 | 5′-TATCGATt | −670 to −677 |
| HP6 | 5′-TATCGAaA | −116 to −123 |
| 5′-TgTCGATA | −132 to −139 | |
| 5′-cATCGAaA | −154 to −161 | |
| 5′-aATCGATt | −1006 to −1013 |
Five suppressor genes for the DREF-induced rough eye phenotype; HP6, pigeon, lace, Xbp-1 and guftagu are candidate DREF target genes, since they carry DRE sequences in their 5′-flanking regions. These five genes have distinct functions as described above. Overexpression of DREF in eye imaginal discs induced multiple effects such as induction of DNA synthesis and apoptosis, inhibition of photoreceptor cell differentiation and loss of pigment cells (20). Although suppression of the rough eye phenotype by mutation of each suppressor genes appeared to be strong by examination with a scanning electron microscopy, inspection of horizontal sections of adult fly eyes showed that the suppression is still partial in most cases (20). Therefore suppression of the DREF-induced rough eye phenotype could be resulted from disturbance of multiple pathways in which many suppressor genes might be involved.
SUPPLEMENTARY DATA
Supplementary Data is available at NAR Online.
FUNDING
Funding for open access charge: KIT.
Conflict of interest statement. None declared.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Dr M. Moore for comments on the English language in the manuscript. This study was partially supported by grants from the KIT.
REFERENCES
- 1.Hirose F, Yamaguchi M, Kuroda K, Omori A, Hachiya T, Ikeda M, Nishimoto Y, Matsukage A. Isolation and characterization of cDNA for DREF, a promoter-activating factor for Drosophila DNA replication-related genes. J. Biol. Chem. 1996;271:3930–3937. doi: 10.1074/jbc.271.7.3930. [DOI] [PubMed] [Google Scholar]
- 2.Ohno K, Hirose F, Sakaguchi K, Nishida Y, Matsukage A. Transcriptional regulation of the Drosophila CycA gene by the DNA replication-related element (DRE) and DRE binding factor (DREF) Nucleic Acids Res. 1996;24:3942–3946. doi: 10.1093/nar/24.20.3942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Takahashi Y, Yamaguchi M, Hirose F, Cotterill S, Kobayashi J, Miyajima S, Matsukage A. DNA replication-related elements cooperate to enhance promoter activity of the drosophila DNA polymerase α 73-kDa subunit gene. J. Biol. Chem. 1996;271:14541–14547. doi: 10.1074/jbc.271.24.14541. [DOI] [PubMed] [Google Scholar]
- 4.Hirose F, Yamaguchi M, Handa H, Inomata Y, Matsukage A. Novel 8-base pair sequence (Drosophila DNA replication-related element) and specific binding factor involved in the expression of Drosophila genes for DNA polymerase α and proliferating cell nuclear antigen. J. Biol. Chem. 1993;268:2092–2099. [PubMed] [Google Scholar]
- 5.Lightfoot K, Maltby L, Duarte R, Veale R, Segev O. Conserved cis-elements bind a protein complex that regulates Drosophila ras2/rop bidirectional expression. Br. J. Cancer. 1994;69:264–273. doi: 10.1038/bjc.1994.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ryu JR, Choi TY, Kwon EJ, Lee WH, Nishida Y, Hayashi Y, Matsukage A, Yamaguchi M, Yoo MA. Transcriptional regulation of the Drosophila-raf proto-oncogene by the DNA replication-related element (DRE)/DRE-binding factor (DREF) system. Nucleic Acids Res. 1997;25:794–799. doi: 10.1093/nar/25.4.794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sawado T, Hirose F, Takahashi Y, Sasaki T, Shinomiya T, Sakaguchi K, Matsukage A, Yamaguchi M. The DNA replication-related element (DRE)/DRE-binding factor system is a transcriptional regulator of the Drosophila E2F gene. J. Biol. Chem. 1998;273:26042–26051. doi: 10.1074/jbc.273.40.26042. [DOI] [PubMed] [Google Scholar]
- 8.Huikeshoven H, Cotterill S. Cloning and characterisation of the gene for the large subunit of the DNA primase from Drosophila melanogaster. Biochim. Biophys. Acta. 1999;1445:359–362. doi: 10.1016/s0167-4781(99)00063-9. [DOI] [PubMed] [Google Scholar]
- 9.Okudaira K, Ohno K, Yoshida H, Asano M, Hirose F, Yamaguchi M. Transcriptional regulation of the Drosophila orc2 gene by the DREF pathway. Biochim. Biophys. Acta. 2005;1732:23–30. doi: 10.1016/j.bbaexp.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 10.Sharkov NV, Ramsay G, Katzen AL. The DNA replication-related element-binding factor (DREF) is a transcriptional regulator of the Drosophila myb gene. Gene. 2002;297:209–219. doi: 10.1016/s0378-1119(02)00890-9. [DOI] [PubMed] [Google Scholar]
- 11.Yamaguchi M, Hayashi Y, Nishimoto Y, Hirose F, Matsukage A. A nucleotide sequence essential for the function of DRE, a common promoter element for Drosophila DNA replication-related genes. J. Biol. Chem. 1995;270:15808–15814. doi: 10.1074/jbc.270.26.15808. [DOI] [PubMed] [Google Scholar]
- 12.Yamaguchi M, Hirose F, Matsukage A. Roles of multiple promoter elements of the proliferating cell nuclear antigen gene during Drosophila development. Genes Cells. 1996;1:47–58. doi: 10.1046/j.1365-2443.1996.03003.x. [DOI] [PubMed] [Google Scholar]
- 13.Hochheimer A, Zhou S, Zheng S, Holmes MC, Tjian R. TRF2 associates with DREF and directs promoter-selective gene expression in Drosophila. Nature. 2002;420:439–445. doi: 10.1038/nature01167. [DOI] [PubMed] [Google Scholar]
- 14.Hirose F, Ohshima N, Kwon EJ, Yoshida H, Yamaguchi M. Drosophila Mi-2 negatively regulates dDREF by inhibiting its DNA-binding activity. Mol. Cell Biol. 2002;22:5182–5193. doi: 10.1128/MCB.22.14.5182-5193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hayashi Y, Kato M, Seto H, Yamaguchi M. Drosophila distal-less negatively regulates dDREF by inhibiting its DNA binding activity. Biochim. Biophys. Acta. 2006;1759:359–366. doi: 10.1016/j.bbaexp.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 16.Ohler U, Liao GC, Niemann H, Rubin GM. Computational analysis of core promoters in the Drosophila genome. Genome Biol. 2002;3:0087. doi: 10.1186/gb-2002-3-12-research0087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matsukage A, Hirose F, Hayashi Y, Hamada K, Yamaguchi M. The DRE sequence TATCGATA, a putative promoter-activating element for Drosophila melanogaster cell-proliferation-related genes. Gene. 1995;166:233–236. doi: 10.1016/0378-1119(95)00586-2. [DOI] [PubMed] [Google Scholar]
- 18.Jasper H, Benes V, Atzberger A, Sauer S, Ansorge W, Bohmann D. A genomic switch at the transition from cell proliferation to terminal differentiation in the Drosophila eye. Dev. Cell. 2002;3:511–521. doi: 10.1016/s1534-5807(02)00297-6. [DOI] [PubMed] [Google Scholar]
- 19.Hirose F, Yamaguchi M, Matsukage A. Targeted expression of the DNA binding domain of DRE-binding factor, a Drosophila transcription factor, attenuates DNA replication of the salivary gland and eye imaginal disc. Mol. Cell Biol. 1999;19:6020–6028. doi: 10.1128/mcb.19.9.6020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hirose F, Ohshima N, Shiraki M, Inoue YH, Taguchi O, Nishi Y, Matsukage A, Yamaguchi M. Ectopic expression of DREF induces DNA synthesis, apoptosis, and unusual morphogenesis in the Drosophila eye imaginal disc: possible interaction with Polycomb and trithorax group proteins. Mol. Cell Biol. 2001;21:7231–7242. doi: 10.1128/MCB.21.21.7231-7242.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yoshida H, Kwon E, Hirose F, Otsuki K, Yamada M, Yamaguchi M. DREF is required for EGFR signalling during Drosophila wing vein development. Genes Cells. 2004;9:935–944. doi: 10.1111/j.1365-2443.2004.00775.x. [DOI] [PubMed] [Google Scholar]
- 22.Hyun J, Jasper H, Bohmann D. DREF is required for efficient growth and cell cycle progression in Drosophila imaginal discs. Mol. Cell Biol. 2005;25:5590–5598. doi: 10.1128/MCB.25.13.5590-5598.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kal AJ, Mahmoudi T, Zak NB, Verrijzer CP. The Drosophila brahma complex is an essential coactivator for the trithorax group protein zeste. Genes Dev. 2000;14:1058–1071. [PMC free article] [PubMed] [Google Scholar]
- 24.Nakamura K, Ida H, Yamaguchi M. Transcriptional regulation of the Drosophila moira and osa genes by the DREF pathway. Nucleic Acids Res. 2008;36:3905–3915. doi: 10.1093/nar/gkn291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takahashi Y, Hirose F, Matsukage A, Yamaguchi M. Identification of three conserved regions in the DREF transcription factors from Drosophila melanogaster and Drosophila virilis. Nucleic Acids Res. 1999;27:510–516. doi: 10.1093/nar/27.2.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spradling AC. Drosophila: A Practical Approach. In: Roberts DB, editor. Oxford: IRL Press; 1986. pp. 175–197. [Google Scholar]
- 27.Robertson HM, Preston CR, Philips RW, Johnson-Schlitz DM, Benz WK, Engels WR. A stable genomic source of P-element transposase in Drosophila melanogaster. Genetics. 1988;118:461–470. doi: 10.1093/genetics/118.3.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- 29.Date T, Yamaguchi M, Hirose F, Nishimoto Y, Tanihara K, Matsukage A. Expression of active rat DNA polymerase β in Escherichia coli. Biochemistry. 1988;27:2983–2990. doi: 10.1021/bi00408a048. [DOI] [PubMed] [Google Scholar]
- 30.Cordingley MG, Callahan PL, Sardana VV, Garsky VM, Colonno RJ. Substrate requirements of human rhinovirus 3C protease for peptide cleavage in vitro. J. Biol. Chem. 1990;265:9062–9065. [PubMed] [Google Scholar]
- 31.Hirose F, Yamaguchi M, Kuroda K, Omori A, Hachiya T, Ikeda M, Nishimoto Y, Matsukage A. Isolation and characterization of cDNA for DREF, a promoter-activating factor for Drosophila DNA replication-related genes. J. Biol. Chem. 1996;271:3930–3937. doi: 10.1074/jbc.271.7.3930. [DOI] [PubMed] [Google Scholar]
- 32.Zink B, Engstrom Y, Gehring WJ, Paro R. Direct interaction of the Polycomb protein with Antennapedia regulatory sequences in polytene chromosomes of Drosophila melanogaster. EMBO J. 1991;10:153–162. doi: 10.1002/j.1460-2075.1991.tb07931.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pisano C, Bonaccorsi S, Gatti M. The kl-3 loop of the Y chromosome of Drosophila melanogaster binds a tektin-like protein. Genetics. 1993;133:569–579. doi: 10.1093/genetics/133.3.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ida H, Yoshida H, Nakamura K, Yamaguchi M. Identification of the Drosophila eIF4A gene as a target of the DREF transcription factor. Exp. Cell Res. 2007;313:4208–4220. doi: 10.1016/j.yexcr.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 35.Morrison TB, Weiss JJ, Wittwer CT. Quantification of low-copy transcripts by continuous SYBR Green I monitoring during amplification. Biotechniques. 1998;24:954–958. 960, 962. [PubMed] [Google Scholar]
- 36.Yamaguchi M, Hirose F, Inoue YH, Shiraki M, Hayashi Y, Nishi Y, Matsukage A. Ectopic expression of human p53 inhibits entry into S phase and induces apoptosis in the Drosophila eye imaginal disc. Oncogene. 1999;18:6767–6775. doi: 10.1038/sj.onc.1203113. [DOI] [PubMed] [Google Scholar]
- 37.Greil F, de Wit E, Bussemaker HJ, van Steensel B. HP1 controls genomic targeting of four novel heterochromatin proteins in Drosophila. EMBO J. 2007;26:741–751. doi: 10.1038/sj.emboj.7601527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Choi YJ, Choi TY, Yamaguchi M, Matsukage A, Kim YS, Yoo MA. Transcriptional regulation of the Drosophila caudal homeobox gene by DRE/DREF. Nucleic Acids Res. 2004;32:3734–3742. doi: 10.1093/nar/gkh688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mahoney PA, Weber U, Onofrechuk P, Biessmann H, Bryant PJ, Goodman CS. The fat tumor suppressor gene in Drosophila encodes a novel member of the cadherin gene superfamily. Cell. 1991;67:853–868. doi: 10.1016/0092-8674(91)90359-7. [DOI] [PubMed] [Google Scholar]
- 40.Clark HF, Brentrup D, Schneitz K, Bieber A, Goodman C, Noll M. Dachsous encodes a member of the cadherin superfamily that controls imaginal disc morphogenesis in Drosophila. Genes Dev. 1995;9:1530–1542. doi: 10.1101/gad.9.12.1530. [DOI] [PubMed] [Google Scholar]
- 41.Greaves S, Sanson B, White P, Vincent JP. A screen for identifying genes interacting with armadillo, the Drosophila homolog of β-catenin. Genetics. 1999;153:1753–1766. doi: 10.1093/genetics/153.4.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roose J, Clevers H. TCF transcription factors: molecular switches in carcinogenesis. Biochim. Biophys. Acta. 1999;1424:M23–37. doi: 10.1016/s0304-419x(99)00026-8. [DOI] [PubMed] [Google Scholar]
- 43.van de Wetering M, Cavallo R, Dooijes D, van Beest M, van Es J, Loureiro J, Ypma A, Hursh D, Jones T, Bejsovec A, et al. Armadillo coactivates transcription driven by the product of the Drosophila segment polarity gene dTCF. Cell. 1997;88:789–799. doi: 10.1016/s0092-8674(00)81925-x. [DOI] [PubMed] [Google Scholar]
- 44.Kwon E, Hayashi Y, Otsuki K, Hirose F, Nishida Y, Yoo MA, Yamaguchi M. Armadillo/Pangolin regulates PCNA and DREF promoter activities. Biochim. Biophys. Acta. 2004;1679:256–262. doi: 10.1016/j.bbaexp.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 45.Adachi-Yamada T, Gotoh T, Sugimura I, Tateno M, Nishida Y, Onuki T, Date H. De novo synthesis of sphingolipids is required for cell survival by down-regulating c-Jun N-terminal kinase in Drosophila imaginal discs. Mol. Cell Biol. 1999;19:7276–7286. doi: 10.1128/mcb.19.10.7276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Phan VH, Herr DR, Panton D, Fyrst H, Saba JD, Harris GL. Disruption of sphingolipid metabolism elicits apoptosis-associated reproductive defects in Drosophila. Dev. Biol. 2007;309:329–341. doi: 10.1016/j.ydbio.2007.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kolodkin AL, Pickup AT, Lin DM, Goodman CS, Banerjee U. Characterization of Star and its interactions with sevenless and EGF receptor during photoreceptor cell development in Drosophila. Development. 1994;120:1731–1745. doi: 10.1242/dev.120.7.1731. [DOI] [PubMed] [Google Scholar]
- 48.Perrimon N, Perkins LA. There must be 50 ways to rule the signal: the case of the Drosophila EGF receptor. Cell. 1997;89:13–16. doi: 10.1016/s0092-8674(00)80177-4. [DOI] [PubMed] [Google Scholar]
- 49.Hsiung F, Griffis ER, Pickup A, Powers MA, Moses K. Function of the Drosophila TGF-α homolog Spitz is controlled by Star and interacts directly with Star. Mech. Dev. 2001;107:13–23. doi: 10.1016/s0925-4773(01)00432-4. [DOI] [PubMed] [Google Scholar]
- 50.Souid S, Lepesant JA, Yanicostas C. The xbp-1 gene is essential for development in Drosophila. Dev. Genes Evol. 2007;217:159–167. doi: 10.1007/s00427-006-0124-1. [DOI] [PubMed] [Google Scholar]
- 51.Mistry H, Wilson BA, Roberts IJ, O’Kane CJ, Skeath JB. Cullin-3 regulates pattern formation, external sensory organ development and cell survival during Drosophila development. Mech. Dev. 2004;121:1495–1507. doi: 10.1016/j.mod.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 52.Phuong Thao DT, Ida H, Yoshida H, Yamaguchi M. Identification of the Drosophila skpA gene as a novel target of the transcription factor DREF. Exp. Cell Res. 2006;312:3641–3650. doi: 10.1016/j.yexcr.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 53.Masson P, Lundgren J, Young P. Drosophila proteasome regulator REGγ: transcriptional activation by DNA replication-related factor DREF and evidence for a role in cell cycle progression. J. Mol. Biol. 2003;327:1001–1012. doi: 10.1016/s0022-2836(03)00188-8. [DOI] [PubMed] [Google Scholar]
- 54.Feldman RM, Correll CC, Kaplan KB, Deshaies RJ. A complex of Cdc4p, Skp1p, and Cdc53p/cullin catalyzes ubiquitination of the phosphorylated CDK inhibitor Sic1p. Cell. 1997;91:221–230. doi: 10.1016/s0092-8674(00)80404-3. [DOI] [PubMed] [Google Scholar]
- 55.Skowyra D, Craig KL, Tyers M, Elledge SJ, Harper JW. F-box proteins are receptors that recruit phosphorylated substrates to the SCF ubiquitin-ligase complex. Cell. 1997;91:209–219. doi: 10.1016/s0092-8674(00)80403-1. [DOI] [PubMed] [Google Scholar]
- 56.Hershko A, Ciechanover A. The ubiquitin system. Annu. Rev. Biochem. 1998;67:425–479. doi: 10.1146/annurev.biochem.67.1.425. [DOI] [PubMed] [Google Scholar]
- 57.Matsukage A, Hirose F, Yoo MA, Yamaguchi M. The DRE/DREF transcriptional regulatory system: a master key for cell proliferation. Biochim. Biophys. Acta. 2008;1779:81–89. doi: 10.1016/j.bbagrm.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 58.Chan HY, Brogna S, O'Kane CJ. Dribble, the Drosophila KRR1p homologue, is involved in rRNA processing. Mol. Biol. Cell. 2001;12:1409–1419. doi: 10.1091/mbc.12.5.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sasaki T, Toh EA, Kikuchi Y. Yeast Krr1p physically and functionally interacts with a novel essential Kri1p, and both proteins are required for 40S ribosome biogenesis in the nucleolus. Mol. Cell Biol. 2000;20:7971–7979. doi: 10.1128/mcb.20.21.7971-7979.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kapp LD, Lorsch JR. The molecular mechanics of eukaryotic translation. Annu. Rev. Biochem. 2004;73:657–704. doi: 10.1146/annurev.biochem.73.030403.080419. [DOI] [PubMed] [Google Scholar]
- 61.LeFebvre AK, Korneeva NL, Trutschl M, Cvek U, Duzan RD, Bradley CA, Hershey JW, Rhoads RE. Translation initiation factor eIF4G-1 binds to eIF3 through the eIF3e subunit. J. Biol. Chem. 2006;281:22917–22932. doi: 10.1074/jbc.M605418200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Itoh N, Ohta K, Ohta M, Kawasaki T, Yamashina I. The nucleotide sequence of a gene for a putative ribosomal protein S31 of Drosophila. Nucleic Acids Res. 1989;17:2121. doi: 10.1093/nar/17.5.2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lambertsson A. The minute genes in Drosophila and their molecular functions. Adv. Genet. 1998;38:69–134. doi: 10.1016/s0065-2660(08)60142-x. [DOI] [PubMed] [Google Scholar]
- 64.Linder P, Lasko PF, Ashburner M, Leroy P, Nielsen PJ, Nishi K, Schnier J, Slonimski PP. Birth of the D-E-A-D box. Nature. 1989;337:121–122. doi: 10.1038/337121a0. [DOI] [PubMed] [Google Scholar]
- 65.Pestova TV, Kolupaeva VG, Lomakin IB, Pilipenko EV, Shatsky IN, Agol VI, Hellen CU. Molecular mechanisms of translation initiation in eukaryotes. Proc. Natl Acad. Sci. USA. 2001;98:7029–7036. doi: 10.1073/pnas.111145798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Blum S, Schmid SR, Pause A, Buser P, Linder P, Sonenberg N, Trachsel H. ATP hydrolysis by initiation factor 4A is required for translation initiation in Saccharomyces cerevisiae. Proc. Natl Acad. Sci. USA. 1992;89:7664–7668. doi: 10.1073/pnas.89.16.7664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yamamoto K, Sonoda M. Self-interaction of heterochromatin protein 1 is required for direct binding to histone methyltransferase, SUV39H1. Biochem. Biophys. Res. Commun. 2003;301:287–292. doi: 10.1016/s0006-291x(02)03021-8. [DOI] [PubMed] [Google Scholar]
- 68.Seeler JS, Marchio A, Sitterlin D, Transy C, Dejean A. Interaction of SP100 with HP1 proteins: a link between the promyelocytic leukemia-associated nuclear bodies and the chromatin compartment. Proc. Natl Acad. Sci. USA. 1998;95:7316–7321. doi: 10.1073/pnas.95.13.7316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lehming N, Le Saux A, Schuller J, Ptashne M. Chromatin components as part of a putative transcriptional repressing complex. Proc. Natl Acad. Sci. USA. 1998;95:7322–7326. doi: 10.1073/pnas.95.13.7322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ryan RF, Schultz DC, Ayyanathan K, Singh PB, Friedman JR, Fredericks WJ, Rauscher FJ., 3rd KAP-1 corepressor protein interacts and colocalizes with heterochromatic and euchromatic HP1 proteins: a potential role for Kruppel-associated box-zinc finger proteins in heterochromatin-mediated gene silencing. Mol. Cell Biol. 1999;19:4366–4378. doi: 10.1128/mcb.19.6.4366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Song K, Jung Y, Jung D, Lee I. Human Ku70 interacts with heterochromatin protein 1α. J. Biol. Chem. 2001;276:8321–8327. doi: 10.1074/jbc.M008779200. [DOI] [PubMed] [Google Scholar]
- 72.Ye Q, Callebaut I, Pezhman A, Courvalin JC, Worman HJ. Domain-specific interactions of human HP1-type chromodomain proteins and inner nuclear membrane protein LBR. J. Biol. Chem. 1997;272:14983–14989. doi: 10.1074/jbc.272.23.14983. [DOI] [PubMed] [Google Scholar]
- 73.Kametaka A, Takagi M, Hayakawa T, Haraguchi T, Hiraoka Y, Yoneda Y. Interaction of the chromatin compaction-inducing domain (LR domain) of Ki-67 antigen with HP1 proteins. Genes Cells. 2002;7:1231–1242. doi: 10.1046/j.1365-2443.2002.00596.x. [DOI] [PubMed] [Google Scholar]
- 74.Badugu R, Shareef MM, Kellum R. Novel Drosophila heterochromatin protein 1 (HP1)/origin recognition complex-associated protein (HOAP) repeat motif in HP1/HOAP interactions and chromocenter associations. J. Biol. Chem. 2003;278:34491–34498. doi: 10.1074/jbc.M305262200. [DOI] [PubMed] [Google Scholar]
- 75.Giot L, Bader JS, Brouwer C, Chaudhuri A, Kuang B, Li Y, Hao YL, Ooi CE, Godwin B, Vitols E, et al. A protein interaction map of Drosophila melanogaster. Science. 2003;302:1727–1736. doi: 10.1126/science.1090289. [DOI] [PubMed] [Google Scholar]
- 76.Secombe J, Li L, Carlos L, Eisenman RN. The Trithorax group protein Lid is a trimethyl histone H3K4 demethylase required for dMyc-induced cell growth. Genes Dev. 2007;21:537–551. doi: 10.1101/gad.1523007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lee N, Zhang J, Klose RJ, Erdjument-Bromage H, Tempst P, Jones RS, Zhang Y. The trithorax-group protein Lid is a histone H3 trimethyl-Lys4 demethylase. Nat. Struct. Mol. Biol. 2007;14:341–343. doi: 10.1038/nsmb1216. [DOI] [PubMed] [Google Scholar]
- 78.Eissenberg JC, Lee MG, Schneider J, Ilvarsonn A, Shiekhattar R, Shilatifard A. The trithorax-group gene in Drosophila little imaginal discs encodes a trimethylated histone H3 Lys4 demethylase. Nat. Struct. Mol. Biol. 2007;14:344–346. doi: 10.1038/nsmb1217. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.