To the Editor
We and others recently demonstrated that the development of allergen-induced airway hyperreactivity (AHR) in mice requires the presence of a novel type of T cell, called invariant natural killer T (iNKT) cells.1,2 iNKT cells represent a distinct lineage of T cells that express characteristics of both conventional T cells and natural killer cells and express a highly conserved T-cell receptor (TCR) α chain: Vα14-Jα18 in mice and Vα24-JαQ in human subjects.3 Unlike conventional T cells, which recognize protein antigens, iNKT cells recognize glycolipid antigens presented by the nonpolymorphic MHC class I-like molecule CD1d.3 Although a critical role for iNKT cells has been clearly demonstrated in murine models of asthma, it is not yet certain whether iNKT cells play a similarly vital role in human subjects in the development of AHR, a cardinal feature of asthma.
We therefore examined the function of iNKT cells in asthma in primates by challenging cynomolgus monkeys with the exquisitely specific iNKT cell-activating agent α-galactocylceramide (α-GalCer). α-GalCer directly activates iNKT cells by specifically binding to the invariant TCR of iNKT cells in mice and human subjects3 and, when administered into the lungs, causes AHR in wild-type but not in iNKT cell–deficient mice.4 Because α-GalCer is extraordinarily specific in directly activating iNKT cells, but not TH2 cells, airway epithelial cells, or smooth muscle cells (it has no effects in natural killer T cell-deficient mice), α--GalCer is extremely useful in studying the role of iNKT cells in isolation of TH2 cells. Furthermore, monkeys provide a very useful model for studying human asthma because monkeys and human subjects are closely related in terms of their genomes, respiratory physiology, and immune responses5 and because airway challenge of human subjects with α-GalCer at this time poses unacceptable risks.
We challenged 4 monkeys with α-GalCer or vehicle using a crossover design (approved by the animal care and use commit-tees at the Children’s Hospital Boston and the Charles River Laboratory), which minimized the effects of genetic variation and the differing past histories in each of the outbred monkeys. In particular, because each monkey served as its own control animal, the crossover design allowed us to normalize the different baseline levels of AHR that each monkey possessed and thus minimized the number of animals required for generating statistically significant results. Each adult cynomolgus monkey was anesthetized, intubated, and challenged with nebulized α-GalCer or vehicle. For safety reasons and to avoid any possible toxic effects of α-GalCer that might cause AHR, a dose-escalation approach was used, starting with a very low dose of α-GalCer (150 µg/m2) similar to the lowest doses used intravenously in phase I safety studies in human subjects with solid tumors. Only one dose of α-GalCer was administered to the monkeys before evaluation of AHR because after activation with α-GalCer, iNKT cells become anergic temporarily.6 Twenty-four hours after challenge, the monkeys were reintubated and assessed for AHR by measuring lung resistance in response to increasing concentrations of methacholine.
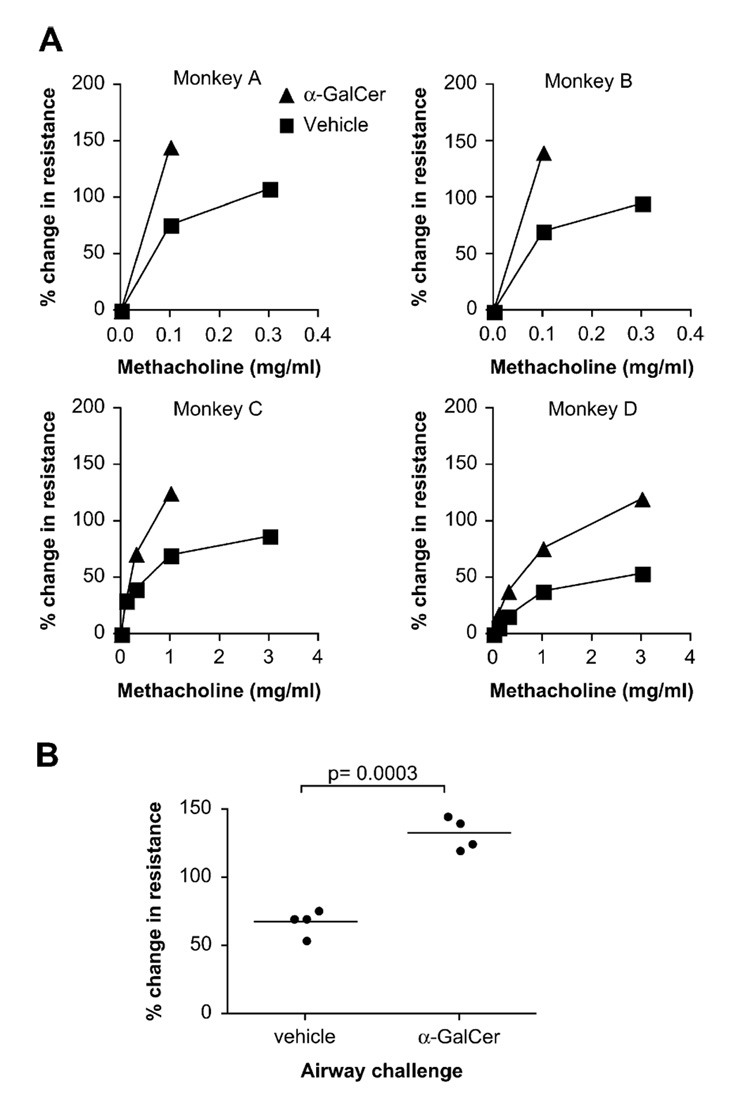
Surprisingly, with the first very low inhaled dose of α-GalCer (150 µg/m2), 3 of the 4 monkeys had significantly increased AHR compared with that seen after challenge with vehicle control (Fig 1, A, monkeys A, C, and D). The fourth monkey (monkey B), which did not respond to the first dose of α-GalCer, had a significant AHR response after later receiving a higher dose of α--GalCer (600 µg/m2; Fig 1, A, monkey B). When combined, data for the 4 monkeys showed that α-GalCer inhalation induced a significantly higher percentage change in lung resistance from baseline compared with that after vehicle control (P = .0003, paired t test comparing results at the highest dose of methacholine used after α-GalCer treatment; Fig 1, B). Although the primary outcome of the study (enhanced AHR in response to methacholine) was achieved, secondary outcomes (increased eosinophils, neutrophils, iNKT cells, and IL-4 levels in the bronchoalveolar lavage fluid in response to α-GalCer), while trending toward an increase, did not reach statistical significance, possibly because the dose of α-GalCer was too low due to safety considerations or due to the small sample size.
FIG 1.
Challenge of monkeys with α-GalCer induces AHR. A, AHR was measured in each monkey after challenge with α-GalCer or vehicle. The percentage change in lung resistance from baseline in response to increasing concentrations of methacholine is shown. B, Combining data from 4 monkeys, α-GalCer induced a significantly higher percentage change in lung resistance from baseline compared with that caused by vehicle control (P = .0003).
Nevertheless, these data clearly demonstrate that direct activation of pulmonary iNKT cells with α-GalCer in monkeys resulted in the development of significant AHR. Furthermore, these results are consistent with observations in mice demonstrating that iNKT cells are required for the development of allergen-induced AHR1 and that administration of α-GalCer intranasally to naive mice also results in the development of AHR.4 Because α-GalCer functioned to induce AHR without allergen administration, even in MHC class II−/− mice,4 which lack conventional CD4+ T cells, these studies with α-GalCer indicate that iNKT cells can induce AHR, even in the absence of TH2 cells. As such, these results suggest that iNKT cells could be important in regulating both allergic and nonallergic forms of asthma. Finally, because treatment outcomes observed in monkeys often predict outcomes that might be observed in human subjects treated similarly, our results provide the first functional in vivo evidence in primates that iNKT cells might play a critical role in the pathogenesis of human asthma.
Our findings in monkeys are also noteworthy because the role of iNKT cells in human asthma has become controversial because the particular number of iNKT cells in the lungs has been disputed. 7,8 However, 6 independent studies have shown that the number of iNKT cells present in the lungs of patients with asthma is greater than that in the lungs of healthy individuals,6,7 although other investigators have been unable to find significant numbers of iNKT cells in the lungs of patients with asthma.8 In our current studies we found that the number of iNKT cells in the bronchialveolar lavage fluid of monkeys before α-GalCer challenge was low (only 0.3% to 0.6% of the CD3+ cells), which is similar to that in nonasthmatic individuals and naive mice. The fact that the administration of α-GalCer induced AHR in monkeys (and in naive mice) suggests that even small numbers of iNKT cells in the lungs, when activated, can potently induce AHR and that pulmonary iNKT cells, even when present in small numbers, might indeed be critical effector cells in human asthma.
Our results in monkeys are especially important in understanding the pathophysiology of human asthma, not only because of the close relationship between monkeys and human subjects but also because iNKT cells, which link innate and adaptive immunity, present a novel and unexpected paradigm in asthma that is difficult to study in human subjects. iNKT cells express several features of innate immunity, including the capacity to very rapidly produce cytokines on activation and the expression of a germ-line-encoded receptor (the invariant TCR) that is remarkably conserved in sequence and function across species.3 This conservation suggests that the invariant TCR of iNKT cells serves as a pattern recognition receptor for glycolipids from pathogens, such as Borrelia burgdorferi, Ehrlichia muris, and Leishmania species.3 Furthermore, we suggest that exogenous glycolipids from pulmonary microbes or from inhaled plant pollens9 or endogenous glycolipids induced in the lungs by means of pulmonary inflammation or injury10 might activate iNKT cells and drive the development of AHR. Further studies of this paradigm in primates, both in monkeys and human subjects, are likely to provide important insight into the pathogenesis of human asthma and lead to the discovery of novel therapeutic measures for patients with asthma.
Acknowledgments
We thank the National Institutes of Health tetramer facility for providing CD1d tetramers, and we thank Nealia Khan, Harvard-MIT Data Center, for help with the statistical analysis.
Supported by grants from the National Institutes of Health (AI26322, HL63248) and an award from the Bunning Food Allergy Project.
Disclosure of potential conflict of interest: P. B. Savage has consulting arrangements with Ceragenix and Innate Immune, Inc, and has received research support from the National Institutes of Health, Ceragenix, and Innate Immune, Inc. R. H. DeKruyff has received research support from the National Institutes of Health. D. T. Umetsu has received research support from the National Institutes of Health and is a consultant for Innate Immune, Inc. The rest of the authors have declared that they have no conflict of interest.
REFERENCES
- 1.Akbari O, Stock P, Meyer E, Kronenberg M, Sidobre S, Nakayama T, et al. Essential role of NKT cells producing IL-4 and IL-13 in the development of allergen-induced airway hyperreactivity. Nat Med. 2003;9:582–588. doi: 10.1038/nm851. [DOI] [PubMed] [Google Scholar]
- 2.Lisbonne M, Diem S, de Castro Keller A, Lefort J, Araujo LM, Hachem P, et al. Cutting edge: invariant V alpha 14 NKT cells are required for allergen-induced airway inflammation and hyperreactivity in an experimental asthma model. J Immunol. 2003;171:1637–1641. doi: 10.4049/jimmunol.171.4.1637. [DOI] [PubMed] [Google Scholar]
- 3.Bendelac A, Savage PB, Teyton L. The biology of NKT cells. Annu Rev Immunol. 2007;25:297–336. doi: 10.1146/annurev.immunol.25.022106.141711. [DOI] [PubMed] [Google Scholar]
- 4.Meyer EH, Goya S, Akbari O, Berry GJ, Savage PB, Kronenberg M, et al. Glyco-lipid activation of invariant T cell receptor+ NK T cells is sufficient to induce airway hyperreactivity independent of conventional CD4+ T cells. Proc Natl Acad Sci U S A. 2006;103:2782–2787. doi: 10.1073/pnas.0510282103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coffman RL, Hessel EM. Nonhuman primate models of asthma. J Exp Med. 2005;201:1875–1879. doi: 10.1084/jem.20050901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meyer EH, DeKruyff RH, Umetsu DT. T cells and NKT cells in the pathogenesis of asthma. Annu Rev Med. 2008;59:281–292. doi: 10.1146/annurev.med.59.061506.154139. [DOI] [PubMed] [Google Scholar]
- 7.Akbari O, Faul JL, Hoyte EG, Berry GJ, Wahlstrom J, Kronenberg M, et al. CD4+ invariant T-cell-receptor+ natural killer T cells in bronchial asthma. N Engl J Med. 2006;354:1117–1129. doi: 10.1056/NEJMoa053614. [DOI] [PubMed] [Google Scholar]
- 8.Vijayanand P, Seumois G, Pickard C, Powell RM, Angco G, Sammut D, et al. Invariant natural killer T cells in asthma and chronic obstructive pulmonary disease. N Engl J Med. 2007;356:1410–1422. doi: 10.1056/NEJMoa064691. [DOI] [PubMed] [Google Scholar]
- 9.Agea E, Russano A, Bistoni O, Mannucci R, Nicoletti I, Corazzi L, et al. Human CD1-restricted T cell recognition of lipids from pollens. J Exp Med. 2005;202:295–308. doi: 10.1084/jem.20050773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pichavant M, Goya S, Meyer EH, Johnston RA, Kim HY, Matangkasombut P, et al. Ozone exposure in a mouse model induces airway hyperreactivity that requires the presence of natural killer T cells and IL-17. J Exp Med. 2008 doi: 10.1084/jem.20071507. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]