Abstract
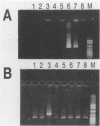
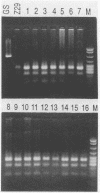
An oligotyping methodology was devised by using the polymerase chain reaction and sequence-specific oligonucleotide probe hybridization in order to discriminate the A and B variants of human herpesvirus 6 (HHV-6). Comparative DNA sequence analysis of portions of the U1102 (variant A) and Z29 (variant B) genomes revealed polymorphic regions which allowed for the synthesis of variant-specific and consensus oligonucleotide probes. These probes were found to hybridize exclusively to their respective HHV-6 variants. This strategy was then further tested by evaluating 16 clinical isolates derived from patients undergoing bone marrow transplantation to determine the subtype prevalence of HHV-6 infection in these patients. All clinical isolates were documented to be of variant B, indicating that the majority of bone marrow transplantation patients may be preferentially infected with this HHV-6 subtype. This oligotyping strategy may be useful in defining the relative prevalence of HHV-6A and HHV-6B infections in patient populations potentially at risk for HHV-6 disease.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ablashi D. V., Balachandran N., Josephs S. F., Hung C. L., Krueger G. R., Kramarsky B., Salahuddin S. Z., Gallo R. C. Genomic polymorphism, growth properties, and immunologic variations in human herpesvirus-6 isolates. Virology. 1991 Oct;184(2):545–552. doi: 10.1016/0042-6822(91)90424-a. [DOI] [PubMed] [Google Scholar]
- Aubin J. T., Collandre H., Candotti D., Ingrand D., Rouzioux C., Burgard M., Richard S., Huraux J. M., Agut H. Several groups among human herpesvirus 6 strains can be distinguished by Southern blotting and polymerase chain reaction. J Clin Microbiol. 1991 Feb;29(2):367–372. doi: 10.1128/jcm.29.2.367-372.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balachandran N., Amelse R. E., Zhou W. W., Chang C. K. Identification of proteins specific for human herpesvirus 6-infected human T cells. J Virol. 1989 Jun;63(6):2835–2840. doi: 10.1128/jvi.63.6.2835-2840.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter-Lowe L. A., Hunter J. B., Casper J. T., Gorski J. HLA gene amplification and hybridization analysis of polymorphism. HLA matching for bone marrow transplantation of a patient with HLA-deficient severe combined immunodeficiency syndrome. J Clin Invest. 1989 Aug;84(2):613–618. doi: 10.1172/JCI114206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burd E. M., Knox K. K., Carrigan D. R. Human herpesvirus-6-associated suppression of growth factor-induced macrophage maturation in human bone marrow cultures. Blood. 1993 Mar 15;81(6):1645–1650. [PubMed] [Google Scholar]
- Carrigan D. R., Drobyski W. R., Russler S. K., Tapper M. A., Knox K. K., Ash R. C. Interstitial pneumonitis associated with human herpesvirus-6 infection after marrow transplantation. Lancet. 1991 Jul 20;338(8760):147–149. doi: 10.1016/0140-6736(91)90137-e. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewhurst S., McIntyre K., Schnabel K., Hall C. B. Human herpesvirus 6 (HHV-6) variant B accounts for the majority of symptomatic primary HHV-6 infections in a population of U.S. infants. J Clin Microbiol. 1993 Feb;31(2):416–418. doi: 10.1128/jcm.31.2.416-418.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drobyski W. R., Dunne W. M., Burd E. M., Knox K. K., Ash R. C., Horowitz M. M., Flomenberg N., Carrigan D. R. Human herpesvirus-6 (HHV-6) infection in allogeneic bone marrow transplant recipients: evidence of a marrow-suppressive role for HHV-6 in vivo. J Infect Dis. 1993 Mar;167(3):735–739. doi: 10.1093/infdis/167.3.735. [DOI] [PubMed] [Google Scholar]
- Josephs S. F., Salahuddin S. Z., Ablashi D. V., Schachter F., Wong-Staal F., Gallo R. C. Genomic analysis of the human B-lymphotropic virus (HBLV). Science. 1986 Oct 31;234(4776):601–603. doi: 10.1126/science.3020691. [DOI] [PubMed] [Google Scholar]
- Knox K. K., Carrigan D. R. In vitro suppression of bone marrow progenitor cell differentiation by human herpesvirus 6 infection. J Infect Dis. 1992 May;165(5):925–929. doi: 10.1093/infdis/165.5.925. [DOI] [PubMed] [Google Scholar]
- Krueger G. R., Koch B., Ramon A., Ablashi D. V., Salahuddin S. Z., Josephs S. F., Streicher H. Z., Gallo R. C., Habermann U. Antibody prevalence to HBLV (human herpesvirus-6, HHV-6) and suggestive pathogenicity in the general population and in patients with immune deficiency syndromes. J Virol Methods. 1988 Sep;21(1-4):125–131. doi: 10.1016/0166-0934(88)90059-6. [DOI] [PubMed] [Google Scholar]
- Lawrence G. L., Chee M., Craxton M. A., Gompels U. A., Honess R. W., Barrell B. G. Human herpesvirus 6 is closely related to human cytomegalovirus. J Virol. 1990 Jan;64(1):287–299. doi: 10.1128/jvi.64.1.287-299.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lusso P., Markham P. D., Tschachler E., di Marzo Veronese F., Salahuddin S. Z., Ablashi D. V., Pahwa S., Krohn K., Gallo R. C. In vitro cellular tropism of human B-lymphotropic virus (human herpesvirus-6). J Exp Med. 1988 May 1;167(5):1659–1670. doi: 10.1084/jem.167.5.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellett P. E., Lindquester G. J., Feorino P., Lopez C. Genomic heterogeneity of human herpesvirus 6 isolates. Adv Exp Med Biol. 1990;278:9–18. doi: 10.1007/978-1-4684-5853-4_2. [DOI] [PubMed] [Google Scholar]
- Pruksananonda P., Hall C. B., Insel R. A., McIntyre K., Pellett P. E., Long C. E., Schnabel K. C., Pincus P. H., Stamey F. R., Dambaugh T. R. Primary human herpesvirus 6 infection in young children. N Engl J Med. 1992 May 28;326(22):1445–1450. doi: 10.1056/NEJM199205283262201. [DOI] [PubMed] [Google Scholar]
- Russler S. K., Tapper M. A., Knox K. K., Liepins A., Carrigan D. R. Pneumonitis associated with coinfection by human herpesvirus 6 and Legionella in an immunocompetent adult. Am J Pathol. 1991 Jun;138(6):1405–1411. [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Salahuddin S. Z., Ablashi D. V., Markham P. D., Josephs S. F., Sturzenegger S., Kaplan M., Halligan G., Biberfeld P., Wong-Staal F., Kramarsky B. Isolation of a new virus, HBLV, in patients with lymphoproliferative disorders. Science. 1986 Oct 31;234(4776):596–601. doi: 10.1126/science.2876520. [DOI] [PubMed] [Google Scholar]
- Schirmer E. C., Wyatt L. S., Yamanishi K., Rodriguez W. J., Frenkel N. Differentiation between two distinct classes of viruses now classified as human herpesvirus 6. Proc Natl Acad Sci U S A. 1991 Jul 1;88(13):5922–5926. doi: 10.1073/pnas.88.13.5922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segondy M., Astruc J., Atoui N., Echenne B., Robert C., Agut H. Herpesvirus 6 infection in young children. N Engl J Med. 1992 Oct 8;327(15):1099–1100. doi: 10.1056/NEJM199210083271514. [DOI] [PubMed] [Google Scholar]
- Takahashi K., Sonoda S., Higashi K., Kondo T., Takahashi H., Takahashi M., Yamanishi K. Predominant CD4 T-lymphocyte tropism of human herpesvirus 6-related virus. J Virol. 1989 Jul;63(7):3161–3163. doi: 10.1128/jvi.63.7.3161-3163.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood W. I., Gitschier J., Lasky L. A., Lawn R. M. Base composition-independent hybridization in tetramethylammonium chloride: a method for oligonucleotide screening of highly complex gene libraries. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1585–1588. doi: 10.1073/pnas.82.6.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt L. S., Balachandran N., Frenkel N. Variations in the replication and antigenic properties of human herpesvirus 6 strains. J Infect Dis. 1990 Oct;162(4):852–857. doi: 10.1093/infdis/162.4.852. [DOI] [PubMed] [Google Scholar]
- Yamanishi K., Okuno T., Shiraki K., Takahashi M., Kondo T., Asano Y., Kurata T. Identification of human herpesvirus-6 as a causal agent for exanthem subitum. Lancet. 1988 May 14;1(8594):1065–1067. doi: 10.1016/s0140-6736(88)91893-4. [DOI] [PubMed] [Google Scholar]
- Yoshikawa T., Suga S., Asano Y., Nakashima T., Yazaki T., Sobue R., Hirano M., Fukuda M., Kojima S., Matsuyama T. Human herpesvirus-6 infection in bone marrow transplantation. Blood. 1991 Sep 1;78(5):1381–1384. [PubMed] [Google Scholar]