Abstract
Purpose
A meta-analytic approach was used to estimate the frequency of: (a) microsatellite instability-high (MSI-H) phenotype in unselected ovarian cancers and (b) various histologic subtypes of mismatch repair (MMR)-deficient epithelial ovarian cancers.
Methods
A systematic search of the Medline electronic database was conducted to identify articles published between January 1, 1966, and December 31, 2007, that examined MMR deficiency in ovarian cancers. Data were extracted on the study population, sample size, MSI-H frequency, and histology of MMR-deficient ovarian tumors.
Results
The pooled proportion of MSI-H ovarian cancers was 0.12 [95% confidence interval (CI), 0.08–0.17] from 18 studies with 977 cases. The proportion of histologic subtypes in the pooled analysis from 15 studies with 159 cases was serous at 0.32 (95% CI, 0.20–0.44), mucinous at 0.19 (95% CI, 0.12–0.27), endometrioid at 0.29 (95% CI, 0.22–0.36), clear cell at 0.18 (95% CI, 0.09–0.28), and mixed at 0.24 (95% CI, 0.07–0.47). There was significant heterogeneity between studies.
Conclusions
The frequency of the MSI-H phenotype in unselected ovarian cancers approximates 12%. MMR-deficient ovarian cancers also seem to be characterized by an overrepresentation of nonserous histologic subtypes. Knowledge of histologic subtype may aid clinicians in identifying the relatively large proportion of ovarian cancers due to MMR defects; such knowledge has potential implications for medical management.
Ovarian cancer ranks fifth in both cancer incidence and mortality in U.S. women, and has the highest mortality rate among gynecologic cancers (1). Overall, an estimated 5% to 12% of invasive ovarian cancers may be attributed to hereditary susceptibility (2). Of the inherited cases, ~10% to 15% are believed to be due to germline mutations in genes involved in the mismatch repair (MMR) pathway, which lead to hereditary nonpolyposis colorectal cancer (HNPCC; ref. 3).
The MMR pathway is well defined in both inherited (4–9) and sporadic (10–12) cancer pathogenesis. Data suggest that identification of MMR-deficient ovarian cancers may be of diagnostic, prognostic, and therapeutic utility (10, 13–15). One molecular marker that is useful in the identification of HNPCC-associated and/or MMR-deficient tumors is microsatellite instability (MSI). Microsatellites are short, polymorphic DNA sequences distributed across the genome (16). MSI is caused by an underlying defect in the MMR pathway (17–19). Initial studies that sought to estimate the prevalence of MSI in ovarian cancer used varying definitions of MSI, making interstudy comparisons difficult. To standardize the definition of MSI, the National Cancer Institute (NCI) in 1997 introduced guidelines for the detection of MSI in colorectal cancer (20). The NCI recommended that a panel of five microsatellite loci including two mononucleotide repeats (Bat25 and Bat26) and three dinucleotide repeats (D2S123, D5S346, and D17S250) be investigated in tumors. Tumors were classified as having high-level MSI (MSI-H) if two or more of the five markers exhibited variations in microsatellite sequence length.
The reported prevalence of MSI-H status (defined by instability in two or more markers studied) in unselected ovarian cancers has ranged from 0% to 37% (10, 11, 21–36; Table 1). This wide variation reflects differences in several factors, including sample size, number and type of microsatellite loci investigated, and criteria used to define MSI phenotype. Because MSI-H status is an indicator of MMR-deficient ovarian tumors, a more precise frequency estimate of this phenomenon is needed.
Table 1.
Frequency of MSI-H phenotype in unselected ovarian cancers
| Reference | Study location | No. markers | Sample size | No. MSI-H |
|---|---|---|---|---|
| Allen et al., 2000 (21) | Buffalo, NY | 4 | 26 | 1 (4%) |
| Alvi et al., 2001 (22) | St. Louis, MO | 5 | 43 | 3 (7%) |
| Buller et al., 2001 (23) | Iowa City, IA | 6 | 116 | 24 (20%) |
| Codegoni et al., 1999 (24) | Milan, Italy | 8 | 31 | 8 (26%) |
| Dellas et al., 2004 (25) | Basel, Switzerland | 5 | 66 | 20 (30%) |
| Fujita et al., 1995 (36) | Osaka, Japan | 4 | 47 | 8 (17%) |
| Geisler et al., 2003 (10) | Iowa City, IA | 6 | 107 | 21 (20%) |
| Gras et al., 2001 (11) | Barcelona, Spain | 5 | 42 | 2 (5%) |
| Han et al., 1993 (27) | Seoul, Korea | 4 | 19 | 1 (5%) |
| Iwabuchi et al., 1995 (28) | New Haven, CT | 66 | 95 | 6 (6%) |
| King et al., 1995 (29) | New Haven, CT and Friedburg, Germany | 2 | 41 | 7 (17%) |
| Kobayashi et al., 1995 (30) | Hokkaido, Japan | 5 | 68 | 2 (3%) |
| Krajinovic et al., 1998 (31) | Montreal, Canada | 8 | 12 | 2 (17%) |
| Osborne et al., 1994 (32) | Cambridge, United Kingdom | 9 | 25 | 2 (8%) |
| Shih et al., 1998 (33) | Queensland, Australia | 69 | 31 | 0 (0%) |
| Sood et al., 1996 (34) | Iowa City, IA | 10 | 68 | 25 (37%) |
| Sood et al., 2001 (35) | Iowa City, IA | 14 | 109 | 13 (12%) |
| Tangir et al., 1996 (36) | Boston, MA | 13 | 31 | 0 (0%) |
Classification of ovarian cancers by histologic subtype may also be important in identifying MMR-deficient ovarian cancers. In developed countries, ~90% of malignant ovarian tumors are epithelial, and 10% are nonepithelial (37). Most epidemiologic studies have focused on epithelial ovarian cancers because they are the predominant subtype. Approximately 55% to 70% of all epithelial ovarian cancers are serous, 3% to 9% are mucinous, 8% to 15% are endometrioid, 7% to 13% are clear cell, 4% to 6% are mixed, and some are undifferentiated (38, 39).
Translational Relevance
This systematic review and meta-analysis estimates the frequency of high-level microsatellite instability (MSI-H) in unselected ovarian cancers as 12%, suggesting that defects in the mismatch repair (MMR) pathway account for a relatively large proportion of ovarian cancers. In the era of personalized medicine, MSI-H status may provide valuable etiologic and diagnostic information, which may eventually be of prognostic and therapeutic utility, as seen in MSI-H colorectal cancers. The results of this meta-analysis also reveal an overrepresentation of nonserous histologies in MMR-deficient tumors. The clinical relevance of these findings is that they may increase clinical awareness of MMR-deficient tumors; such awareness may aid in the identification of this subtype of tumors, having potential implications for medical management.
Information regarding the histologic subtypes of MMR-associated ovarian cancers is derived from studies that identified MMR-deficient cases based on (a) clinical criteria and/or germline mutation analysis or (b) MSI or MMR protein expression analyses. Such studies suggest that MMR-deficient ovarian cancers are characterized by an overrepresentation of the less common nonserous histologies (i.e., endometrioid, mucinous, and clear cell; refs. 3, 10, 11, 13, 25, 26, 29, 35, 40–47; Table 2).
Table 2.
Histologic subtypes of MMR-deficient ovarian cancers
| Reference | Sample size | Serous | Mucinous | Endometrioid | Clear cell |
|---|---|---|---|---|---|
| HNPCC-associated ovarian cancers | |||||
| Aarnio et al., 1999 (40) | 13 | 4 | 2 | 1 | 2 |
| Bewtra et al., 1992 (3) | 4 | 1 | 0 | 1 | 2 |
| Crijnen et al., 2005 (13) | 26 | 12 | 1 | 4 | 1 |
| Ichikawa et al., 1999 (43) | 4 | 2 | 1 | 1 | 0 |
| Stratton et al., 1999 (46) | 2 | 0 | 1 | 1 | 0 |
| Watson et al., 2001 (47) | 79 | 17 | 7 | 13 | 7 |
| MSI-H ovarian cancers | |||||
| Chiaravalli et al., 2001 (41) | 4 | 0 | 3 | 1 | 0 |
| Dellas et al., 2004 (25) | 20 | 11 | 3 | 3 | 1 |
| Fujita et al., 1995 (26) | 8 | 2 | 1 | 5 | 0 |
| Geisler et al., 2003 (10) | 21 | 12 | 2 | 6 | 0 |
| Gras et al., 2001 (11) | 2 | 0 | 0 | 1 | 1 |
| King et al., 1995 (29) | 7 | 2 | 0 | 2 | 1 |
| Sood et al., 2001 (35) | 13 | 7 | N/A | N/A | N/A |
| Ovarian cancers with loss of MMR protein expression | |||||
| Domanska et al., 2007 (42) | 6 | 0 | 1 | 3 | 2 |
| Malander et al., 2006 (44) | 3 | 0 | 1 | 0 | 1 |
| Rosen et al., 2006 (45) | 7 | 0 | 0 | 1 | 2 |
| Undifferentiated | Unspecified | Mixed | Nonepithelial* | Nonserous proportions† |
|---|---|---|---|---|
| 0 | 4 | 0 | 0 | 5/9 (56%) |
| 0 | 0 | 0 | 0 | 3/4 (75%) |
| 1 | 1 | 0 | 4 | 7/19 (37%)‡ |
| 0 | 0 | 0 | 0 | 2/4 (50%) |
| 0 | 0 | 0 | N/A | 2/2 (100%) |
| 0 | 26 | 4 | 5 | 31/48 (65%) |
| 0 | 0 | 0 | 0 | 4/4 (100%) |
| 0 | 2 | 0 | N/A | 7/18 (39%) |
| 0 | 0 | 0 | N/A | 6/8 (75%) |
| 0 | 1 | 0 | 0 | 8/20 (40%) |
| 0 | 0 | 0 | 0 | 2/2 (100%) |
| 0 | 0 | 1 | 1 | 4/6 (67%) |
| N/A | N/A | N/A | N/A | 6/13 (46%) |
| 0 | 0 | 0 | N/A | 6/6 (100%) |
| 0 | 0 | 1 | N/A | 3/3 (100%) |
| 0 | 0 | 3 | 1 | 6/6 (100%) |
Abbreviation: N/A, not applicable.
Studies where only epithelial ovarian cancers were included, N/A (not applicable) is indicated in this column, as nonepithelial ovarian cancers were not included in these studies.
Unspecified adenocarcinomas and nonepithelial cancers were not included within the denominator.
Two histologic subtypes were unknown, thus not included in the percentage.
NOTE: This reference included within the table was omitted from the analysis presented within the paper owing to lack of information regarding the specific histologic subtypes of the non-serious cancers.
To provide a reliable estimate of MSI-H frequency (aim 1), we conducted a meta-analysis of studies of unselected ovarian cancers. Furthermore, to characterize the histologic profile of MMR-deficient ovarian cancers, we did a meta-analysis of studies that investigated histologic subtypes in subgroups of MMR-deficient ovarian cancers (aim 2).
Materials and Methods
Identification of relevant studies
A systematic search of the Medline (PubMed) electronic database was conducted to identify articles published in English between January 1, 1966, and December 31, 2007 that examined MMR deficiency in ovarian cancers. The following search terms were used: “ovarian cancer,” “microsatellite instability” or “MSI-H,” “immunohistochemistry” or “IHC,” “hereditary nonpolyposis colorectal cancer syndrome” or “HNPCC,” and “mismatch-repair.” Additionally, references in each article were searched to identify potentially missed studies. When multiple articles were published by the same authors or groups, the most recent article was selected. Review articles without original data and single case reports were excluded.
Study selection
For aim 1, case series of unselected ovarian cancers in which MSI analysis was done were included. Eligible studies defined MSI-H status on the basis of instability of two markers. Case series of ovarian cancers that investigated MSI in selected subgroups were excluded. Because epithelial ovarian cancers make up the majority of ovarian cancers (37), we sought to only include data regarding epithelial subtypes. For studies that investigated both epithelial and nonepithelial subtypes (23, 29), only data pertaining to epithelial subtypes were included in the pooled analysis. For studies that did not clearly delineate the type of ovarian cancers that were investigated (epithelial versus nonepithelial; refs. 22, 27, 30, 31), attempts were made to contact the authors to verify the respective distributions.
For aim 2, case series of ovarian cancer that reported the histologic characteristics of MMR-deficient epithelial ovarian cancers were included. Studies that only categorized histologic subtype as serous or nonserous, without further classification of nonserous subtypes were excluded (35). For studies that reported data on both epithelial and nonepithelial subtypes (3, 10, 11, 13, 15, 29, 40, 41, 43, 45), only data pertaining to epithelial subtypes were included in the overall pooled analysis.
Data extraction
All reports considered for inclusion in the analysis were reviewed jointly by two reviewers (T.P. and J.P.W.) to ensure the accuracy of all recorded information. Discrepancies in data abstraction were resolved by consulting a third reviewer. For both aims, the following information was abstracted from eligible studies: first author, publication year, type of case series, and sample size. For aim 1, the number of markers investigated and the number and percentage of MSI-H cases were abstracted. For aim 2, the numbers and percentages of histologic subtypes were extracted.
Statistical analysis
For the purpose of meta-analysis, the proportions were transformed into quantities according to the Freeman-Tukey variant of the arcsine square root transformed proportion (48). The pooled proportion was calculated as the back-transformation of the weighted mean of the transformed proportions, using the random effects model proposed by DerSimonian-Laird (49).
A formal statistical test for heterogeneity using the I2 test (50) was done. The heterogeneity and robustness of pooled proportions were explored by conducting sensitivity and subgroup analyses.
For aim 1, MSI-H frequency study-specific estimates were pooled and are reported as proportions and 95% confidence intervals (95% CI). For aim 2, the histologic characteristics of MMR-deficient epithelial ovarian tumors were pooled and reported as proportions and 95% CI.
The possibility of publication bias was assessed for both aims using the Begg-Mazumdar adjusted rank correlation test and Egger funnel plot tests (51, 52). The meta-analysis was conducted using StatsDirect statistical software (53), and was done according to the guidelines for Quality of Reporting of Meta-analyses (54, 55).
Results
MSI frequency
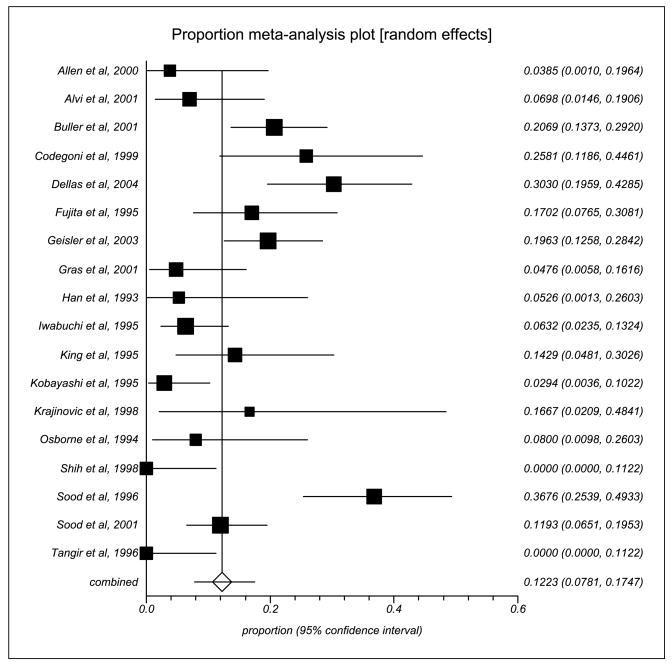
Overall, there were 18 studies with 977 cases that investigated MSI-H phenotype in unselected ovarian cancers. The characteristics of these 18 studies are summarized in Table 1. The proportional meta-analysis showed a pooled proportion of 0.12 (95% CI, 0.08–0.17; Fig. 1). There was significant heterogeneity between studies (I2 = 81%). The P value for the Begg-Mazumdar test was of borderline significance (P = 0.06), suggesting the possibility of publication bias.
Fig. 1.
Proportion of MSI in unselected ovarian cancers: a meta-analysis plot.
Sensitivity analyses were conducted to explore the robustness of this observation. After removing those studies (n = 4; refs. 25, 28, 30, 34) whose proportion and 95% CI were outliers, and the studies that had 0% proportion of MSI-H phenotype in unselected ovarian cancers (n = 2; refs. 33, 36), the recalculated pooled proportion of MSI-H phenotype in unselected ovarian cancers was 0.14 (95% CI, 0.10–0.18). The I2 was 44%, indicating no significant heterogeneity between studies.
Additionally, to determine a more precise estimate of the pooled proportion of epithelial ovarian cancers with MSI-H, a separate sensitivity analysis was done by only removing those studies (n = 4; refs. 22, 27, 30, 31) that did not specify the histopathologic characteristics (epithelial versus nonepithelial) of the study sample. The recalculated proportion of MSI-H phenotype in unselected epithelial ovarian cancers was 0.13 (95% CI, 0.08–0.20). Finally, a sensitivity analysis was done by removing the four studies conducted at the same center (n = 4; refs. 10, 23, 33, 34), and the recalculated proportion of MSI-H phenotype was 0.10 (95% CI, 0.05–0.15).
Histology of MMR-deficient tumors
Fifteen studies covering a total of 159 patients investigated histologic subtype in MMR-deficient epithelial ovarian cancers (Table 2). The pooled estimates for the various proportions of histologic subtypes were serous at 0.32 (95% CI, 0.20–0.44), mucinous at 0.19 (95% CI, 0.12–0.27), endometrioid at 0.29 (95% CI, 0.22–0.36), clear cell at 0.18 (95% CI, 0.09–0.28), and mixed at 0.24 (95% CI, 0.07–0.47). These proportions were similar when analyzing HNPCC-associated ovarian cancers (as defined by positive family history or germline mutation status) and MSI-H ovarian cancers (Table 3). However, the proportion of nonserous cancers was much higher for ovarian cancers with loss of MMR protein expression, due to a lack of serous cancers observed within the very limited number of studies in this category (i.e., 3 studies comprising a total of 16 cases). Nevertheless, the data suggest that the skewed distribution across histologic types also occurs within this subgroup, but the limited sample size within this category precludes a definitive statement to this effect. Significant between-study heterogeneity was seen for the serous and mixed subtypes. However, a sensitivity analysis could not be done due to a lack of distinct quality of reporting standards as well as the limited sample size, particularly of the group comprising ovarian cancers with loss of MMR protein expression. The P value for the Begg-Mazumdar test was not significant (P = 0.76), indicating an absence of publication bias.
Table 3.
Results of meta-analysis of histologic subtypes of MMR deficient ovarian cancers
| HNPCC-associated ovarian cancers |
MSI-H ovarian cancers |
Ovarian cancers with loss of MMR expression |
Pooled analysis of all 3 categories |
|
|---|---|---|---|---|
| Proportion (95% CI) | Proportion (95% CI) | Proportion (95% CI) | Proportion (95% CI) | |
| Serous | 0.42 (0.29–0.55) | 0.36 (0.18–0.57) | 0 (0) | 0.32 (0.20–0.44) |
| Nonserous | 0.57 (0.44–0.70) | 0.063 (0.42–0.81) | 0.95 (0.81–0.99) | 0.68 (0.56–0.80) |
| Mucinous | 0.16 (0.08–0.25) | 0.22 (0.07–0.42) | 0.26 (0.05–0.55) | 0.19 (0.12–0.27) |
| Endometrioid | 0.25 (0.17–0.35) | 0.32 (0.21–0.45) | 0.34 (0.10–0.64) | 0.29 (0.22–0.36) |
| Clear cell | 0.17 (0.07–0.30) | 0.10 (0.009–0.27) | 0.35 (0.15–0.58) | 0.18 (0.09–0.28) |
| Undifferentiated | 1 study | — | — | 1 study |
| Mixed | 1 study | 1 study | 1 study | 0.24 (0.07–0.47) |
NOTE: The table includes MMR-deficient ovarian cancers identified based on (a) positive family history and/or germline mutation analysis, (b) MSI-H status and/or (c) lack of MMR protein expression, stratified by each category, as well as the pooled analysis.
Additional pooled analyses for the 10 studies that investigated both epithelial and nonepithelial MMR-deficient ovarian cancers were done (3, 10, 11, 13, 15, 29, 40, 41, 43, 45). The results of the pooled analyses from these studies revealed that nonepithelial subtypes made up 10% of cases, and the distribution of epithelial subtypes was similar to the overall pooled estimate in aim 2 (data not shown). Further analysis was done to include all studies of epithelial ovarian cancers that could be stratified based on serous and nonserous histologic subtypes. This comprised the 15 studies included in aim 2, as well as 1 additional study (35). Results showed that 60% of cases were of nonserous histologies (data not shown), which is similar to the overall pooled results.
Discussion
Although there has been wide variation in the frequency of the MSI-H phenotype in ovarian cancer based on previous studies, our analysis confirms that the MMR pathway may be etiologically important in a significant proportion of ovarian tumors, with an expected frequency of 12%. Furthermore, results from our pooled meta-analysis suggest an overrepresentation of nonserous histologies in MMR-deficient ovarian tumors.
The results of our analyses suggest that the expected frequency of MSI-H in unselected ovarian cancers is between 8% to 17%. However, of the 18 studies of unselected ovarian cancer patients, 11 had a sample size <60 and 8 were done before 1997. Of the seven studies with a sample size >60, one was a Japanese study of 68 cases (30) with a MSI-H frequency of 3%. This low estimate may reflect ethnic variation in the frequency of alleles that influence MSI. Another U.S.-based study had a sample size of 95 (28) and a MSI-H frequency of 6%. Because this was published before the development of the five NCI-standardized markers, this may be an underestimate. The remaining five studies (10, 23, 25, 34, 35) were U.S.-based; four were conducted at the same center (10, 23, 34, 35) and had sample sizes between 66 and 116 and MSI frequencies between 12% and 37%.
Significant between-study heterogeneity was identified when evaluating MSI-H frequency. Some degree of heterogeneity is expected given differences in the characteristics of the populations studied and differences in the numbers and types of markers used to evaluate MSI, especially in studies done before the development of standardized markers. We attempted to evaluate the characteristics of the populations studied (age ranges, racial/ethnic background of cases), but the majority of studies did not report this information. Additionally, although it is theoretically possible that studies evaluating a higher number of microsatellite markers may detect a higher percentage of MSI-H ovarian cancers, the data suggest otherwise. This is evidenced by the observation that two studies that used >60 markers (28, 33) had MSI-H frequencies of 0% and 6%, whereas one study that used 14 markers (including the five NCI-recommended markers) reported that the NCI-recommended markers allowed identification of >90% of MSI-H ovarian cancers, and the addition of only one marker, NME1, would have allowed detection of all MSI-H cases in their series (35).
Studies that have investigated MMR-associated ovarian cancers based on family history or germline mutation status have generally suggested an overrepresentation of nonserous histologies (3, 13, 40, 43, 46, 47), but these studies have been limited by small sample sizes. Specifically, of the six published studies that reported histopathologic subtypes, five had sample sizes <30. Watson et al. (47) have done the largest study to date investigating HNPCC-associated ovarian cancers (n = 79), and although there was a suggestion of overrepresentation of nonserous histologies, 26 cases had unspecified adenocarcinoma, limiting the interpretation of results. Similarly, studies of MMR-associated ovarian cancers detected through MSI or MMR protein expression studies suggest overrepresentation of endometrioid, mucinous, and clear cell histologic subtypes. Specifically, most (11, 26, 29, 41), but not all (25, 35), studies of MSI-H ovarian cancers found an overrepresentation of nonserous histologies. Although based on small sample sizes, all studies of MMR protein expression in ovarian cancers that have stratified on histopathologic subtypes (42, 44, 45) have reported nonserous histologies in those with loss of expression. Taken together, these data suggest an overrepresentation of endometrioid, mucinous, and clear cell subtypes in ovarian cancers with MMR defects, consistent with the findings of the current meta-analysis.
The two most common HNPCC-associated cancers are colorectal and endometrial cancer. It is of interest that histologic subtypes overrepresented in these HNPCC-associated tumors are mucinous subtypes in colorectal cancer (56) and endometrioid subtypes in endometrial cancer (57), which are analogous to two overrepresented nonserous subtypes in epithelial ovarian cancers (i.e., mucinous and endometrioid). Thus, our results are consistent with studies investigating histologic subtypes of other MMR-deficient tumor types.
Epithelial ovarian cancers themselves reflect a heterogeneous group of diseases, as etiologic differences seem to exist according to the histologic subtypes (58–64). It is possible that the histologic subtypes overrepresented in MMR-deficient ovarian cancers may be attributed to a particular combination of genetic and environmental risk factors. It has been postulated that factors that increase total ovulatory months, thereby increasing overall hormone exposure, may serve as promoters of tumorigenesis for all histologic subtypes, whereas those having other mechanisms of action may serve as initiators of tumorigenesis for certain histologic subtypes (60). Specifically, evidence suggests that endometriosis may increase the risk for both endometrioid and clear cell tumors (65–67), whereas hormone replacement therapy may increase the risk for only endometrioid tumors (68, 69). Furthermore, smoking may increase the risk for the invasive mucinous tumors but not other subtypes (60, 61, 70). Future efforts should be placed toward further characterization of the hormonal and environmental factors of women with MMR-deficient ovarian cancers to better understand the gene-environment interactions that may have contributed to these cancers. Similar studies that have been conducted to investigate established and hypothesized risk factors for MMR-deficient colorectal cancers have suggested that lack of estrogen (71–73) and cigarette smoking (72, 74, 75) increase the risk of MSI-H tumors, although results have not been consistent (76).
As with any systematic review and meta-analysis, there are several sources of potential bias that may affect the results. One issue relates to whether all relevant articles were identified. The breadth of the search strategy and limited restrictions on the inclusion criteria led us to believe that few published studies have been missed. Publication bias occurs when studies reporting significant or positive findings are more likely to be published than studies reporting null findings (51, 52). Based on the Begg-Mazumdar and Egger funnel plot tests, there was not strong evidence for publication bias in this meta-analysis.
Several limitations need to be considered when interpreting results from this meta-analysis. With regard to aim 1, our investigation was limited by the small sample sizes of most studies, many of which were done before the standardization of the MSI-H phenotype definition. Furthermore, the study populations were ethnically diverse; ethnic variation in the distribution of MMR-deficient ovarian cancers potentially limits the generalizability of our findings. Additionally, although we attempted to only report MSI data on epithelial ovarian cancers, we were unable to verify the histopathologic profile of ovarian cancers in four of the included studies (22, 27, 30, 31). However, the sensitivity analysis that was done by omitting these four studies revealed a higher pooled proportion of MSI-H cases. Thus, inclusion of these studies in the overall pooled estimate provides a conservative estimate of the frequency of MSI-H epithelial ovarian cancers. Regarding aim 2, pathologists may differ in the interpretation of histologic subtypes, as evidenced by studies in other tumor types (77, 78). The potential misclassification of subtypes attributed to interobserver variability in interpretation may have biased findings from this meta-analysis. If such misclassification occurred, however, it seems most probable that it would be nondifferential.
Other limitations in our analyses included the problem related to the occurrence of zero counts in study strata. For example, if the proportion of a certain type of ovarian cancer is 0%, then there will be a zero in one cell and the inevitable result will be a formula requiring division by zero, which is an undefined mathematical operation that is impossible to calculate (79). To address this issue, it has been suggested that 0.5 be added to the numerator and the denominator, which works optimally in the case of 2 × 2 tables (80) but introduces a systematic bias in the case of single proportions (79). Another proposed solution has been to randomly subtract 0.5 from the denominator and the numerator of an equal number of proportions not having zero. However the validity of this method has not yet been tested (79). Therefore, in the absence of methodology to address this issue, a sensitivity analysis could not be done.
The findings from our study suggest that 12% of unselected ovarian cancers are MSI-H. These results provide a more precise frequency estimate than previously available and confirm the MMR pathway as an etiologically important cause of ovarian tumorigenesis. Furthermore, our finding that nonserous histologies are overrepresented in this subset of tumors has potential clinical utility in the identification of MMR-deficient ovarian cancer cases.
In the era of personalized medicine, MSI-H status may provide valuable etiologic and diagnostic information, which may eventually be of prognostic and therapeutic utility, as seen in MSI-H colorectal cancers (81–85). Specifically, it is possible that certain chemotherapeutic regimens exist to improve treatment efficacy and reduce drug toxicity specifically in MMR-deficient ovarian cancers (86–90). These findings are clinically relevant, as they may increase clinician awareness of this subset of tumors, having potential implications for medical management. This evidence highlights the importance of further study of this understudied subgroup of cancers.
Acknowledgments
Grant support: R01CA111914 and K07CA108987 from the National Cancer Institute.
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.American Cancer Society. Cancer facts and figures 2006. Atlanta: American Cancer Society; 2006. [Google Scholar]
- 2.Boyd J, Rubin SC. Hereditary ovarian cancer: molecular genetics and clinical implications. Gynecol Oncol. 1997;64:196–206. doi: 10.1006/gyno.1996.4572. [DOI] [PubMed] [Google Scholar]
- 3.Bewtra C, Watson P, Conway T, Read-Hippee C, Lynch HT. Hereditary ovarian cancer: a clinicopathological study. Int J Gynecol Pathol. 1992;11:180–7. doi: 10.1097/00004347-199207000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Akiyama Y, Sato H, Yamada T, et al. Germ-line mutation of the hMSH6/GTBP gene in an atypical hereditary nonpolyposis colorectal cancer kindred. Cancer Res. 1997;57:3920–3. [PubMed] [Google Scholar]
- 5.Bronner CE, Baker SM, Morrison PT, et al. Mutation in the DNA mismatch repair gene homologue hMLH1 is associated with hereditary non-polyposis colon cancer. Nature. 1994;368:258–61. doi: 10.1038/368258a0. [DOI] [PubMed] [Google Scholar]
- 6.Leach FS, Nicolaides NC, Papadopoulos N, et al. Mutations of a mutS homolog in hereditary nonpolyposis colorectal cancer. Cell. 1993;75:1215–25. doi: 10.1016/0092-8674(93)90330-s. [DOI] [PubMed] [Google Scholar]
- 7.Miyaki M, Konishi M, Tanaka K, et al. Germline mutation of MSH6 as the cause of hereditary nonpolyposis colorectal cancer. Nat Genet. 1997;17:271–2. doi: 10.1038/ng1197-271. [DOI] [PubMed] [Google Scholar]
- 8.Nicolaides NC, Papadopoulos N, Liu B, et al. Mutations of two PMS homologues in hereditary nonpolyposis colon cancer. Nature. 1994;371:75–80. doi: 10.1038/371075a0. [DOI] [PubMed] [Google Scholar]
- 9.Papadopoulos N, Nicolaides NC, Wei YF, et al. Mutation of a mutL homolog in hereditary colon cancer. Science. 1994;263:1625–9. doi: 10.1126/science.8128251. [DOI] [PubMed] [Google Scholar]
- 10.Geisler JP, Goodheart MJ, Sood AK, Holmes RJ, Hatterman-Zogg MA, Buller RE. Mismatch repair gene expression defects contribute to microsatellite instability in ovarian carcinoma. Cancer. 2003;98:2199–206. doi: 10.1002/cncr.11770. [DOI] [PubMed] [Google Scholar]
- 11.Gras E, Catasus L, Arguelles R, et al. Microsatellite instability, MLH-1 promoter hypermethylation, and frameshift mutations at coding mononucleotide repeat microsatellites in ovarian tumors. Cancer. 2001;92:2829–36. doi: 10.1002/1097-0142(20011201)92:11<2829::aid-cncr10094>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 12.Strathdee G, Appleton K, Illand M, et al. Primary ovarian carcinomas display multiple methylator phenotypes involving known tumor suppressor genes. Am J Pathol. 2001;158:1121–7. doi: 10.1016/S0002-9440(10)64059-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crijnen TE, Janssen-Heijnen ML, Gelderblom H, et al. Survival of patients with ovarian cancer due to a mismatch repair defect. Fam Cancer. 2005;4:301–5. doi: 10.1007/s10689-005-6573-2. [DOI] [PubMed] [Google Scholar]
- 14.Massey A, Offman J, Macpherson P, Karran P. DNA mismatch repair and acquired cisplatin resistance in E. coli and human ovarian carcinoma cells DNA. Repair (Amst) 2003;2:73–89. doi: 10.1016/s1568-7864(02)00187-8. [DOI] [PubMed] [Google Scholar]
- 15.Watson P, Butzow R, Lynch HT, et al. The clinical features of ovarian cancer in hereditary nonpolyposis colorectal cancer. Gynecol Oncol. 2001;82:223–8. doi: 10.1006/gyno.2001.6279. [DOI] [PubMed] [Google Scholar]
- 16.Thibodeau SN, Bren G, Schaid D. Microsatellite instability in cancer of the proximal colon. Science. 1993;260:816–9. doi: 10.1126/science.8484122. [DOI] [PubMed] [Google Scholar]
- 17.Parsons R, Li GM, Longley MJ, et al. Hypermutability and mismatch repair deficiency in RER+ tumor cells. Cell. 1993;75:1227–36. doi: 10.1016/0092-8674(93)90331-j. [DOI] [PubMed] [Google Scholar]
- 18.Perucho M. Cancer of the microsatellite mutator phenotype. Biol Chem. 1996;377:675–84. [PubMed] [Google Scholar]
- 19.Umar A, Boyer JC, Thomas DC, et al. Defective mismatch repair in extracts of colorectal and endometrial cancer cell lines exhibiting microsatellite instability. J Biol Chem. 1994;269:14367–70. [PubMed] [Google Scholar]
- 20.Boland CR, Thibodeau SN, Hamilton SR, et al. A National Cancer Institute Workshop on Microsatellite Instability for cancer detection and familial predisposition: development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res. 1998;58:5248–57. [PubMed] [Google Scholar]
- 21.Allen HJ, DiCioccio RA, Hohmann P, Piver MS, Tworek H. Microsatellite instability in ovarian and other pelvic carcinomas. Cancer Genet Cytogenet. 2000;117:163–6. doi: 10.1016/s0165-4608(99)00167-3. [DOI] [PubMed] [Google Scholar]
- 22.Alvi AJ, Rader JS, Broggini M, Latif F, Maher ER. Microsatellite instability and mutational analysis of transforming growth factor β receptor type II gene (TGFBR2) in sporadic ovarian cancer. Mol Pathol. 2001;54:240–3. doi: 10.1136/mp.54.4.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Buller RE, Shahin MS, Holmes RW, Hatterman M, Kirby PA, Sood AK. p53 Mutations and microsatellite instability in ovarian cancer: Yin and yang. Am J Obstet Gynecol. 2001;184:891–902. doi: 10.1067/mob.2001.113856. discussion -3. [DOI] [PubMed] [Google Scholar]
- 24.Codegoni AM, Bertoni F, Colella G, et al. Microsatellite instability and frameshift mutations in genes involved in cell cycle progression or apoptosis in ovarian cancer. Oncol Res. 1999;11:297–301. [PubMed] [Google Scholar]
- 25.Dellas A, Puhl A, Schraml P, et al. Molecular and clinicopathological analysis of ovarian carcinomas with and without microsatellite instability. Anticancer Res. 2004;24:361–9. [PubMed] [Google Scholar]
- 26.Fujita M, Enomoto T, Yoshino K, et al. Microsatellite instability and alterations in the hMSH2 gene in human ovarian cancer. Int J Cancer. 1995;64:361–6. doi: 10.1002/ijc.2910640602. [DOI] [PubMed] [Google Scholar]
- 27.Han HJ, Yanagisawa A, Kato Y, Park JG, Nakamura Y. Genetic instability in pancreatic cancer and poorly differentiated type of gastric cancer. Cancer Res. 1993;53:5087–9. [PubMed] [Google Scholar]
- 28.Iwabuchi H, Sakamoto M, Sakunaga H, et al. Genetic analysis of benign, low-grade, and high-grade ovarian tumors. Cancer Res. 1995;55:6172–80. [PubMed] [Google Scholar]
- 29.King BL, Carcangiu ML, Carter D, et al. Microsatellite instability in ovarian neoplasms. Br J Cancer. 1995;72:376–82. doi: 10.1038/bjc.1995.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kobayashi K, Sagae S, Kudo R, Saito H, Koi S, Nakamura Y. Microsatellite instability in endometrial carcinomas. Genes Chromosomes Cancer. 1995;14:128–32. doi: 10.1002/gcc.2870140207. [DOI] [PubMed] [Google Scholar]
- 31.Krajinovic M, Richer C, Gorska-Flipot I, et al. Genomic loci susceptible to replication errors in cancer cells. Br J Cancer. 1998;78:981–5. doi: 10.1038/bjc.1998.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Osborne RJ, Leech V. Polymerase chain reaction allelotyping of human ovarian cancer. Br J Cancer. 1994;69:429–38. doi: 10.1038/bjc.1994.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shih YC, Kerr J, Hurst TG, Khoo SK, Ward BG, Chenevix-Trench G. No evidence for microsatellite instability from allelotype analysis of benign and low malignant potential ovarian neoplasms. Gynecol Oncol. 1998;69:210–3. doi: 10.1006/gyno.1998.5014. [DOI] [PubMed] [Google Scholar]
- 34.Sood AK, Buller RE. Genomic instability in ovarian cancer: a reassessment using an arbitrarily primed polymerase chain reaction. Oncogene. 1996;13:2499–504. [PubMed] [Google Scholar]
- 35.Sood AK, Holmes R, Hendrix MJ, Buller RE. Application of the National Cancer Institute international criteria for determination of microsatellite instability in ovarian cancer. Cancer Res. 2001;61:4371–4. [PubMed] [Google Scholar]
- 36.Tangir J, Loughride NS, Berkowitz RS, et al. Frequent microsatellite instability in epithelial borderline ovarian tumors. Cancer Res. 1996;56:2501–5. [PubMed] [Google Scholar]
- 37.Sankaranarayanan R, Ferlay J. Worldwide burden of gynaecological cancer: the size of the problem. Best Pract Res Clin Obstet Gynaecol. 2006;20:207–25. doi: 10.1016/j.bpobgyn.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 38.Quirk JT, Natarajan N. Ovarian cancer incidence in the United States, 1992–1999. Gynecol Oncol. 2005;97:519–23. doi: 10.1016/j.ygyno.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 39.Seidman JD, Horkayne-Szakaly I, Haiba M, Boice CR, Kurman RJ, Ronnett BM. The histologic type and stage distribution of ovarian carcinomas of surface epithelial origin. Int J Gynecol Pathol. 2004;23:41–4. doi: 10.1097/01.pgp.0000101080.35393.16. [DOI] [PubMed] [Google Scholar]
- 40.Aarnio M, Sankila R, Pukkala E, et al. Cancer risk in mutation carriers of DNA-mismatch-repair genes. Int J Cancer. 1999;81:214–8. doi: 10.1002/(sici)1097-0215(19990412)81:2<214::aid-ijc8>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 41.Chiaravalli AM, Furlan D, Facco C, et al. Immunohistochemical pattern of hMSH2/hMLH1 in familial and sporadic colorectal, gastric, endometrial and ovarian carcinomas with instability in microsatellite sequences. Virchows Arch. 2001;438:39–48. doi: 10.1007/s004280000325. [DOI] [PubMed] [Google Scholar]
- 42.Domanska K, Malander S, Masback A, Nilbert M. Ovarian cancer at young age: the contribution of mismatch-repair defects in a population-based series of epithelial ovarian cancer before age 40. Int J Gynecol Cancer. 2007;17:789–93. doi: 10.1111/j.1525-1438.2007.00875.x. [DOI] [PubMed] [Google Scholar]
- 43.Ichikawa Y, Lemon SJ, Wang S, et al. Microsatellite instability and expression of MLH1 and MSH2 in normal and malignant endometrial and ovarian epithelium in hereditary nonpolyposis colorectal cancer family members. Cancer Genet Cytogenet. 1999;112:2–8. doi: 10.1016/s0165-4608(98)00252-0. [DOI] [PubMed] [Google Scholar]
- 44.Malander S, Rambech E, Kristoffersson U, et al. The contribution of the hereditary nonpolyposis colorectal cancer syndrome to the development of ovarian cancer. Gynecol Oncol. 2006;101:238–43. doi: 10.1016/j.ygyno.2005.10.029. [DOI] [PubMed] [Google Scholar]
- 45.Rosen DG, Cai KQ, Luthra R, Liu J. Immunohistochemical staining of hMLH1 and hMSH2 reflects microsatellite instability status in ovarian carcinoma. Mod Pathol. 2006;19:1414–20. doi: 10.1038/modpathol.3800672. [DOI] [PubMed] [Google Scholar]
- 46.Stratton JF, Thompson D, Bobrow L, et al. The genetic epidemiology of early-onset epithelial ovarian cancer: a population-based study. Am J Hum Genet. 1999;65:1725–32. doi: 10.1086/302671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Watson P, Lynch HT. Cancer risk in mismatch repair gene mutation carriers. Fam Cancer. 2001;1:57–60. doi: 10.1023/a:1011590617833. [DOI] [PubMed] [Google Scholar]
- 48.Stuart A, Ord J. Kendall’s advanced theory of statistics. 6. London: Edward Arnold; 1994. [Google Scholar]
- 49.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 50.Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–101. [PubMed] [Google Scholar]
- 52.Egger M, Smith DG, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.StatsDirect Ltd. StatsDirect statistical software. England: 2005. [Google Scholar]
- 54.Vandenbroucke JP, von Elm E, Altman DG, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. Epidemiology. 2007;18:805–35. doi: 10.1097/EDE.0b013e3181577511. [DOI] [PubMed] [Google Scholar]
- 55.Moher D, Cook D, Eastwood S, Olkin I, Rennie D, Stroup S. Improving the quality of reports of meta-analyses of randomised controlled trials: the QUOROM statement. Lancet. 1999;354:1896–900. doi: 10.1016/s0140-6736(99)04149-5. [DOI] [PubMed] [Google Scholar]
- 56.Shia J, Ellis NA, Paty PB, et al. Value of histopathology in predicting microsatellite instability in hereditary nonpolyposis colorectal cancer and sporadic colorectal cancer. Am J Surg Pathol. 2003;27:1407–17. doi: 10.1097/00000478-200311000-00002. [DOI] [PubMed] [Google Scholar]
- 57.Broaddus RR, Lynch HT, Chen LM, et al. Pathologic features of endometrial carcinoma associated with HNPCC: a comparison with sporadic endometrial carcinoma. Cancer. 2006;106:87–94. doi: 10.1002/cncr.21560. [DOI] [PubMed] [Google Scholar]
- 58.Chiaffarino F, Parazzini F, Bosetti C, et al. Risk factors for ovarian cancer histotypes. Eur J Cancer. 2007;43:1208–13. doi: 10.1016/j.ejca.2007.01.035. [DOI] [PubMed] [Google Scholar]
- 59.Eltabbakh GH, Natarajan N, Piver MS, Mettlin CJ. Epidemiologic differences between women with borderline ovarian tumors and women with epithelial ovarian cancer. Gynecol Oncol. 1999;74:103–7. doi: 10.1006/gyno.1999.5459. [DOI] [PubMed] [Google Scholar]
- 60.Kurian AW, Balise RR, McGuire V, Whittemore AS. Histologic types of epithelial ovarian cancer: have they different risk factors? Gynecol Oncol. 2005;96:520–30. doi: 10.1016/j.ygyno.2004.10.037. [DOI] [PubMed] [Google Scholar]
- 61.Marchbanks PA, Wilson H, Bastos E, Cramer DW, Schildkraut JM, Peterson HB. Cigarette smoking and epithelial ovarian cancer by histologic type. Obstet Gynecol. 2000;95:255–60. doi: 10.1016/s0029-7844(99)00531-1. [DOI] [PubMed] [Google Scholar]
- 62.Parazzini F, Chiaffarino F, Negri E, et al. Risk factors for different histological types of ovarian cancer. Int J Gynecol Cancer. 2004;14:431–6. doi: 10.1111/j.1048-891x.2004.14302.x. [DOI] [PubMed] [Google Scholar]
- 63.Risch HA, Marrett LD, Jain M, Howe GR. Differences in risk factors for epithelial ovarian cancer by histologic type. Results of a case-control study. Am J Epidemiol. 1996;144:363–72. doi: 10.1093/oxfordjournals.aje.a008937. [DOI] [PubMed] [Google Scholar]
- 64.Tung KH, Goodman MT, Wu AH, et al. Reproductive factors and epithelial ovarian cancer risk by histologic type: a multiethnic case-control study. Am J Epidemiol. 2003;158:629–38. doi: 10.1093/aje/kwg177. [DOI] [PubMed] [Google Scholar]
- 65.Kobayashi H, Sumimoto K, Kitanaka T, et al. Ovarian endometrioma – risks factors of ovarian cancer development. Eur J Obstet Gynecol Reprod Biol. 2008;138(2):187–93. doi: 10.1016/j.ejogrb.2007.06.017. [DOI] [PubMed] [Google Scholar]
- 66.Prowse AH, Manek S, Varma R, et al. Molecular genetic evidence that endometriosis is a precursor of ovarian cancer. Int J Cancer. 2006;119:556–62. doi: 10.1002/ijc.21845. [DOI] [PubMed] [Google Scholar]
- 67.Somigliana E, Vigano P, Parazzini F, Stoppelli S, Giambattista E, Vercellini P. Association between endometriosis and cancer: a comprehensive review and a critical analysis of clinical and epidemiological evidence. Gynecol Oncol. 2006;101:331–41. doi: 10.1016/j.ygyno.2005.11.033. [DOI] [PubMed] [Google Scholar]
- 68.Risch HA. Estrogen replacement therapy and risk of epithelial ovarian cancer. Gynecol Oncol. 1996;63:254–7. doi: 10.1006/gyno.1996.0315. [DOI] [PubMed] [Google Scholar]
- 69.Weiss NS, Lyon JL, Krishnamurthy S, Dietert SE, Liff JM, Daling JR. Noncontraceptive estrogen use and the occurrence of ovarian cancer. J Natl Cancer Inst. 1982;68:95–8. [PubMed] [Google Scholar]
- 70.Modugno F, Ness RB, Cottreau CM. Cigarette smoking and the risk of mucinous and nonmucinous epithelial ovarian cancer. Epidemiology. 2002;13:467–71. doi: 10.1097/00001648-200207000-00016. [DOI] [PubMed] [Google Scholar]
- 71.Breivik J, Lothe RA, Meling GI, Rognum TO, Borresen-Dale AL, Gaudernack G. Different genetic pathways to proximal and distal colorectal cancer influenced by sex-related factors. Int J Cancer. 1997;74:664–9. doi: 10.1002/(sici)1097-0215(19971219)74:6<664::aid-ijc18>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 72.Slattery ML, Curtin K, Anderson K, et al. Associations between cigarette smoking, lifestyle factors, and microsatellite instability in colon tumors. J Natl Cancer Inst. 2000;92:1831–6. doi: 10.1093/jnci/92.22.1831. [DOI] [PubMed] [Google Scholar]
- 73.Slattery ML, Potter JD, Curtin K, et al. Estrogens reduce and withdrawal of estrogens increase risk of microsatellite instability-positive colon cancer. Cancer Res. 2001;61:126–30. [PubMed] [Google Scholar]
- 74.Yang P, Cunningham JM, Halling KC, et al. Higher risk of mismatch repair-deficient colorectal cancer in α(1)-antitrypsin deficiency carriers and cigarette smokers. Mol Genet Metab. 2000;71:639–45. doi: 10.1006/mgme.2000.3089. [DOI] [PubMed] [Google Scholar]
- 75.Wu AH, Shibata D, Yu MC, Lai MY, Ross RK. Dietary heterocyclic amines and microsatellite instability in colon adenocarcinomas. Carcinogenesis. 2001;22:1681–4. doi: 10.1093/carcin/22.10.1681. [DOI] [PubMed] [Google Scholar]
- 76.Diergaarde B, Vrieling A, van Kraats AA, van Muijen GN, Kok FJ, Kampman E. Cigarette smoking and genetic alterations in sporadic colon carcinomas. Carcinogenesis. 2003;24:565–71. doi: 10.1093/carcin/24.3.565. [DOI] [PubMed] [Google Scholar]
- 77.Farmer ER, Gonin R, Hanna MP. Discordance in the histopathologic diagnosis of melanoma and melanocytic nevi between expert pathologists. Hum Pathol. 1996;27:528–31. doi: 10.1016/s0046-8177(96)90157-4. [DOI] [PubMed] [Google Scholar]
- 78.Terry MB, Neugut AI, Bostick RM, Potter JD, Haile RW, Fenoglio-Preiser CM. Reliability in the classification of advanced colorectal adenomas. Cancer Epidemiol Biomarkers Prev. 2002;11:660–3. [PubMed] [Google Scholar]
- 79.Einarson TR. Pharmacoeconomic applications of meta-analysis for single groups using antifungal onychomycosis lacquers as an example. Clin Ther. 1997;19:559–69. doi: 10.1016/s0149-2918(97)80140-3. discussion 38–9. [DOI] [PubMed] [Google Scholar]
- 80.Sheehe PR. Combination of log relative risk in retrospective studies of disease. Am J Public Health Nations Health. 1966;56:1745–50. doi: 10.2105/ajph.56.10.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Elsaleh H, Joseph D, Grieu F, Zeps N, Spry N, Iacopetta B. Association of tumour site and sex with survival benefit from adjuvant chemotherapy in colorectal cancer. Lancet. 2000;355:1745–50. doi: 10.1016/S0140-6736(00)02261-3. [DOI] [PubMed] [Google Scholar]
- 82.Gryfe R, Kim H, Hsieh ET, et al. Tumor microsatellite instability and clinical outcome in young patients with colorectal cancer. N Engl J Med. 2000;342:69–77. doi: 10.1056/NEJM200001133420201. [DOI] [PubMed] [Google Scholar]
- 83.Ribic CM, Sargent DJ, Moore MJ, et al. Tumor microsatellite-instability status as a predictor of benefit from fluorouracil-based adjuvant chemotherapy for colon cancer. N Engl J Med. 2003;349:247–57. doi: 10.1056/NEJMoa022289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Warusavitarne J, Schnitzler M. The role of chemotherapy in microsatellite unstable (MSI-H) colorectal cancer. Int J Colorectal Dis. 2007;22:739–48. doi: 10.1007/s00384-006-0228-0. [DOI] [PubMed] [Google Scholar]
- 85.Watanabe T, Wu TT, Catalano PJ, et al. Molecular predictors of survival after adjuvant chemotherapy for colon cancer. N Engl J Med. 2001;344:1196–206. doi: 10.1056/NEJM200104193441603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Aebi S, Kurdi-Haidar B, Gordon R, et al. Loss of DNA mismatch repair in acquired resistance to cisplatin. Cancer Res. 1996;56:3087–90. [PubMed] [Google Scholar]
- 87.Brown R, Hirst GL, Gallagher WM, et al. hMLH1 expression and cellular responses of ovarian tumour cells to treatment with cytotoxic anticancer agents. Oncogene. 1997;15:45–52. doi: 10.1038/sj.onc.1201167. [DOI] [PubMed] [Google Scholar]
- 88.Marcelis CL, Van Der Putten HW, Tops C, Lutgens LC, Moog U. Chemotherapy resistant ovarian cancer in carriers of an hMSH2 mutation? Fam Cancer. 2001;1:109–11. doi: 10.1023/a:1013865323890. [DOI] [PubMed] [Google Scholar]
- 89.Scartozzi M, De Nictolis M, Galizia E, et al. Loss of hMLH1 expression correlates with improved survival in stage III–IV ovarian cancer patients. Eur J Cancer. 2003;39:1144–9. doi: 10.1016/s0959-8049(03)00197-7. [DOI] [PubMed] [Google Scholar]
- 90.Strathdee G, MacKean MJ, Illand M, Brown R. A role for methylation of the hMLH1 promoter in loss of hMLH1 expression and drug resistance in ovarian cancer. Oncogene. 1999;18:2335–41. doi: 10.1038/sj.onc.1202540. [DOI] [PubMed] [Google Scholar]