Abstract
Isl1 and Nkx2–5 expressing cardiovascular progenitors play pivotal roles in cardiogenesis. Previously reported Cre-based fate mapping studies showed that Isl1 progenitors contribute predominantly to the derivatives of the second heart field, and Nkx2–5 progenitors contributed mainly to the cardiomyocyte lineage. However, partial recombination of Cre reporter genes can complicate interpretation of Cre fate mapping experiments. We found that a Gata4-based Cre-activated reporter was recombined by Isl1Cre and Nkx2–5Cre in a substantially broader domain than previously reported using standard Cre-activated reporters. The expanded Isl1 and Nkx2–5 cardiac fate maps were remarkably similar, and included extensive contributions to cardiomyocyte, endocardial, and smooth muscle lineages in all four cardiac chambers. These data indicate that Isl1 is expressed in progenitors of both primary and secondary heart fields, and that Nkx2–5 is expressed in progenitors of cardiac endothelium and smooth muscle, in addition to cardiomyocytes. These results have important implications for our understanding of cardiac lineage diversification in vivo, and for the interpretation of Cre-based fate maps.
Keywords: Fate map, Cre reporter, secondary heart field, cardiogenesis, cardiac progenitor
Introduction
The mature heart is mainly comprised of cells belonging to cardiomyocyte, endothelial, and smooth muscle lineages. The diversification of these lineages from progenitors cells has become an area of intensive investigation (reviewed in ref. Bruneau and Black, 2007). Progress has been driven by two major approaches. One approach has been to determine the developmental fate of progenitor cells in vivo. In mammals this is most commonly achieved by expression of Cre recombinase in progenitor cells. Cells descended from these progenitors are heritably and irreversibly marked by recombination of Cre-activated reporter genes (Soriano, 1999). Using this approach, Isl1 progenitors were found to contribute to cardiomyocyte, smooth muscle cells (SMCs), and endothelial cells (ECs) (Moretti et al., 2006). Isl1-marked cells contributed extensively to right ventricle (RV), outflow tract (OT), and atria, with a reduced contribution to left ventricle (LV) (Cai et al., 2003; Yang et al., 2006; Sun et al., 2007). This has been a key observation supporting the existence of two distinct cardiac progenitor populations, one that gives rise to left ventricle (first heart field or FHF), and one that gives rise to right ventricle (RV), outflow tract (OT), and atria (second heart field or SHF) (Buckingham et al., 2005). Using a similar fate-mapping strategy, Nkx2–5-expressing progenitors were shown to primarily give rise to the cardiomyocyte lineage, with an additional but infrequent contribution to the endothelial lineage (Moses et al., 2001; Stanley et al., 2002).
A second approach has been to analyze the in vitro differentiation potential of cardiac progenitors, isolated by their expression of marker genes such as Isl1 or Nkx2–5. Consistent with in vivo fate mapping, these in vitro studies showed that Isl1+ progenitors differentiated into cardiomyocyte, SMC, and EC lineages (Moretti et al., 2006), and that Nkx2–5+ progenitors differentiated into cardiomyocytes (Wu et al., 2006). However, gaps between in vivo fate-mapping and in vitro differentiation studies remain. Fate mapping studies did not demonstrate descent of most LV cardiomyocytes from Isl1+ progenitors. Nkx2–5-expressing progenitors differentiated into SMCs in addition to cardiomyocytes in vitro (Wu et al., 2006), but an SMC fate for Nkx2–5 cells has not been noted in vivo.
Different floxed loci exhibit differential susceptibility to Cre recombination (Novak et al., 2000; Vooijs et al., 2001). Incomplete recombination of Cre-dependent reporters has the potential to significantly influence Cre-based fate-mapping experiments. We recently described a Gata4-based reporter, Gata4flap, that was more susceptible to Cre recombination than a Rosa26-based reporter (Zhou et al., 2008). Because Gata4 is expressed in the major lineages of the developing and mature heart (Fig. 1 and Heikinheimo et al., 1994), this Gata4-based reporter can be used to report on the cardiac fates of Cre-expressing precursors. Using Gata4flap, we showed that Isl1 and Nkx2–5 expressing progenitors contribute extensively to the proepicardium. Here, we use this reporter to reassess the cardiac fates of Isl1 and Nkx2–5 progenitors. We found that the cardiac fate maps of Isl1 and Nkx2–5 are significantly broader that previously reported (Moses et al., 2001; Stanley et al., 2002; Cai et al., 2003; Yang et al., 2006; Sun et al., 2007). These results have important implications for our understanding of lineage diversification in the developing heart, and for the interpretation of recombinase-based fate mapping experiments.
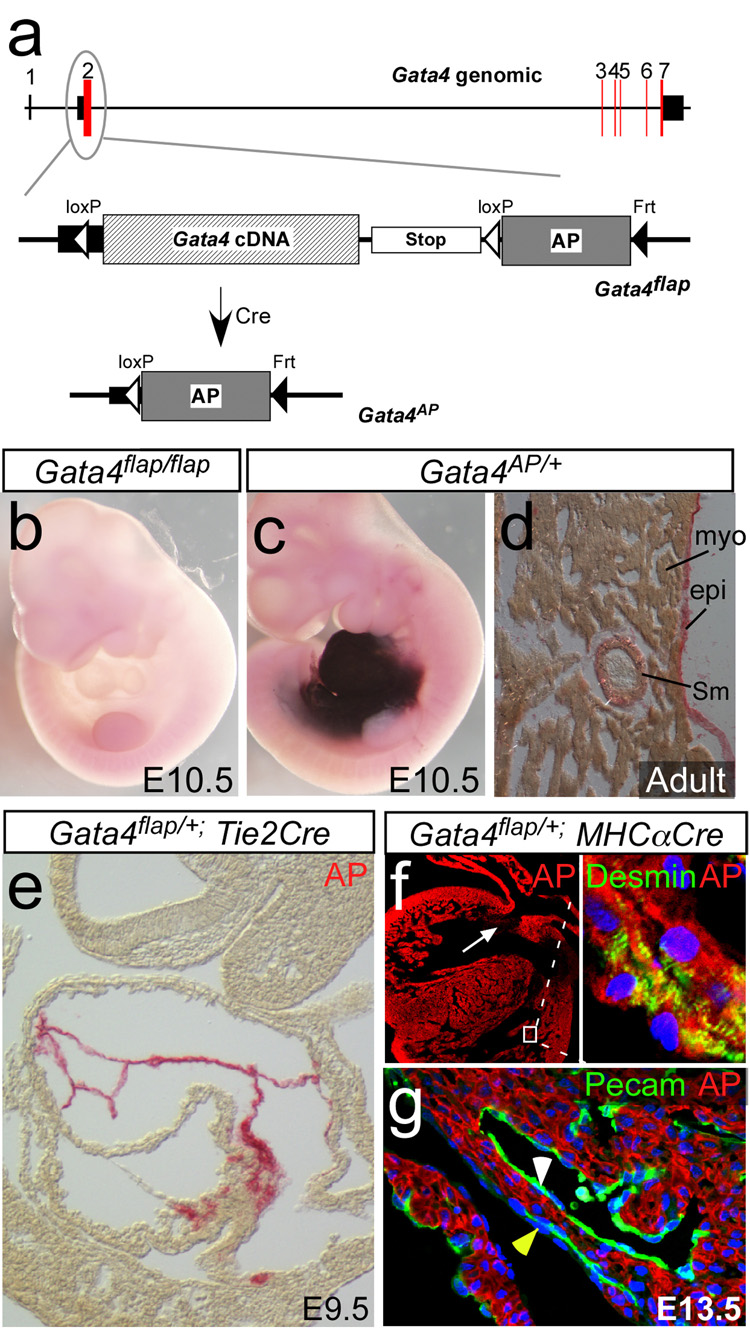
Figure 1. Gata4flap, a reporter of cardiac Cre activity.
a. Schematic depicting the structure of the Gata4 genomic locus, and the knockin Gata4flap allele. Red boxes indicate coding regions, and black boxes untranslated regions. AP, human placental alkaline phosphatase. b. Lack of AP activity in Gata4flap/flap embryos. c. AP activity in Gata4AP/+ embryo. d. AP expression in Gata4AP/+ adult heart. e. Tie2Cre activated Gata4flap in the endocardium but not myocardium. f–g. MHC〈Cre recombined Gata4flap selectively in cardiomyocytes (Myo). Cells of the endocardium (white arrowhead, g), epicardium (yellow arrowhead, g) and endocardial cushions (white arrow, f) were not recombined. AP was detected with BCIP/NBT in b,c and Permanent Red in d–g.
Materials and Methods
Mice
Gata4flox, Gata4flap, Rosa26fsLz, EIIaCre, Tie2Cre, Nkx2–5Cre, MHCαCre, and Isl1Cre mice were described previously (Lakso et al., 1996; Agah et al., 1997; Mao et al., 1999; Kisanuki et al., 2001; Moses et al., 2001; Pu et al., 2004; Yang et al., 2006; Zhou et al., 2008). Mice were used according to protocols approved by the Institutional Animal Care and Use Committee.
Histological Analysis
Detection of β-galactosidase and human placental alkaline phosphatase (AP) was performed as described (Lobe et al., 1999). AP activity was visualized with either BCIP/NBT (purple; Roche) or Permanent Red (red; Dako). For fluorescent imaging, Permanent Red was detected with Cy5 filters. Isl1, Tnnt, desmin, smooth muscle actin, and PECAM antibodies were from Iowa Developmental Hybridoma Bank, Neomarkers, Biomedia, Sigma, and BD Biosciences, respectively.
Results
We previously described generation of a Gata4-based Cre reporter, Gata4flap, in which Cre-mediated Gata4 inactivation is coupled with expression of the reporter gene alkaline phosphatase (AP) (Zhou et al., 2008). Gata4flap contains a loxP-Gata4 cDNA–transcriptional stop-loxP cassette followed by an AP cDNA at the endogenous Gata4 start codon (Fig. 1a). Prior to Cre-mediated recombination, endogenous Gata4 regulatory elements drive transcription of Gata4 cDNA. Gata4flap/flap mice were viable and fertile, indicating that the Gata4 cDNA functionally replaced Gata4 expression from the native gene. Cre recombinase excises the Gata4 cDNA and transcriptional stop signal, permitting expression of AP under control of endogenous Gata4 regulatory elements. In the absence of Cre, no AP activity was detected (Fig. 1b). Germline Cre recombination by EIIaCre generated Gata4AP mice, which expressed AP in most cells of the developing and adult heart, including cardiomyocytes, SMCs, and ECs (Fig. 1c–d). Cardiac and extracardiac AP expression (Fig. 1 and Fig. S1–S2) were consistent with previously reported expression of Gata4 (Arceci et al., 1993; Heikinheimo et al., 1994; Rivera-Feliciano et al., 2006), indicating that Gata4AP faithfully reports on Gata4 expression. Because Gata4 is expressed in most cells of the heart, within this domain Gata4flap/+ can be used to report on tissue-specific Cre activity. Tissue specific expression of Cre recombinase by Tie2Cre, MHCαCre, and cTNTCre transgenes selectively activated Gata4flap in ECs and cardiomyocytes, respectively, matching previously reported patterns of Cre activity driven by these transgenes within the heart (Fig. 1e–g and (Zhou et al., 2008)) Collectively, these data validate the Gata4flap allele.
Different genetic loci are known to vary widely in their susceptibility to Cre-mediated recombination (Novak et al., 2000; Vooijs et al., 2001). We found that Gata4flap was more susceptible to Cre recombination than Rosa26fsLz (Fig. S3; p < 0.001). This increased sensitivity permitted detection of an expanded contribution of Isl1+ and Nkx2–5+ progenitors to the proepicardium (Zhou et al., 2008). We therefore investigated whether or not the Gata4flap-based cardiac fate maps of Isl1Cre or Nkx2–5Cre would differ from those previously reported (Moses et al., 2001; Stanley et al., 2002; Cai et al., 2003; Yang et al., 2006; Sun et al., 2007)
Extensive contribution of Isl1+ progenitors to FHF and SHF derivatives of the fetal and postnatal heart
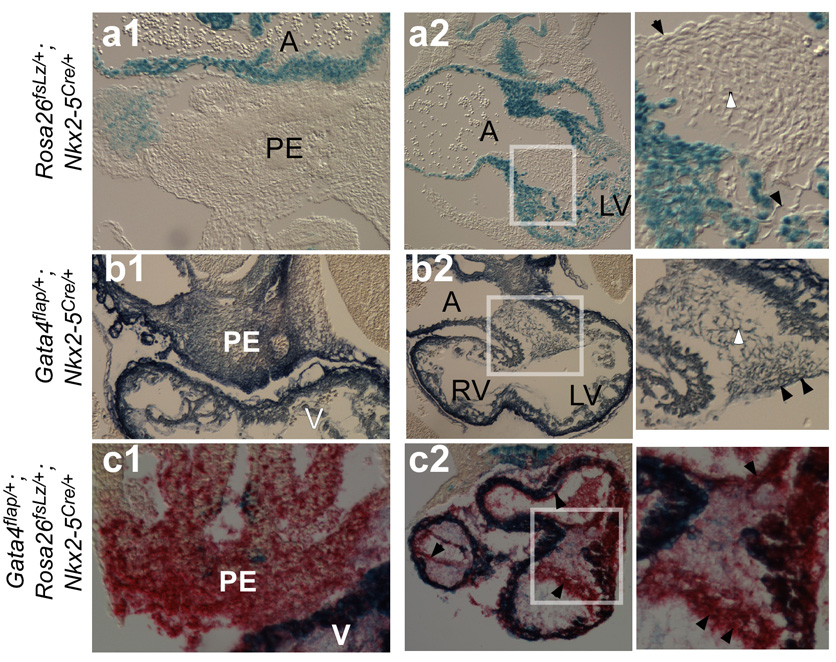
We used Gata4flap to determine the sites where Isl1Cre recombined Gata4, comparing the results to a second Cre-activated reporter, Rosa26fsLz (Mao et al., 1999). Consistent with previous reports (Cai et al., 2003; Moretti et al., 2006), Isl1Cre efficiently recombined Rosa26fsLz in cardiomyocytes, ECs, and endocardial cushion mesenchyme of OT and RV, but did not efficiently recombine Rosa26fsLz in LV or atrioventricular (AV) endocardial cushions (Fig. 2a; Table 1). However, the Gata4flap-based fate map was strikingly different (Fig. 2b; Table 1). Isl1Cre efficiently recombined Gata4flap in LV, as well as in RV and OT. In addition, Isl1Cre efficiently recombined Gata4flap, but not Rosa26fsLz, in proepicardium and in ECs and mesenchymal cells of the AV endocardial cushions (Fig. 2a–b; Table 1).
Figure 2. Developmental fates of Isl1+ progenitors in the developing heart.
E9.5 Rosa26fsLz/+; Isl1Cre/+ (a), Gata4flap/+; Isl1Cre+ (b), and Gata4flap/+; Rosa26fsLz/+; Isl1Cre/+ (c) embryos were stained for β-galactosidase (blue, a), AP (red, b), or both (blue and red, c; overlap appears as dark blue). Embryos were then transversely sectioned. Note that Gata4flap was activated much more extensively than Rosa26fsLz in LV and AV cushion (askterisk and inset, a2 vs b2). Black arrowheads indicate endocardium marked by Isl1Cre. Arrows indicate LV myocardium, which strongly expressed AP but only expressed LacZ in a mosaic pattern. Abbreviations are as in Figure 1.
Table 1.
Recombination domains of Isl1Cre and Nkx2–5Cre, as determined by Rosa26fsLz and Gata4flap Cre-activated reporters.
| Structure | Location | Lineage | Isl1Cre | Nkx2–5Cre | ||||
|---|---|---|---|---|---|---|---|---|
| Rosa26fsLz | Gata4flap | Rosa26fsLz | Gata4flap | |||||
| Myocardium | A | CM | + | ++++ | ++++ | ++++ | ||
| EC | +/− | ++++ | − | ++++ | ||||
| LV | CM | + | ++++ | ++++ | ++++ | |||
| EC | +/− | ++++ | − | ++++ | ||||
| RV | CM | ++++ | ++++ | ++++ | ++++ | |||
| EC | +++ | ++++ | − | ++++ | ||||
| AV Canal |
CM | +/− | ++++ | ++++ | ++++ | |||
| EC | +/− | ++++ | − | ++++ | ||||
| Mes | +/− | ++++ | − | ++++ | ||||
| Outflow Tract |
CM | ++++ | ++++ | ++++ | ++++ | |||
| EC | +++ | ++++ | − | ++++ | ||||
| Mes | ++++ | ++++ | − | ++++ | ||||
| Proepicardium | + | ++++ | + | ++++ | ||||
| Coronary |
SMC | ND | +++ | ND | +++ | |||
| EC | ND | +++ | ND | ++ | ||||
A, atria; CM, cardiomyocyte; LV, left ventricle; RV, right ventricle; Mes, mesenchyme. Frequency of Cre-marked cells was qualitatively scored from “−“ (no contribution detected) to “++++” (nearly all cells marked). ND, not determined.
We considered the possibility that genetic differences, such as Gata4 genotype or strain background, may have led to divergent results. We compared Isl1Cre fate maps for Gata4flap and Rosa26fsLz reporters in the same embryo (Fig. 2c). The differences between the two reporters persisted within the same embryo, excluding genetic differences as the cause of divergent Gata4flap and Rosa26fsLz fate maps.
We also considered whether differences in reporter gene promoter activity or AP versus LacZ detection thresholds led to the more extensive Gata4flap fate map. Cre recombination acts as a binary on/off switch, while the reporter gene promoter determines the strength of reporter gene expression. Therefore, both possibilities can be tested by examining reporter gene expression after germline reporter gene activation by EIIaCre (Lakso et al., 1996). Germline recombination of both Gata4flap and Rosa26fsLz resulted in strong expression of both reporters throughout the heart, including LV (Fig. S4) (Mao et al., 1999; Soriano, 1999; Stanley et al., 2002). These data indicate that when activated both reporters provide a robust signal throughout the heart.
The divergent fate maps reported by Rosa26fsLz and Gata4flap led us to examine the fate of Isl1+ progenitors with a third Cre-activated reporter, Z/Red (Vintersten et al., 2004), in which the strong CAG promoter drives expression of red fluorescent protein (RFP) after Cre recombination. Isl1Cre efficiently activated RFP expression in OT myocardium, less efficiently in RV myocardium, and rarely in LV myocardium (Fig. S5). Thus, each of the three Cre reporters yielded distinct Isl1 fate maps. These data reinforce the conclusion that recombinase-based fate maps require a nuanced interpretation that considers the properties of the Cre-dependent reporter used (see Discussion).
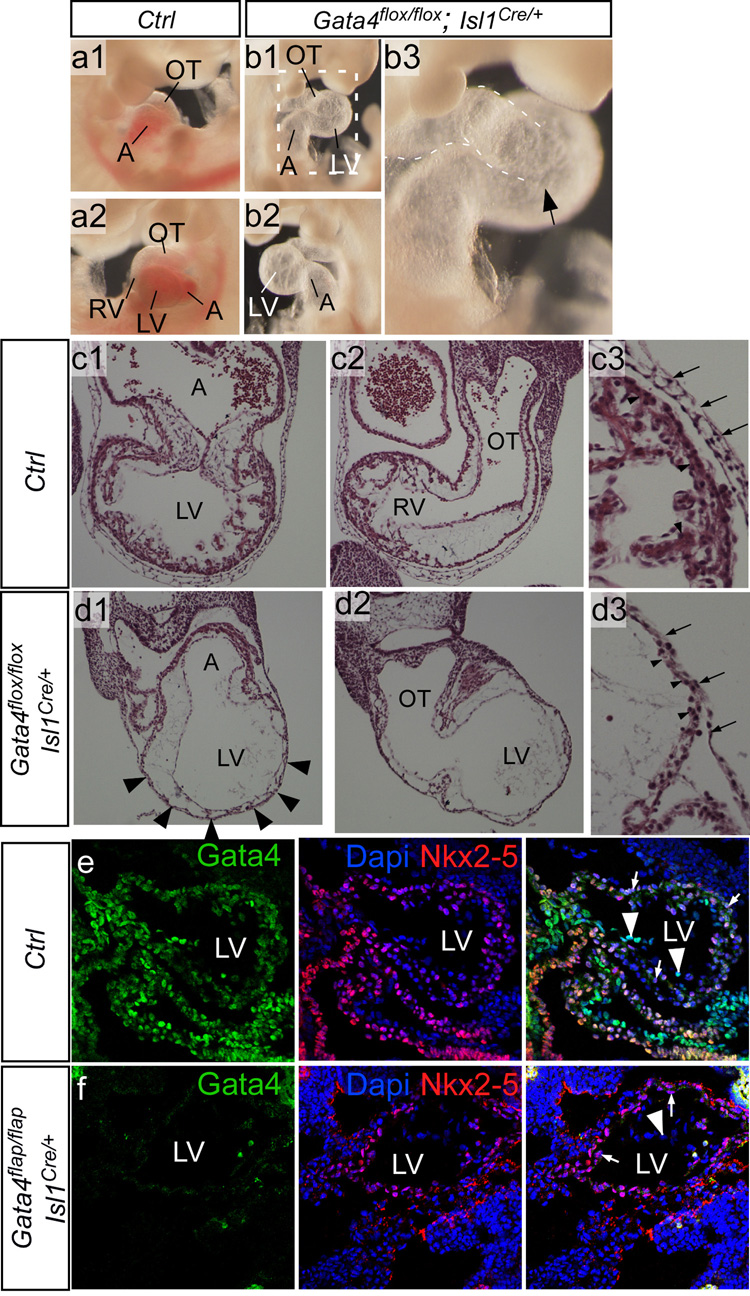
Having established that Gata4flap is extensively recombined throughout the heart by Isl1Cre, we next examined the phenotype resulting from Gata4 knockout by Isl1Cre. Isl1Cre inactivation of Gata4flap or an independently constructed Gata4 conditional allele, Gata4flox (Pu et al., 2004), resulted in similar phenotypes (Fig. 3 and S6). These embryos had markedly hypoplastic to absent RVs (Fig. 3a–b) and severe thinning of the LV myocardium (Fig. 3c–d). There was a paucity of myocardial trabeculation. This phenotype was highly reminiscent of hearts in which Gata4 was inactivated throughout the myocardium by Nkx2–5Cre (Zeisberg et al., 2005). Consistent with extensive Gata4 recombination by Isl1Cre in LV, Gata4 protein was not detectable in Gata4flap/flap; Isl1Cre/+ LV endocardium or cardiomyocytes (Fig. 3e–f). Gata4 loss of function did not lead to ectopically increased Isl1Cre activity in LV, because Rosa26fsLz remained largely inactive in Gata4flap/flap; Rosa26fsLz; Isl1Cre/+ LV (Fig. S7). In contrast to Gata4 inactivation by Isl1Cre, a different, SHF-restricted Cre transgene based on a Mef2c enhancer selectively inactivated Gata4 in RV and OFT (Rojas et al., 2008). This impaired RV/OFT development, but spared the LV, suggesting that abnormal LV development following Gata4 inactivation by Isl1Cre was unlikely to be an indirect consequence of Gata4 inactivation in SHF. Together, these data indicate that Isl1Cre efficiently knocked out Gata4 throughout the derivatives of both primary and secondary heart fields and suggest that caution should be exercised when using Isl1Cre to achieve gene inactivation restricted to the second heart field.
Figure 3. Inactivation of Gata4 by Isl1Cre.
a–b. Gross morphology of control and mutant embryos at E10.5. Mutant embryos were growth retarded and often had large pericardial effusions (not shown). Removal of the pericardial sac showed a bulbous and translucent appearing LV, and absence or severe hypoplasia of the RV (arrow, b3). c–d. Histological transverse sections of E10.5 control and mutant embryos. The mutant embryos had severe thinning of the LV myocardium and absence of myocardial trabeculation (arrowheads, d1). The morphological RV was severely hypoplastic (not shown) to absent (d2). c3-d3 show higher magnification of LV myocardium. Arrows indicate pericardium, arrowheads LV myocardium. A, atrium. LV and RV, left and right ventricle. OT, outflow tract. e–f. Loss of Gata4 immunoreactivity in Isl1Cre-recombined mutant LV. Sagittal frozen sections of control and Gata4flap/flap; Isl1Cre/+ mutant E9.5 littermate hearts. Gata4 was expressed in cardiomyocytes (arrows) and endothelium (arrowheads) of control LV. In Gata4flap/flap; Isl1Cre/+ hearts, Gata4 immunoreactivity was not detected in either cardiomyocytes or endothelium of LV. Nkx2–5 expression was unchanged in the mutant, demonstrating preservation of antigen integrity in mutant as well as control samples.
Given the expanded fate map of Isl1+ progenitors in the embryonic heart demonstrated by Gata4flap, we re-examined the fate map of Isl1+ progenitors in the postnatal heart (Fig. 4; Table 1). In the absence of Cre, we did not detect AP activity in postnatal Gata4flap/+ hearts (data not shown). In Gata4flap/+; Isl1Cre/+ hearts, most cardiomyocytes were marked by Isl1Cre, although there was a region of LV apex that was mosaic for AP expression (Fig. 4a–b). This was not due to mosaic Gata4 promoter activity, because Gata4AP was expressed by all cardiomyocytes (data not shown). Most likely, Isl1-driven Cre expression in progenitors of these cells was insufficient for recombination of Gata4flap.
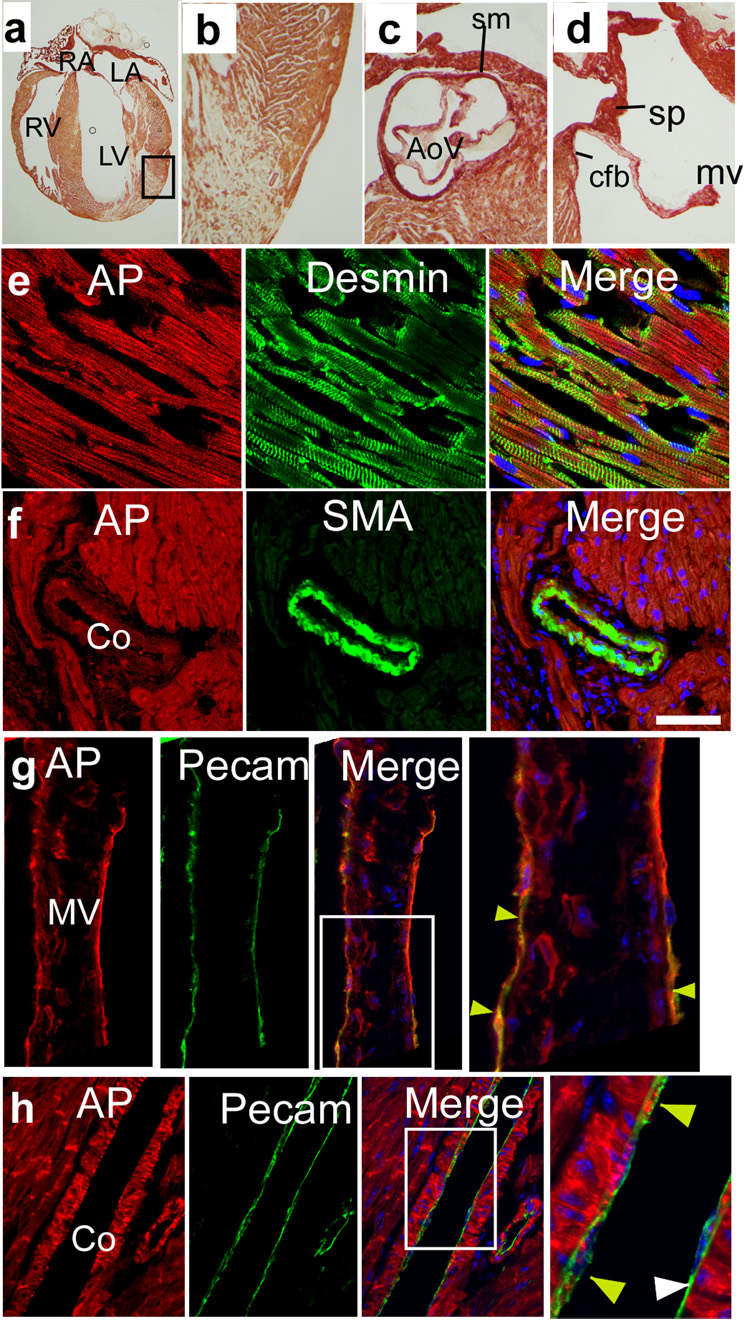
Figure 4. Developmental fates of Isl1+ progenitors in the postnatal heart.
Frozen sections of postnatal Gata4flap/+; Isl1Cre/+ hearts, stained for AP activity (red) and lineage markers. Isl1Cre activated Gata4flap in cardiomyocytes (a–b), in the base of the aorta and in the semilunar valves (c), and in derivatives of the AV endocardial cushions (d). AP activity colocalized with markers of cardiomyocytes (desmin, e), coronary SMCs (smooth muscle α-actin (SMA), f), and valve and coronary ECs (Pecam, yellow arrowheads, g and h). Coronary ECs were mosaic for AP staining (white arrowheads indicate Pecam+ cells that were AP negative). AoV, aortic valve. cfb, central fibrous body. co, coronary. mv, mitral valve. sm, smooth muscle. sp, atrial septum primum.
Isl1Cre labeled smooth muscle at the base of the aorta (Fig. 4c) and in the coronary arteries (Fig. 4f). Septum primum, the crux of the heart, and portions of the heart valves, all derivatives of the endocardial cushions, were efficiently recombined by Isl1Cre in the mature heart (Fig. 4c–d), consistent with Isl1Cre labeling of the cushions of the fetal heart (Fig. 1). Chamber and valve endocardium were likewise marked by Isl1Cre (Fig. 4g), while coronary ECs expressed AP in a mosaic pattern (Fig. 4h).
Nkx2–5 progenitors contribute extensively to cardiomyocyte, smooth muscle, and endothelial lineages
Nkx2–5 has also emerged as an important marker of multi-potent cardiac progenitor cells (Kattman et al., 2006; Moretti et al., 2006; Wu et al., 2006). Consistent with previous reports (Moses et al., 2001; Stanley et al., 2002), Nkx2–5Cre activated Rosa26fsLz predominantly in cardiomyocytes (Fig. 5a; Table 1). Rosa26fsLz was not efficiently activated in endocardium or endocardial cushion mesenchyme. However, studies have suggested that Nkx2–5 progenitors have the potential to differentiate into a broader set of lineages than reported by Cre-labeling using Rosa26fsLz (Moretti et al., 2006; Wu et al., 2006). Therefore, we used Gata4flap to reassess the fate of Nkx2–5 expressing cells. Gata4flap revealed a considerably broader Nkx2–5Cre fate map. Nkx2–5Cre efficiently recombined Gata4flap in endocardium and endocardial cushion mesenchyme, as well as in cardiomyocytes (Fig. 5b; Table 1). Nkx2–5Cre also recombined Gata4flap, but not Rosa26fsLz, in proepicardium (Fig. 5a–b). These findings were not due to genetic differences, because Gata4flap and Rosa26fsLz reporters yielded divergent results within the same embryo (Fig. 5c).
Figure 5. Developmental fates of Nkx2–5+ progenitors in the developing heart.
Transverse paraffin sections of embryos (a–b, E10.0; c, E9.5) stained in whole mount for LacZ (blue) or AP (BCIP/NBT in b, Permanent Red in c). Boxed regions of AV endocardial cushions are enlarged in right panels. Nkx2–5Cre activated both reporters in cardiomyocytes (dark blue). Nkx2–5Cre activation of Gata4flap was markedly more efficient than Rosa26fsLz in endocardium (black arrowhead), endocardial cushion mesenchyme (white arrowhead), and proepicardium (PE).
Next, we reanalyzed the contribution of Nkx2–5+ progenitors to lineages of the adult heart. (Fig. 6; Table 1). Nkx2–5Cre marked virtually all cardiomyocytes (Fig. 6a–e). Smooth muscle of the coronary arteries and the base of the aorta were labeled (Fig. 6c and 7f). Chamber and valve endocardium and coronary endothelium were also marked by Nkx2–5Cre (Fig. 6g–h). The heart valves and endocardial-cushion-derived portions of the atrial and ventricular septae were labeled (Fig. 6c–d and 7g), consistent with the extensive contribution of Nkx2–5+ progenitors to the fetal endocardial cushions (Fig. 5b–c). These data indicate Nkx2–5+ progenitors contribute to cardiomyocyte, SMC, and EC lineages of the developing and postnatal heart.
Figure 6. Developmental fates of Nkx2–5+ progenitors in the postnatal heart.
Gata4flap fate map of Nkx2–5 in adult heart. Frozen sections were stained for AP activity (red) and lineage markers. Nkx2–5Cre activated Gata4flap in cardiomyocytes (a–b), in the base of the aorta and in the semilunar valves (c), and in derivatives of the AV endocardial cushions (d). AP activity colocalized with markers of cardiomyocytes (e), coronary smooth muscle (f), valve endocardium (yellow arrowheads, g), and coronary ECs (yellow arrowheads, h). Endothelium was mosaic for AP staining (white arrowheads indicate Pecam+ cells that were AP negative). ad, adventitial tissue around aortic root, AoR, aortic root, tv, tricuspid valve. Other abbreviations as in Figure 4.
Discussion
Interpretation of recombinase-based fate mapping experiments
Recombinase-based fate mapping has become an important strategy for defining progenitor-descendant relationships in mammalian development. However, interpretation of such experiments is complex. Our data demonstrate that susceptibility of the Cre-dependent reporter to recombination significantly influences Cre-based fate mapping results. While Cre is expressed in temporally and spatially graded patterns, activation of a Cre-dependent reporter is a binary readout in which progenitors surpassing a Cre exposure threshold become activated. The specific threshold depends on the Cre-dependent reporter and the cellular context. The importance of this thresholding effect on Cre-dependent reporter readouts is illustrated in Fig. S8. Reporters that are more susceptible to recombination reveal a broader fate map that includes progenitors with lower level or transient Cre expression, while less sensitive reporters reveal a more restricted fate map that corresponds to progenitors with higher level or duration of Cre expression. An important implication is that lack of Cre reporter activation must be interpreted carefully, because this does not exclude Cre expression in progenitors at levels below the threshold required for reporter recombination. Conversely, the thresholding that occurs in Cre fate-mapping experiments can obscure biologically relevant differences in the level of gene expression, and this may become more problematic with extremely sensitive Cre-dependent reporters.
Our fate mapping results with Isl1Cre and Z/Red, Rosa26fsLz, and Gata4flap reporters illustrate the importance of the Cre reporter in determining the fate map. A likely explanation to the divergent fate maps obtained with these reporters is that more sensitive reporters are activated by lower levels or shorter durations of Cre exposure. In LV progenitors, where Isl1 expression is likely most transient, only Gata4flap was efficiently activated. In RV progenitors, with likely intermediate duration of Isl1 expression, Gata4flap and Rosa26fsLz were efficiently activated, while Z/Red was not. In OT, with the greatest duration of Isl1 expression (Sun et al., 2007), all three reporters were efficiently activated. In this model, each reporter delineates the fate of Isl1-expressing progenitors, but the fate maps differ by the threshold level of Isl1 expression that triggered a positive readout. While the variable readout of different Cre-activated reporters has been noted previously (Novak et al., 2000; Vooijs et al., 2001), our data emphasize the substantial impact this may have on the outcome of fate mapping experiments.
Contribution of Isl1+ and Nkx2–5+ progenitors to the developing heart
Isl1 and Nkx2–5 have emerged as important markers of cardiac progenitor cells (Bruneau and Black, 2007). The contribution of these progenitors to the developing heart has been determined primarily by fate mapping using Cre-activated reporters. We reassessed the fate map of Isl1 and Nkx2–5 expressing progenitors using a Cre-activated reporter based on Gata4, a gene expressed natively in the principal lineages of the heart. The Gata4flap-based fate maps, summarized in Table 1, demonstrated that Isl1 and Nkx2–5 progenitors make substantially greater contributions to the developing and postnatal heart than previously described: (1) Isl1+ progenitors contribute extensively to cardiomyocytes, SMCs (base of aorta; coronaries), and ECs (coronary, chamber, and valve endothelium) of FHF as well as SHF derivatives; (2) Nkx2–5+ progenitors contribute significantly to SMCs (base of aorta; coronaries) and ECs (coronary, chamber, and valve endothelium), in addition to cardiomyocytes; (3) Isl1+ and Nkx2–5+ progenitors contribute extensively to development of both the AV and OT cushions, and to the mature heart valves; (4) Isl1+ and Nkx2–5+ progenitors contribute extensively to proepicardium and its derivatives, the coronary vasculature. These fate-mapping data reconcile differences between in vivo fate mapping and in vitro differentiation studies, putting the in vitro findings in the context of heart development in the intact embryo.
The fate map of Isl1+ progenitors was initially studied with an Isl1IRES-Cre knockin, and showed substantial contribution of Isl1+ progenitors to RV, OT, and atria, with a minor contribution to LV (Cai et al., 2003). Subsequent reanalysis using Isl1Cre (no IRES) and Isl1MerCreMer demonstrated somewhat increased contribution of Isl1+ progenitors to LV, but was interpreted to be consistent with the Isl1IRES-Cre fate map (Yang et al., 2006; Sun et al., 2007). These data suggested that FHF and SHF progenitors differ by their expression of Isl1, supporting the hypothesis that FHF and SHF progenitors are distinct. On the other hand, previous reports have described Isl1 expression in precardiac mesoderm (Yuan and Schoenwolf, 2000; Brade et al., 2007; Prall et al., 2007), the presumptive location of FHF progenitors. However, at present Isl1 expression in FHF progenitor cells cannot be definitively demonstrated, because specific markers of this population are lacking. Our fate mapping data, based on Isl1Cre (no IRES) and Gata4flap, resolve this discrepancy between Isl1 expression and Isl1 fate, and indicate that LV progenitors, like RV/OT/atrial progenitors, express Isl1. The phenotype of Isl1 null embryos, which have severe underdevelopment of the residual (left) ventricle in addition to aberrant RV/OT morphogenesis (Cai et al., 2003), suggests that Isl1 expression in LV precursors is functionally significant. Whether the heart originates from two progenitor pools with distinct molecular signatures, or a single progenitor pool that differentiates in temporally and spatially distinct manners into right and left heart structures, remains controversial (Abu-Issa et al., 2004; Moorman et al., 2007). Our results indicate that Isl1 is expressed in both left and right heart precursors, and therefore Isl1 expression does not qualitatively distinguish SHF and FHF progenitors.
Precardiac mesoderm contains precursors of both endocardial and myocardial lineages (Linask and Lash, 1993; Sugi and Markwald, 1995). Whether these lineages arise from a common precursor in vivo is not known. In vitro studies identified a multipotent progenitor marked by expression of Isl1 and Nkx2–5 that could differentiate into cardiomyocyte, endothelial, and smooth muscle lineages (Moretti et al., 2006; Wu et al., 2006). Our data are consistent with these studies, and indicate that most endocardial and myocardial cells of the developing heart arise from progenitors that express Nkx2–5 and Isl1. The contribution of Isl1- or Nkx2–5-expressing progenitors to the endothelial lineage was noted previously (Stanley et al., 2002; Moretti et al., 2006; Sun et al., 2007), although the extent of contribution appeared to be less than noted in this study. Transient Nkx2–5 expression in endocardial progenitors might be functionally significant for endocardial differentiation, as suggested by the failure of Nkx2–5 knockout endocardium to subspecialize to form endocardial cushions (Tanaka et al., 1999).
We found that smooth muscle at the base of the aorta, and in the wall of some large and small coronary vessels, derives from Nkx2–5+ and Isl1+ progenitors. Isl1+ progenitors were previously noted to contribute to the smooth muscle lineage in vivo (Moretti et al., 2006; Sun et al., 2007). Most smooth muscle within the myocardium originates from precursors located in the proepicardium (Wilm et al., 2005). Because prior fate-mapping studies did not detect Isl1Cre marking of the proepicardium, it was concluded that the Isl1Cre-marked smooth muscle population arose from a distinct source. However, our fate-mapping studies indicate that Isl1Cre efficiently labels the proepicardium (Fig. 2 and (Zhou et al., 2008)), suggesting that the Isl1-derived smooth muscle likely arises from a proepicardial intermediate. A smooth muscle fate of Nkx2–5+ progenitors was not previously noted in vivo, but is consistent with the differentiation potential of Nkx2–5+ progenitors observed in vitro (Wu et al., 2006). However, not all coronary endothelium or smooth muscle was labeled by Nkx2–5Cre or Isl1Cre. Whether this mosaicism reflects heterogeneous origins of coronary vascular cells, or incomplete Cre-marking, is unclear.
We observed a broad overlap of Nkx2–5 and Isl1 fate maps. This extensive overlap was reflected in the similarity of mutant embryos in which Gata4 was inactivated by Nkx2–5Cre or Isl1Cre. The overlapping fate maps suggest that most cells of the mature heart traverse a developmental pathway that includes at least transient expression of both Nkx2–5 and Isl1. Based on these data, one might speculate that the multipotent Isl1+/Nkx2–5+ progenitor recently identified by Moretti and colleagues (Moretti et al., 2006) represents a common cardiac progenitor of cardiomyocyte, SMC, and EC lineages in all four cardiac chambers.
Supplementary Material
Acknowledgments
Sources of Funding
This work was supported by a grant from NIH NHLBI (SCCOR 1 PO1 HL074734) and by a charitable donation from Edward Marran and Karen Carpenter. BZ was supported by a postdoctoral fellowship from the American Heart Association.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abu-Issa R, Waldo K, Kirby ML. Heart fields: one, two or more? Dev Biol. 2004;272:281–285. doi: 10.1016/j.ydbio.2004.05.016. [DOI] [PubMed] [Google Scholar]
- Agah R, Frenkel PA, French BA, Michael LH, Overbeek PA, Schneider MD. Gene recombination in postmitotic cells. Targeted expression of Cre recombinase provokes cardiac-restricted, site-specific rearrangement in adult ventricular muscle in vivo. J Clin Invest. 1997;100:169–179. doi: 10.1172/JCI119509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arceci RJ, King AA, Simon MC, Orkin SH, Wilson DB. Mouse GATA-4: a retinoic acid-inducible GATA-binding transcription factor expressed in endodermally derived tissues and heart. Mol Cell Biol. 1993;13:2235–2246. doi: 10.1128/mcb.13.4.2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brade T, Gessert S, Kuhl M, Pandur P. The amphibian second heart field: Xenopus islet-1 is required for cardiovascular development. Dev Biol. 2007 doi: 10.1016/j.ydbio.2007.08.004. [DOI] [PubMed] [Google Scholar]
- Bruneau BG, Black BL. The heart's Da Vinci code: a Renaissance at Keystone. Development. 2007;134:1631–1633. doi: 10.1242/dev.002014. [DOI] [PubMed] [Google Scholar]
- Buckingham M, Meilhac S, Zaffran S. Building the mammalian heart from two sources of myocardial cells. Nat Rev Genet. 2005;6:826–835. doi: 10.1038/nrg1710. [DOI] [PubMed] [Google Scholar]
- Cai CL, Liang X, Shi Y, Chu PH, Pfaff SL, Chen J, Evans S. Isl1 identifies a cardiac progenitor population that proliferates prior to differentiation and contributes a majority of cells to the heart. Dev Cell. 2003;5:877–889. doi: 10.1016/s1534-5807(03)00363-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heikinheimo M, Scandrett JM, Wilson DB. Localization of transcription factor GATA-4 to regions of the mouse embryo involved in cardiac development. Dev Biol. 1994;164:361–373. doi: 10.1006/dbio.1994.1206. [DOI] [PubMed] [Google Scholar]
- Kattman SJ, Huber TL, Keller GM. Multipotent flk-1+ cardiovascular progenitor cells give rise to the cardiomyocyte, endothelial, and vascular smooth muscle lineages. Dev Cell. 2006;11:723–732. doi: 10.1016/j.devcel.2006.10.002. [DOI] [PubMed] [Google Scholar]
- Kisanuki YY, Hammer RE, Miyazaki J, Williams SC, Richardson JA, Yanagisawa M. Tie2-Cre transgenic mice: a new model for endothelial cell-lineage analysis in vivo. Dev Biol. 2001;230:230–242. doi: 10.1006/dbio.2000.0106. [DOI] [PubMed] [Google Scholar]
- Lakso M, Pichel JG, Gorman JR, Sauer B, Okamoto Y, Lee E, Alt FW, Westphal H. Efficient in vivo manipulation of mouse genomic sequences at the zygote stage. Proc Natl Acad Sci U S A. 1996;93:5860–5865. doi: 10.1073/pnas.93.12.5860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linask KK, Lash JW. Early heart development: dynamics of endocardial cell sorting suggests a common origin with cardiomyocytes. Dev Dyn. 1993;196:62–69. doi: 10.1002/aja.1001960108. [DOI] [PubMed] [Google Scholar]
- Lobe CG, Koop KE, Kreppner W, Lomeli H, Gertsenstein M, Nagy A. Z/AP, a double reporter for cre-mediated recombination. Dev Biol. 1999;208:281–292. doi: 10.1006/dbio.1999.9209. [DOI] [PubMed] [Google Scholar]
- Mao X, Fujiwara Y, Orkin SH. Improved reporter strain for monitoring Cre recombinase-mediated DNA excisions in mice. Proc Natl Acad Sci U S A. 1999;96:5037–5042. doi: 10.1073/pnas.96.9.5037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moorman AF, Christoffels VM, Anderson RH, Van Den Hoff MJ. The heart-forming fields: one or multiple? Philos Trans R Soc Lond B Biol Sci. 2007 doi: 10.1098/rstb.2007.2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moretti A, Caron L, Nakano A, Lam JT, Bernshausen A, Chen Y, Qyang Y, Bu L, Sasaki M, Martin-Puig S, Sun Y, Evans SM, Laugwitz KL, Chien KR. Multipotent embryonic isl1+ progenitor cells lead to cardiac, smooth muscle, and endothelial cell diversification. Cell. 2006;127:1151–1165. doi: 10.1016/j.cell.2006.10.029. [DOI] [PubMed] [Google Scholar]
- Moses KA, Demayo F, Braun RM, Reecy JL, Schwartz RJ. Embryonic expression of an Nkx2–5/Cre gene using ROSA26 reporter mice. Genesis. 2001;31:176–180. doi: 10.1002/gene.10022. [DOI] [PubMed] [Google Scholar]
- Novak A, Guo C, Yang W, Nagy A, Lobe CG. Z/EG, a double reporter mouse line that expresses enhanced green fluorescent protein upon Cre-mediated excision. Genesis. 2000;28:147–155. [PubMed] [Google Scholar]
- Prall OW, Menon MK, Solloway MJ, Watanabe Y, Zaffran S, Bajolle F, Biben C, Mcbride JJ, Robertson BR, Chaulet H, Stennard FA, Wise N, Schaft D, Wolstein O, Furtado MB, Shiratori H, Chien KR, Hamada H, Black BL, Saga Y, Robertson EJ, Buckingham ME, Harvey RP. An Nkx2–5/Bmp2/Smad1 negative feedback loop controls heart progenitor specification and proliferation. Cell. 2007;128:947–959. doi: 10.1016/j.cell.2007.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pu WT, Ishiwata T, Juraszek AL, Ma Q, Izumo S. GATA4 is a dosage-sensitive regulator of cardiac morphogenesis. Developmental Biology. 2004;275:235–244. doi: 10.1016/j.ydbio.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Rivera-Feliciano J, Lee KH, Kong SW, Rajagopal S, Ma Q, Springer Z, Izumo S, Tabin CJ, Pu WT. Development of heart valves requires Gata4 expression in endothelial-derived cells. Development. 2006;133:3607–3618. doi: 10.1242/dev.02519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas A, Kong SW, Agarwal P, Gilliss B, Pu WT, Black BL. GATA4 is a direct transcriptional activator of Cyclin D2 and Cdk4 and is required for cardiomyocyte proliferation in anterior heart field-derived myocardium. Mol Cell Biol. 2008 doi: 10.1128/MCB.00717-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- Stanley EG, Biben C, Elefanty A, Barnett L, Koentgen F, Robb L, Harvey RP. Efficient Cre-mediated deletion in cardiac progenitor cells conferred by a 3'UTR-ires-Cre allele of the homeobox gene Nkx2–5. Int J Dev Biol. 2002;46:431–439. [PubMed] [Google Scholar]
- Sugi Y, Markwald RR. Early endocardial formation originates from precardiac mesoderm as revealed by QH-1 antibody staining. Ital J Anat Embryol. 1995;100 Suppl 1:263–272. [PubMed] [Google Scholar]
- Sun Y, Liang X, Najafi N, Cass M, Lin L, Cai CL, Chen J, Evans SM. Islet 1 is expressed in distinct cardiovascular lineages, including pacemaker and coronary vascular cells. Dev Biol. 2007;304:286–296. doi: 10.1016/j.ydbio.2006.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M, Chen Z, Bartunkova S, Yamasaki N, Izumo S. The cardiac homeobox gene Csx/Nkx2.5 lies genetically upstream of multiple genes essential for heart development. Development. 1999;126:1269–1280. doi: 10.1242/dev.126.6.1269. [DOI] [PubMed] [Google Scholar]
- Vintersten K, Monetti C, Gertsenstein M, Zhang P, Laszlo L, Biechele S, Nagy A. Mouse in red: red fluorescent protein expression in mouse ES cells, embryos, and adult animals. Genesis. 2004;40:241–246. doi: 10.1002/gene.20095. [DOI] [PubMed] [Google Scholar]
- Vooijs M, Jonkers J, Berns A. A highly efficient ligand-regulated Cre recombinase mouse line shows that LoxP recombination is position dependent. EMBO Rep. 2001;2:292–297. doi: 10.1093/embo-reports/kve064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilm B, Ipenberg A, Hastie ND, Burch JB, Bader DM. The serosal mesothelium is a major source of smooth muscle cells of the gut vasculature. Development. 2005;132:5317–5328. doi: 10.1242/dev.02141. [DOI] [PubMed] [Google Scholar]
- Wu SM, Fujiwara Y, Cibulsky SM, Clapham DE, Lien CL, Schultheiss TM, Orkin SH. Developmental origin of a bipotential myocardial and smooth muscle cell precursor in the mammalian heart. Cell. 2006;127:1137–1150. doi: 10.1016/j.cell.2006.10.028. [DOI] [PubMed] [Google Scholar]
- Yang L, Cai CL, Lin L, Qyang Y, Chung C, Monteiro RM, Mummery CL, Fishman GI, Cogen A, Evans S. Isl1Cre reveals a common Bmp pathway in heart and limb development. Development. 2006;133:1575–1585. doi: 10.1242/dev.02322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan S, Schoenwolf GC. Islet-1 marks the early heart rudiments and is asymmetrically expressed during early rotation of the foregut in the chick embryo. Anat Rec. 2000;260:204–207. doi: 10.1002/1097-0185(20001001)260:2<204::AID-AR90>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Zeisberg EM, Ma Q, Juraszek AL, Moses K, Schwartz RJ, Izumo S, Pu WT. Morphogenesis of the right ventricle requires myocardial expression of Gata4. J Clin Invest. 2005;115:1522–1531. doi: 10.1172/JCI23769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou B, Ma Q, Rajagopal S, Wu SM, Domian I, Rivera-Feliciano J, Jiang D, Von Gise A, Ikeda S, Chien KR, Pu WT. Epicardial progenitors contribute to the cardiomyocyte lineage in the developing heart. Nature. 2008;454:109–113. doi: 10.1038/nature07060. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.