Abstract
Purpose
To investigate the relationship between blood pressure (BP) parameters in the habitual position and glaucomatous damage at initial presentation in patients with untreated normal tension glaucoma (NTG).
Methods
Fifty-four eyes from 54 subjects diagnosed with NTG were consecutively enrolled. BP was measured with an automated ambulatory monitoring device in the habitual position during 24-hour in-hospitalization. Patients were classified into three groups: non-dippers, dippers, and over-dippers. corresponded to the degree of reduction in their nocturnal mean arterial pressure (MAP) compared with their diurnal MAP. Regression models were used to evaluate potential risk factors, including: age, pre-admission office intraocular pressure (IOP), central corneal thickness (CCT), and BP parameters. Functional outcome variables for glaucomatous damage included mean deviation (MD) and pattern standard deviation (PSD) on a Humphrey field analyzer (HFA). Anatomic outcome variables were TSNIT score (temporal, superior, nasal, inferior, and temporal) average, superior average, inferior average, and nerve fiber indicator (NFI) score on scanning laser polarimetry with variable corneal compensation (SLP-VCC; GDx-VCC).
Results
Marked systolic blood pressure (SBP), diastolic blood pressure (DBP), and MAP fluctuation were noted in the over-dipper group (p<0.05). A linear regression analysis model revealed that nocturnal trough DBP and MAP, average nocturnal SBP, and MAP were all significantly associated with a decreased average TSNIT score and an increased NFI score.
Conclusions
Nocturnal BP reduction estimated in the habitual position was associated with structural damage in eyes with NTG. This finding may suggest systemic vascular etiology of NTG development associated with nocturnal BP reduction.
Keywords: Blood pressure, Habitual position, Normal tension glaucoma, 24-hour
Various articles have shown that abnormal ocular blood flow (OBF) is a risk factor for glaucoma.1-5 It has also been suggested that a loss of autoregulation of OBF may be present in primary open angle glaucoma (POAG).6
In normal tension glaucoma (NTG), vascular risk factors include: migraine, blood transfusion, Raynaud's phenomenon, and excessive nocturnal blood pressure (BP) reduction.7-10 Nocturnal BP reduction is caused by a reduction in sympathetic activity during the night because of a lower level of circulating catecholamine hormones, which can in turn lead to a decrease in heart rate, cardiac output, and peripheral resistance. Patients with NTG have been reported to show a significantly greater reduction in nocturnal BP compared to healthy subjects11,12; a greater reduction in nocturnal BP was thought to induce more rapid progression of glaucoma.13,14
In glaucoma patients, both systolic blood pressure (SBP) and diastolic blood pressure (DBP) were shown to be more dynamic and volatile during a 24-hour period compared to healthy subjects, especially in the nocturnal period in the habitual position.11,13-16 Therefore, the dynamic change of BP in these glaucomatous eyes might further lead to an unexpected alteration of 24-hour OBF. We recently demonstrated that the instability of this 24-hour ocular perfusion pressure (OPP) during the nocturnal period might be a risk factor for NTG.17,18 However, all BP measurements in those studies were performed in the sitting position. Some may contend that those nonphysiologic BP measurements recorded in the sitting position during the nocturnal period could lead to a biased conclusion. In the present study we investigated the association of the anatomical (scanning laser polarimeter measurements of retinal nerve fiber layer (RNFL) thickness) and functional (HFA parameters) outcomes with the alteration of BP in the habitual position during 24-hour in-hospitalization in eyes with NTG.
To elucidate the mechanism of glaucomatous damage in eyes with NTG and the role of BP measured in the habitual position, we prospectively investigated the 24-hour change of systemic BP by the degree of nocturnal BP reduction relative to diurnal BP. We also evaluated the circadian pattern, peak and trough levels in BP, and mean arterial pressure (MAP) in the habitual position during 24 hours in a group of patients with newly diagnosed NTG. Finally, we evaluated predictor variables of systemic, ocular, and hemodynamic risk factors for advanced glaucomatous damage as detected by the functional and anatomic outcome variables.
Methods
Patients
We performed a prospective evaluation of 54 eyes from 54 consecutive patients (18 men and 36 women) initially diagnosed with NTG (mean age±SD: 59.7±11.2 years), each of whom was seen by a glaucoma specialist (M.S.K.) during the period from February 2007 to January 2008.
All patients diagnosed with NTG on the basis of clinical evaluation and visual field (VF) examination at our glaucoma clinic had undergone in-hospital 24-hour monitoring of BP, as described below. Patients were eligible for the study if their optic nerve had a glaucomatous appearance, including diffuse or focal neural rim thinning, hemorrhage, enlarged cupping or nerve fiber layer defects indicative of glaucoma, in addition to corresponding VF loss; best-corrected visual acuity greater than 20/40; maximum IOP less than 22 mmHg on multiple measurements using Goldmann applanation tonometry (GAT) in the office; normal anterior chamber and openangle on slit-lamp and gonioscopic examination; and glaucomatous VF damage. Patients with evidence of intracranial or otolaryngological lesions, a history of massive hemorrhage or hemodynamic crisis, previous or current use of anti-glaucoma medications, any other ophthalmic disease that could result in VF defects, or a history of diabetes mellitus were excluded. However, patients on anti-hypertensive or other hemodynamically active medications were not excluded.
The central corneal thickness (CCT) of each patient was measured three times using ultrasonic pachymetry (DGH-550, DGH Technology Inc., Exton, PA, USA) at the first visit and the average was calculated. Scanning laser polarimetry (SLP) examination at the initial visit was performed using a commercial GDx-VCC system (software version 5.5.0; Carl Zeiss Meditec, Dublin, CA, USA). The affected eye was selected in patients with unilateral disease if both eyes of a patient showed NTG and met the inclusion criteria, one eye was randomly selected. All procedures conformed to the Declaration of Helsinki and the study was approved by the ethics committee of the Asan medical center. Informed consent was obtained from all participants.
Visual Field Examination
VF examinations were performed with the 24-2 full threshold program or 24-2 Swedish Interactive Thresholding Algorithm (SITA) standard program on the HFA (Carl-Zeiss Meditec, Dublin, CA, USA). Eyes with glaucomatous VF defects were defined as those that met two of the following criteria: (1) a cluster of three points with a probability of less than 5% on a pattern deviation map in at least one hemifield and including at least one point with a probability of less than 1%; or a cluster of two points with a probability of less than 1%; (2) glaucoma hemifield test (GHT) outside 99% of age-specific normal limits; and (3) pattern standard deviation (PSD) outside 95% of the normal limit. We included only those patients who had a reliable VF within 1 month of initial evaluation, defined as a false-positive error <15%, a false-negative error <15%, and a fixation loss <20%. VF data for analysis included mean deviation (MD) and PSD.
Scanning Laser Polarimetry (SLP) Examination of the Retinal Nerve Fiber Layer (RNFL)
SLP imaging was performed in a standardized fashion (GDx-VCC software version 5.5.0) with a circular scan (3.2 mm diameter) centered on the optic disc, as previously described.19 The general principles of SLP with variable corneal polarization compensation (VCC) have been described in detail elsewhere.20,21 Only eyes with a scan quality score of 8 or better were analyzed. Images with atypical retardation patterns (ARP) were excluded. An atypical scan was defined as one that contained atypical and flower-like patterns of elevated retardation values that did not match the expected retardation distribution based on RNFL anatomy and could generate spurious RNFL thickness measurements. The SLP parameters examined were TSNIT (temporal, superior, nasal, inferior, and temporal) average, superior average, inferior average, and nerve fiber indicator (NFI).
Measurement of Systemic Blood Pressure
Twenty-four hour automated ambulatory BP monitoring was performed in all subjects included in this prospective study using a Space Labs ambulatory BP monitor (Space Labs Medical Inc., Redmond, WA, USA). The automated device was used to minimize the variability between BP examiners and to measure physiological BP while patients performed routine activities. SBP, DBP, MAP, and heart rate were obtained every 20 minutes during the day and night. The average values of each time were calculated in the habitual position at 8 AM, 10 AM, 12 PM, 2 PM, 4 PM, 6 PM, 8 PM, 10 PM, 12 AM, 3 AM, and 6 AM. During daytime hours (8 AM to 10 PM) patients stayed indoors and were encouraged to continue normal indoor activities. During nighttime, the patients' rooms were kept dark for a 6-hour period and patients were instructed to sleep in the supine position. Patients were asked to refrain from any physical activities that could affect BP. Meals were provided at 7:30 AM, 12:00 PM, and 6:30 PM, and did not include any alcohol or caffeine.
Calculation of Systemic Hemodynamic Parameters
Twenty-four hour SBP and DBP peak and trough values were defined as the highest and lowest SBP and DBP values recorded during the 24-hour period. SBP and DBP peak and trough values for daytime and nighttime were entered independently in the analysis. MAP was calculated for a specified time (daytime, nighttime, and 24-hour) according to the formula:
MAP=DBP+[1/3×(SBP-DBP)].
Definitions of Non-dippers, Dippers, and Over-dippers
Nocturnal BP reduction was calculated as:
[(diurnal averaged MAP-nocturnal lowest MAP)/diurnal averaged MAP]×100
Patients were then classified into three groups based on the degree of nocturnal BP reduction as follows: non-dippers, <5% nocturnal BP reduction (or higher nocturnal BP than diurnal BP) (n=16); dippers, ≥5% but <10% reduction (n=11); and over-dippers, ≥10% reduction (n=27).22
Statistical Analysis
All statistical tests were performed using a statistical software package (SPSS 15.0 software for Windows; SPSS Inc., Chicago, IL, USA).
Each variable was assessed individually for its relationship to the degree of damage from glaucoma. Predictor variables for analysis included age, pre-admission office IOP, and CCT. Hemodynamic predictor variables included 24-hour, day, and nocturnal averaged MAP. MAP peak and trough day, night, and 24-hour values that were used as predictor variables were included in the analysis. The functional outcome variables were MD and PSD of HFA. The anatomical outcome variables were TSNIT average, superior average, inferior average, and NFI obtained by GDx-VCC. Initially, the relationships between predictor variables and outcome variables were assessed by univariate linear regression models. Differences with a value of p<0.05 were considered statistically significant.
Results
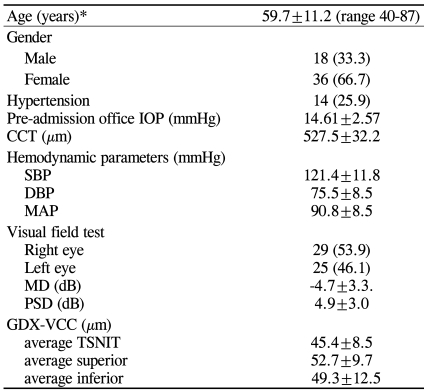
All 54 patients were Asian; 18 (33.3%) were men and 36 (66.7%) were women. The mean age was 59.7±11.2 years (range, 40-87 years) and the mean pre-admission office IOP was 14.61±2.57 mmHg. Fourteen (25.9%) of the patients had a history of hypertension and were taking antihypertensive medications. The mean (± SD) duration of antihypertensive therapy was 9.4±7.78 years. The mean corrected visual acuity in the LogMAR scale was 0.17±0.37; spherical equivalent (SE) was -0.89±2.57 diopters; and CCT was 527.5±32.2 µm. The twenty-four hour average SBP was 121.4±11.8 mmHg, DBP was 75.5±8.5 mmHg, and MAP was 90.8±8.5 mmHg. The average MD and PSD on HFA were -4.7±3.3 dB and 4.9±3.0 dB, respectively. The mean TSNIT average, superior average, and inferior average scores on SLP examination were 45.4± 8.5, 52.7±9.7, and 49.3±12.5 µm, respectively. Table 1 summarizes the overall descriptive statistics for the demographic background variables.
Table 1.
Patient demographic and background variables
CCT=central corneal thickness; SBP=systolic blood pressure; DBP=diastolic blood pressure; MAP=mean arterial pressure; MD=mean deviation; PSD=pattern standard deviation.
*Data are expressed as number (percentage) of subjects or mean±SD; N=54.
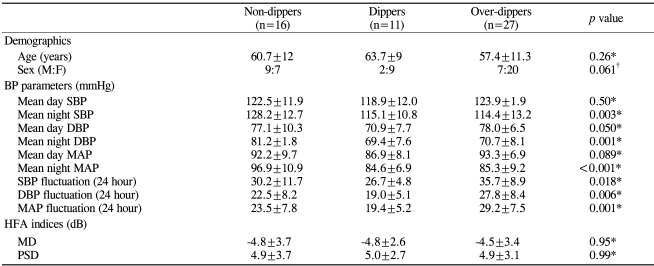
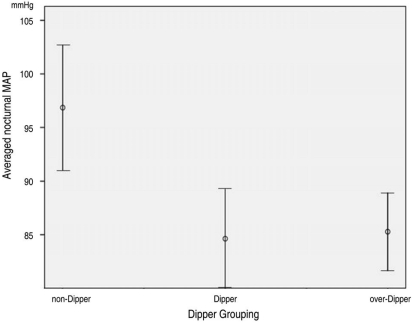
Table 2 presents the patient demographics and BP parameters of the three groups (non-dippers, dippers, and over-dippers) classified by the degree of nocturnal BP reduction. The distribution of age did not differ among the groups (p>0.05, oneway ANOVA), nor were there differences in daytime mean SBP or MAP (p>0.05). However, the mean nocturnal SBP, DBP, MAP, and fluctuation of the SBP, DBP, and MAP were all significantly different among the groups (p<0.05). The range of nocturnal mean MAP in dippers, over-dippers, and non-dippers is shown in Figure 1. HFA indices (MD, PSD) at initial diagnosis of NTG did not differ among the groups (p>0.05).
Table 2.
Patient demographics and parameters of non-dippers, dippers, and over-dippers
IOP=intraocular pressure; BP=blood pressure; SBP=systolic BP; DBP=diastolic BP; MAP=mean arterial pressure.
Data are expressed as the mean±SD and all data were recorded over a 24-hour period.
Fluctuation was defined as: (peak value-trough value).
*One-way ANOVA; †Chi-square test
Fig. 1.
Nocturnal mean MAP comparison among dippers, over-dippers, and non-dippers (95% confidence interval). p<0.001, one-way ANOVA. MAP=mean arterial pressure; ANOVA=analysis of variance.
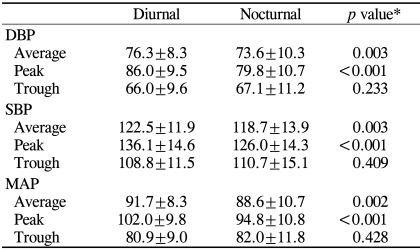
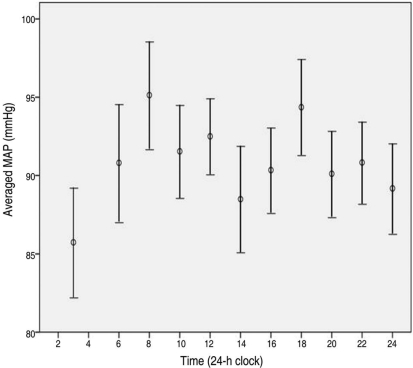
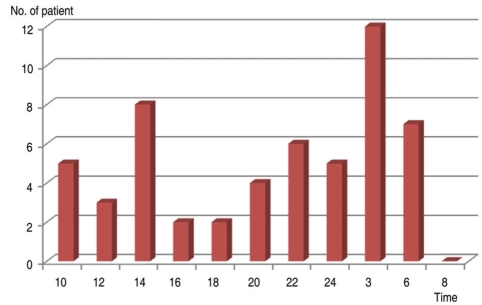
Table 3 summarizes diurnal and nocturnal values of various vascular parameters in our series of NTG patients. Mean SBP, DBP, and MAP showed statistically significant differences between the diurnal and nocturnal periods. Peak SBP, DBP, and MAP also showed the same results. The fluctuation of MAP over 24 hours is shown in Figure 2. Although many patients showed a DBP drop during the nocturnal period, some patients showed a DBP reduction during the diurnal period (Fig. 3).
Table 3.
Comparison of diurnal and nocturnal BP parameters (mmHg)
BP=blood pressure; SBP=systolic BP; DBP=diastolic BP; MAP=mean arterial pressure.
N=54. *paired sample t-test
Fig. 2.
Twenty-four hour variation of MAP (95% confidence interval). MAP=mean arterial pressure.
Fig. 3.
Time of DBP trough. DBP=diastolic blood pressure.
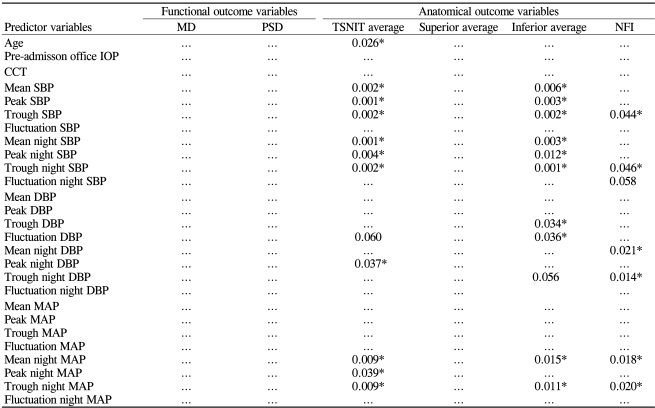
Table 4 presents the results of linear regression analysis between the independent predictor variables and the outcome variables in a univariate model. Trough SBP, trough night SBP, mean night MAP, and trough night MAP showed significant association with TSNIT average, inferior average, and NFI scores assessed by GDx-VCC.
Table 4.
Significance of independent variables (24 hr SBP, DBP, MAP and nighttime SBP, DBP, and MAP parameters) in predicting outcomes of glaucoma: univariate models
MD=mean deviation; PSD=pattern standard deviation; TSNIT=temporal, superior, nasal, inferior, and temporal; NFI=nerve fiber indicator; SE=spherical equivalent; CCT=central corneal thickness; IOP=intraocular pressure; MAP=mean arterial pressure.
*p<0.05; ellipses, not significant (p>0.05)
Discussion
To our knowledge, this is the first report addressing the association between BP and anatomical (GDx-VCC) and functional (VF test) outcomes during a 24-hour period in the habitual position in glaucomatous eyes.
Nocturnal BP depression ('dip') has been reported to be increased in NTG and POAG.23-27 Nocturnal BP drops have been postulated to lead to decreased ocular perfusion and thus to cause optic nerve ischemia in glaucoma.28-32 Of 54 patients with NTG, 27 (50.0%) were classified as over-dippers in our series. The prevalence of over-dippers in the glaucomatous population has not been accurately established because the definition of nocturnal BP reduction varies in the literature and few studies have been performed on glaucomatous subjects. It has been reported that blunted BP and heart rate modulation were frequently observed in NTG subjects.33 Yazici et al.34 found that excessive and repetitive nocturnal BP drops occur more frequently in some patients with NTG compared to patients with high-tension glaucoma (HTG) and ocular hypertension (OHT). In our study, we found 50% of the NTG patients experienced an excessive nighttime 'dip' in BP with ambulatory BP monitoring. Choi et el reported that 41% of NTG patients demonstrated the over-dipper pattern.18 Since the definitions of dippers/over-dippers varies among studies, direct comparison of the percentage of dipper/over-dippers in our study with previous reports might be difficult. However, we confirmed that half of the participants in our NTG study were categorized as over-dippers. This finding may reflect vascular instability as an etiology of NTG.
Previous studies have shown that glaucoma patients exhibited faulty autoregulation of OBF during posture change.35,36 Given this, we performed two separate analyses to determine which, if any, of the measured independent hemodynamic variables were related significantly to glaucomatous damage, in one case during the diurnal period and in the other case during the nocturnal period. Regression analyses revealed that some of the BP parameters measured in the habitual position showed a correlation with structural glaucomatous damage assessed by GDx-VCC. The trough SBP, trough night SBP, mean night DBP, trough night DBP, mean night MAP, and trough night MAP all had a significant relationship with the NFI score. According to our results, a relatively greater number of nocturnal parameters than diurnal parameters were related with structural outcome variables. Since nocturnal BP is expected to vary less than diurnal BP, in some eyes a relative drop of MAP due to excessive BP drop at night may induce OPP below the physiologically required level for certain durations in a repetitive manner. Therefore, these findings may again highlight the importance of unstable circulating blood pressure as a prognostic factor involving an altered circulation at the optic disc and support the aforementioned hypothesis.
Recently, low systolic perfusion pressure and low SBP have emerged as new predictors of glaucoma progression in eyes with both POAG and NTG according to an EMGT report looking at predictors of long-term progression in glaucoma.37 These results are very similar to the findings of our study (Table 4).
The crucial finding of the present study was that lower nighttime BP or MAP were associated with worse glaucomatous damage in patients with NTG. This link between low nocturnal perfusion pressure and glaucomatous damage might be compatible with the findings of previous studies indicating abnormal OBF autoregulation in patients with NTG, based on experiments testing short-term changes of OPP.35,36,38 During changes in posture, glaucoma patients exhibited an abnormal response in blood velocity in the central retinal artery.36 A significant association was also observed between optic nerve hemodynamic parameters and MAP in the glaucoma patients. Fuchsjager-Mayrl et al. reported that MAP was a determinant of rim and cup blood flow in patients with POAG or OHT, but not in healthy control subjects.38 An abnormal association between optic nerve head blood flow, as assessed with laser Doppler flowmetry and systemic BP, was also reported by Grunwald et al.39
Feke et al. found that in some OAG patients, the arterial diameter remained essentially unchanged after 31 minutes in the reclining position. However, the average blood speed and blood flow rate increased by approximately 60% as a result of absence of retinal blood flow autoregulation.35 One could speculate that patients with NTG should initially experience a relative increase in OBF due to posture changes at night while in the supine position. If we assume that NTG patients have faulty or decompensated autoregulation, this sudden change of OBF should be followed by repeated OPP drops associated with excessive BP 'dips' during the sleep period. As shown in Figure 1, over-dippers and dippers had a significantly lower nocturnal MAP than did non-dippers. This abnormal blood flow autoregulation, which occurs at night in dippers and over-dippers, may be a contributing factor to the pathogenesis of glaucomatous optic neuropathy.
Recently, a high percentage of NTG patients were found to exhibit an Alzheimer's disease (AD)-like cerebral perfusion pattern, although none were clinically diagnosed with AD. NTG patients with this AD-like pattern showed a more rapid progression of visual field defects than those with a normal perfusion pattern.40 These findings suggest that NTG and AD may have a common pathologic mechanism. Altered circadian BP rhythm is associated with cerebral blood flow change, resulting in cerebrovascular damage.41-44 Patients with nocturnal hypotension that leads to OPP reduction may also be at an increased risk for developing NTG, as noted in our series. Excessive free radicals derived from ischemia and reperfusion may contribute to reversible or irreversible manifestations of cell injury.45,46 Thus, chronic repetitive circadian OBF variations could result in cumulative ischemia and reperfusion effects, manifesting as RNFL damage.
The fact that our study was designed as a cross-sectional study and included a relatively small number of subjects could be a limitation. However, our finding that nocturnal BP and MAP parameters were associated with glaucomatous structural outcome variables coincided with previous findings and supports our hypothesis that ischemic insults resulting from nocturnal BP drops may play an role in glaucoma development. This hypothesis warrants longitudinal study, including a larger sample size. A second limitation of our study was our inability to generalize our findings to all types of primary open angle glaucoma classified by IOP level, since 24-hour BP monitoring was only performed in patients with IOP <21 mmHg based on GAT. Our patient group consisted of NTG patients based on initial diagnosis at the clinic. Third, in this article we did not monitor 24-hour IOP parameters in the habitual position. Thus, IOP factors could have potentially confounded nocturnal OPP reduction. A previous study revealed that a nocturnal elevation of IOP can be detected in healthy young adults in both the sitting and supine position.47 In our case series many patients showed nocturnal BP drops, but others also showed diurnal trough BP drops (Fig. 3) If BP drop was the sole cause of glaucoma development, there might be a strong correlation between glaucomatous outcome variables and 24-hour trough DBP rather than nocturnal trough DBP. However, our results were more complex. Some other factors may exist which accelerate structural glaucomatous damage at night more than during the day. Nocturnal IOP elevation may be one of the factors that leads to a decrease in OPP and results in ischemic nerve fiber damage. Fourth, our study investigated the relationship between BP parameters and the status of glaucomatous damage in a cross-sectional fashion; we therefore did not longitudinally observe the progression of glaucomatous damage. Rather, our data only reflect the findings at the initial examination. There were no significant correlations between functional outcomes assessed by HFA and BP parameters, while anatomical outcomes (GDx-VCC) showed correlations. We cannot provide an appropriate explanation for this result at this moment. However, functional and structural damage has been reported to occur in different time frames, therefore this relationship needs to be investigated in longitudinal studies.
In conclusion, we found that the nocturnal BP and MAP level measured in the habitual position were correlated with structural parameters of NTG. These findings suggest that relatively large reductions in nocturnal BP may lead to chronic ischemic/reperfusion insults. These cumulative injuries to the ocular tissue may influence the retinal nerve fiber layer thickness and the severity of glaucoma. Future longitudinal studies, including evaluation of systemic and ocular parameters under physiologic conditions, are warranted to study the relationship between OPP and glaucoma progression and development.
Footnotes
The authors have no proprietary interest and financial support in the development or marketing of instruments or equipment mentioned in this article, or any competing instruments or equipment.
References
- 1.Hayeh SS. The optic nerve circulation in health and disease. Exp Eye Res. 1995;61:259–272. doi: 10.1016/s0014-4835(05)80121-6. [DOI] [PubMed] [Google Scholar]
- 2.Flammer J, Orgul S. Optic nerve blood-flow abnormalities in glaucoma. Prog Retin Eye Res. 1998;17:267–289. doi: 10.1016/s1350-9462(97)00006-2. [DOI] [PubMed] [Google Scholar]
- 3.Bresson-Dumont H, Bechetoille A. [Role of arterial blood pressure in the development of glaucomatous lesions] J Fr Ophtalmol. 1996;19:435–442. [PubMed] [Google Scholar]
- 4.Cioffi GA, Sullivan P. The effect of chronic ischemia on the primate optic nerve. Eur J Ophthalmol. 1999;9(suppl 1):S34–S36. doi: 10.1177/112067219900901S12. [DOI] [PubMed] [Google Scholar]
- 5.Costa VP, Harris A, Stefansson E, et al. The effects of antiglaucoma and systemic medications on ocular blood flow. Prog Retin Eye Res. 2003;22:769–805. doi: 10.1016/s1350-9462(03)00064-8. [DOI] [PubMed] [Google Scholar]
- 6.Hafez AS, Bizzarro R, Descovich D, Lesk MR. Correlation between finger blood flow and changes in optic nerve head blood flow following therapeutic intraocular pressure reduction. J Glaucoma. 2005;14:448–454. doi: 10.1097/01.ijg.0000185433.71031.90. [DOI] [PubMed] [Google Scholar]
- 7.Phelps CD, Corbett JJ. Migraine and low-tension glaucoma. A case-control study. Invest Ophthalmol vis Sci. 1985;26:1105–1108. [PubMed] [Google Scholar]
- 8.Morgan RW, Drance SM. Chronic open-angle glaucoma and ocular hypertension. An epidemiological study. Br J Ophthalmol. 1975;59:211–215. doi: 10.1136/bjo.59.4.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Broadway DC, Drance SM. Glaucoma and vasospasm. Br J Ophthalmol. 1998;82:862–870. doi: 10.1136/bjo.82.8.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shimada K, Kawamoto A, Matsubayashi K, et al. Diurnal blood pressure variations and silent cerebrovascular damage in elderly patients with hypertension. J Hypertens. 1992;10:875–878. [PubMed] [Google Scholar]
- 11.Meyer JH, Brandi-Dohrn J, Funk J. Twenty four hour blood pressure monitoring in normal tension glaucoma. Br J Ophthalmol. 1996;80:864–867. doi: 10.1136/bjo.80.10.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bonomi L, Marchini G, Marraffa M, et al. Vascular risk factors for primary open angle glaucoma: the Egna-Neumarkt Study. Ophthalmology. 2000;107:1287–1293. doi: 10.1016/s0161-6420(00)00138-x. [DOI] [PubMed] [Google Scholar]
- 13.Collignon N, Dewe W, Guillaume S, Collignon-Brach J. Ambulatory blood pressure monitoring in glaucoma patients. The nocturnal systolic dip and its relationship with disease progression. Int Ophthalmol. 1998;22:19–25. doi: 10.1023/a:1006113109864. [DOI] [PubMed] [Google Scholar]
- 14.Graham SL, Drance SM. Nocturnal hypotension: role in glaucoma progression. Surv Ophthalmol. 1999;43(Suppl 1):S10–S16. doi: 10.1016/s0039-6257(99)00016-8. [DOI] [PubMed] [Google Scholar]
- 15.Liu JH. Diurnal measurement of intraocular pressure. J Glaucoma. 2001;10:S39–S41. doi: 10.1097/00061198-200110001-00015. [DOI] [PubMed] [Google Scholar]
- 16.Tsukahara S, Sasaki T. Postural change of IOP in normal persons and in patients with primary wide open-angle glaucoma and low-tension glaucoma. Br J Ophthalmol. 1984;68:389–392. doi: 10.1136/bjo.68.6.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Choi J, Jeong J, Cho HS, Kook MS. Effect of nocturnal blood pressure reduction on circadian fluctuation of mean ocular perfusion pressure: a risk factor for normal tension glaucoma. Invest Ophthalmol Vis Sci. 2006;47:831–836. doi: 10.1167/iovs.05-1053. [DOI] [PubMed] [Google Scholar]
- 18.Choi J, Kim KH, Jeong J, et al. Circadian Fluctuation of Mean zOcular Perfusion Pressure Is a Consistent Risk Factor for Normal-Tension Glaucoma. Invest Ophthalmol Vis Sci. 2007;48:104–111. doi: 10.1167/iovs.06-0615. [DOI] [PubMed] [Google Scholar]
- 19.Kook MS, Cho HS, Seong M, Choi J. Scanning laser polarimetry using variable corneal compensation in the detection of glaucoma with localized visual field defects. Ophthalmology. 2005;112:1970–1978. doi: 10.1016/j.ophtha.2005.06.023. [DOI] [PubMed] [Google Scholar]
- 20.Zhou Q, Weinreb RN. Individualized compensation of anterior segment birefringence during scanning laser polarimetry. Invest Ophthalmol Vis Sci. 2002;43:2221–2228. [PubMed] [Google Scholar]
- 21.Weinreb RN, Bowd C, Zangwill LM. Glaucoma detection using scanninglaser polarimetry with variable corneal polarization compensation. Arch Ophthalmol. 2003;121:218–224. doi: 10.1001/archopht.121.2.218. [DOI] [PubMed] [Google Scholar]
- 22.Verdecchia P, Schillaci G, Porcellati S. Dippers versus non dippers. J Hypertens. 1991;9:S42–S44. [PubMed] [Google Scholar]
- 23.Hayreh SS, Podhajsky P, Zimmerman MB. Role of nocturnal arterial hypotension in optic nerve head ischemic disorders. Ophthalmologica. 1999;213:76–96. doi: 10.1159/000027399. [DOI] [PubMed] [Google Scholar]
- 24.Jampol LM, Board RJ, Maumenee AE. Systemic hypotension and glaucomatous changes. Am J Ophthalmol. 1978;85:154–159. doi: 10.1016/s0002-9394(14)75941-0. [DOI] [PubMed] [Google Scholar]
- 25.Meyer JH, Brandi-Dohrn J, Funk J. Twenty four hour blood pressure monitoring in normal tension glaucoma. Br J Ophthalmol. 1996;80:864–867. doi: 10.1136/bjo.80.10.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pannarale G, Pannarale L, Arrico L, et al. Ambulatory blood pressure in patients with glaucoma. Invest Ophthalmol Vis Sci. 1996;37:S30. [Google Scholar]
- 27.Graham SL, Drance SM. Nocturnal hypotension: role in glaucoma progression. Surv Ophthalmol. 1999;43(Suppl 1):S10–S16. doi: 10.1016/s0039-6257(99)00016-8. [DOI] [PubMed] [Google Scholar]
- 28.Gramer E, Tausch M. The risk profile of the glaucomatous patient. Curr Opin Ophthalmol. 1995;6:78–88. doi: 10.1097/00055735-199504000-00013. [DOI] [PubMed] [Google Scholar]
- 29.Berglund G. Goals of antihypertensive therapy, is there a point beyond which pressure reduction is dangerous? Am J Hypertens. 1989;2:586–593. doi: 10.1093/ajh/2.7.586. [DOI] [PubMed] [Google Scholar]
- 30.Criuckshank JM, thorp JM, Zacharias FJ. Benefits and potential harm of lowering high blood pressure. Lancet. 1987;1(8533):581–583. doi: 10.1016/s0140-6736(87)90231-5. [DOI] [PubMed] [Google Scholar]
- 31.Farnett L, Mulrow CD, Linn WD, et al. The J-curve phenomenon and the treatment of hypertension, is there a point beyond which pressre reduction is dangerous? JAMA. 1991;265:489–495. [PubMed] [Google Scholar]
- 32.Rouhiainen HJ, Terasvorta ME. Hemodynamic variables in progressive and non-progressive low tension glaucoma. Acta Ophthalmol. 1990;68:34–36. doi: 10.1111/j.1755-3768.1990.tb01646.x. [DOI] [PubMed] [Google Scholar]
- 33.Riccadonna M, Covi G, Pancera P, et al. Autonomic system activity and 24-hour blood pressure variations in subjects with normal- and high-tension glaucoma. J Glaucoma. 2003;12:156–163. doi: 10.1097/00061198-200304000-00011. [DOI] [PubMed] [Google Scholar]
- 34.Yazici B, Usta E, Erturk H, Dilek K. Comparison of ambulatory blood pressure values in patients with glaucoma and ocular hypertension. Eye. 2003;17:593–598. doi: 10.1038/sj.eye.6700436. [DOI] [PubMed] [Google Scholar]
- 35.Feke GT, Pasquale LR. Retinal Blood Flow Response to Posture Change in Glaucoma Patients Compared with Healthy Subjects. Ophthalmology. 2008;115:246–252. doi: 10.1016/j.ophtha.2007.04.055. [DOI] [PubMed] [Google Scholar]
- 36.Evans DW, Harris A, Garrett M, et al. Glaucoma patients demonstrate faulty autoregulation of ocular blood flow during posture change. Br J Ophthalmol. 1999;83:809–813. doi: 10.1136/bjo.83.7.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leske MC, Heijl A, Hyman L, et al. Predictors of Long-term Progression in the early Manifest glaucoma Trial. Ophthalmology. 2007;114:1965–1972. doi: 10.1016/j.ophtha.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 38.Fuchsjager-Mayrl G, Wally B, Georgopoulos M, et al. Ocular blood flow and systemic blood pressure in patients with primary open-angle glaucoma and ocular hypertension. Invest Ophthalmol Vis Sci. 2004;45:834–839. doi: 10.1167/iovs.03-0461. [DOI] [PubMed] [Google Scholar]
- 39.Grunwald JE, Piltz JR, Hariprasad SM, Dupont J, Maguire aMG. Optic nerver blood flow in glaucoma: effect of systemic hypertension. Am J Ophthalmol. 1999;127:516–522. doi: 10.1016/s0002-9394(99)00028-8. [DOI] [PubMed] [Google Scholar]
- 40.Sugiyama T, Utsunomiya K, Ota H, et al. Comparative study of cerebral blood flow in patients with normal-tension glaucoma and control subjects. Am J Ophthalmol. 2006;141:394–396. doi: 10.1016/j.ajo.2005.08.037. [DOI] [PubMed] [Google Scholar]
- 41.Shimada K, Kawamoto A, Matsubayashi K, et al. Diurnal blood pressure variations and silent cerebrovascular damage in elderly patients with hypertension. J Hypertens. 1992;10:875–878. [PubMed] [Google Scholar]
- 42.Shimada K, Kario K. Altered circadian rhythm of blood pressure and cerebrovascular damage. Blood Press Monit. 1997;2:333–338. [PubMed] [Google Scholar]
- 43.Manabe Y, Murakami T, Iwatsuki K, et al. Nocturnal blood pressure dip in CADASIL. J Neurol Sci. 2001;193:13–16. doi: 10.1016/s0022-510x(01)00636-0. [DOI] [PubMed] [Google Scholar]
- 44.Rufa A, Dotti MT, Franchi M, et al. Systemic blood pressure profile in cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy. Stroke. 2005;36:2554–2558. doi: 10.1161/01.STR.0000190832.17620.25. [DOI] [PubMed] [Google Scholar]
- 45.Aliev G, Obrenovich ME, Seyidova D, de la Torre JC. Exploring ischemia-induced vascular lesions and potential pharmacological intervention strategies. Histol Histopathol. 2005;20:261–273. doi: 10.14670/HH-20.261. [DOI] [PubMed] [Google Scholar]
- 46.Reimer KA, Tanaka M, Murry CE, et al. Evaluation of free radical injury in myocardium. Toxicol Pathol. 1990;18:470–480. [PubMed] [Google Scholar]
- 47.Sayegh FN, Weigelin E. Functional ophthalmodynamometry: comparison between brachial and ophthalmic blood pressure in sitting and supine position. Angiology. 1983;34:176–182. doi: 10.1177/000331978303400303. [DOI] [PubMed] [Google Scholar]