Abstract
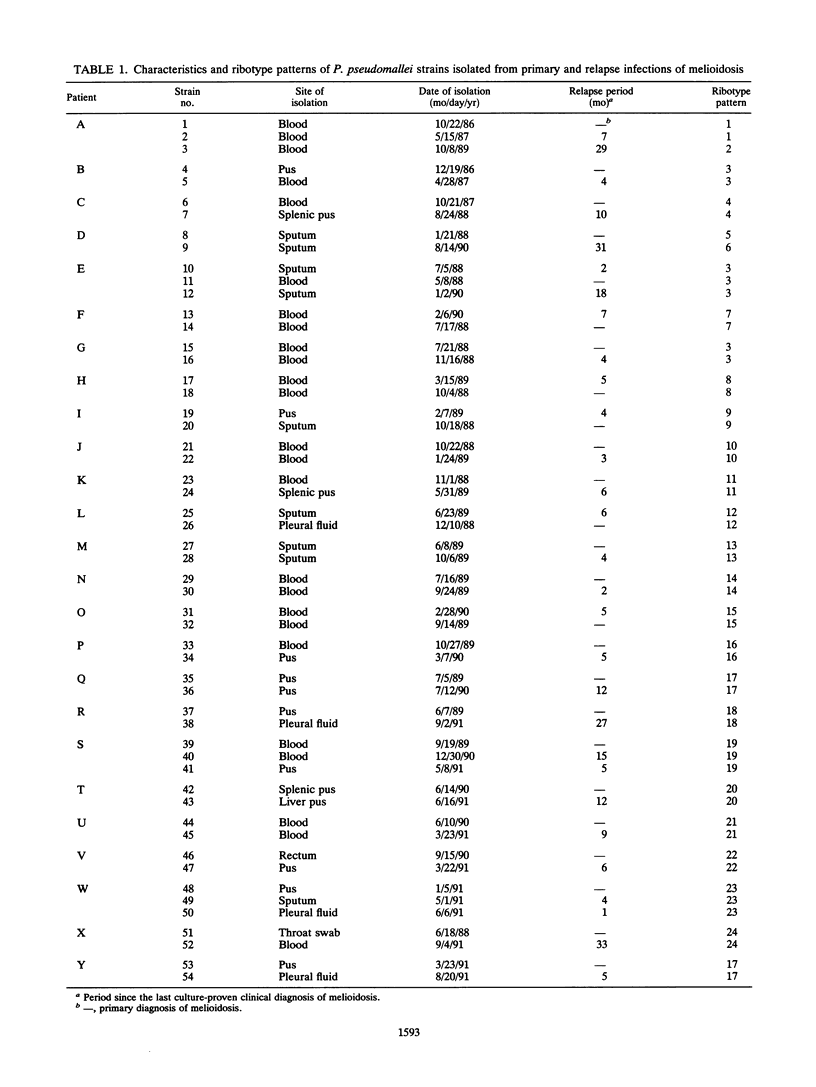
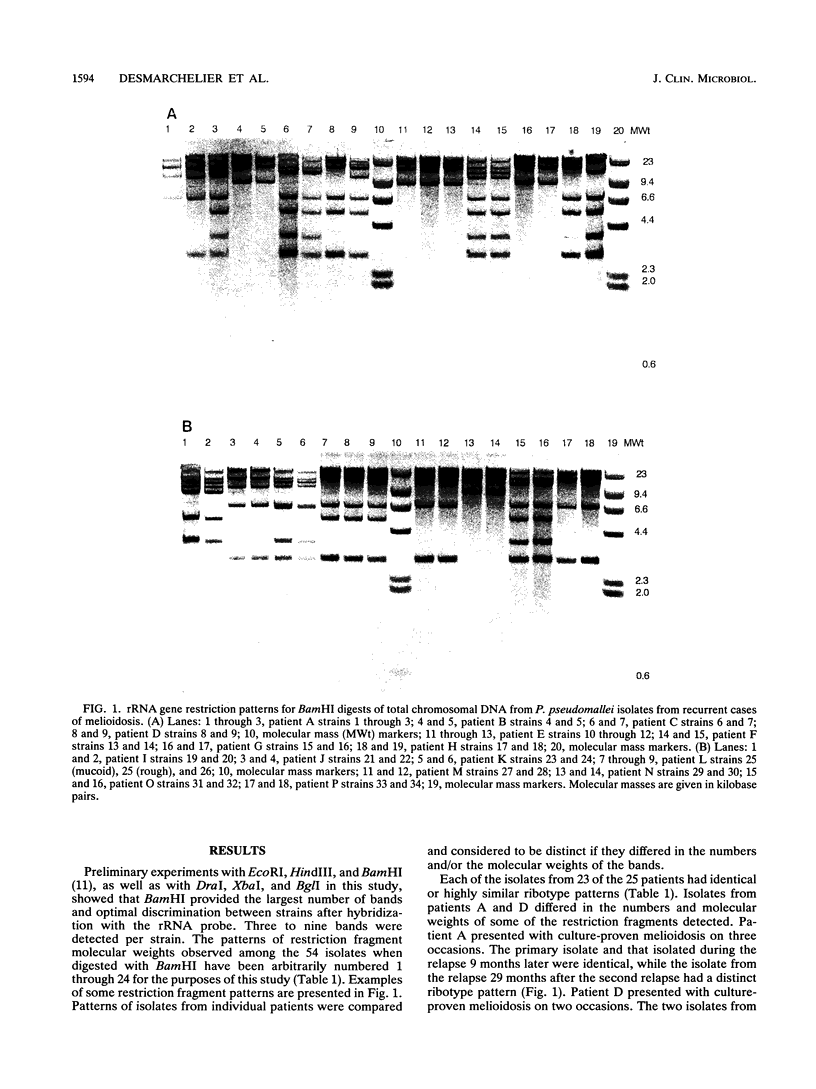
Patients with melioidosis may present with recurrent infections after clinical resolution of their primary illness. Because there has been no satisfactory typing scheme for Pseudomonas pseudomallei, recrudescence could not be distinguished from reinfection. We determined the strain identity of primary and relapse isolates of P. pseudomallei from 25 patients with culture-proven melioidosis to answer whether secondary infections were due to the initial infecting strain or to the acquisition of a new strain. Fifty-four isolates were compared by the patterns of BamHI restriction digests produced after hybridization with a cDNA copy of Escherichia coli rRNA. Twenty-three patients had primary and relapse isolates with identical or highly similar ribotype patterns. The patterns of isolates from two patients were different; the primary and relapse isolates differed by a single fragment for one, and the other had identical primary and first-relapse isolates while the second-relapse isolate was markedly different. The results indicated that recurrent infection probably resulted from endogenous relapse in most of the melioidosis patients studied, although reinfection from an exogenous source was also possible in two cases.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dance D. A., King C., Aucken H., Knott C. D., West P. G., Pitt T. L. An outbreak of melioidosis in imported primates in Britain. Vet Rec. 1992 Jun 13;130(24):525–529. doi: 10.1136/vr.130.24.525. [DOI] [PubMed] [Google Scholar]
- Dance D. A. Melioidosis: the tip of the iceberg? Clin Microbiol Rev. 1991 Jan;4(1):52–60. doi: 10.1128/cmr.4.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier J., Bussy G. Les antigènes thermostables de Pseudomonas pseudomallei et de Malleomyces mallei et leurs communautés. Ann Inst Pasteur (Paris) 1967 Jan;112(1):93–104. [PubMed] [Google Scholar]
- Garaizar J., Kaufmann M. E., Pitt T. L. Comparison of ribotyping with conventional methods for the type identification of Enterobacter cloacae. J Clin Microbiol. 1991 Jul;29(7):1303–1307. doi: 10.1128/jcm.29.7.1303-1307.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill C. W., Harnish B. W. Inversions between ribosomal RNA genes of Escherichia coli. Proc Natl Acad Sci U S A. 1981 Nov;78(11):7069–7072. doi: 10.1073/pnas.78.11.7069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes B., Pinning C. A., Dawson C. A. A probability matrix for the identification of gram-negative, aerobic, non-fermentative bacteria that grow on nutrient agar. J Gen Microbiol. 1986 Jul;132(7):1827–1842. doi: 10.1099/00221287-132-7-1827. [DOI] [PubMed] [Google Scholar]
- Kibbler C. C., Roberts C. M., Ridgway G. L., Spiro S. G. Melioidosis in a patient from Bangladesh. Postgrad Med J. 1991 Aug;67(790):764–766. doi: 10.1136/pgmj.67.790.764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingston C. W. Chronic or latent melioidosis. Med J Aust. 1971 Sep 18;2(12):618–621. doi: 10.5694/j.1326-5377.1971.tb92445.x. [DOI] [PubMed] [Google Scholar]
- Leelarasamee A., Bovornkitti S. Melioidosis: review and update. Rev Infect Dis. 1989 May-Jun;11(3):413–425. doi: 10.1093/clinids/11.3.413. [DOI] [PubMed] [Google Scholar]
- Lew A. E., Desmarchelier P. M. Molecular typing of Pseudomonas pseudomallei: restriction fragment length polymorphisms of rRNA genes. J Clin Microbiol. 1993 Mar;31(3):533–539. doi: 10.1128/jcm.31.3.533-539.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mays E. E., Ricketts E. A. Melioidosis: recrudescence associated with bronchogenic carcinoma twenty-six years following initial geographic exposure. Chest. 1975 Aug;68(2):261–263. doi: 10.1378/chest.68.2.261. [DOI] [PubMed] [Google Scholar]
- Morrison R. E., Lamb A. S., Craig D. B., Johnson W. M. Melioidosis: a reminder. Am J Med. 1988 May;84(5):965–967. doi: 10.1016/0002-9343(88)90080-0. [DOI] [PubMed] [Google Scholar]
- Newland R. C. Chronic melioidosis: a case in Sydney. Pathology. 1969 Apr;1(2):149–152. doi: 10.3109/00313026909061049. [DOI] [PubMed] [Google Scholar]
- Salvi R. J., Ahroon W., Saunders S. S., Arnold S. A. Evoked potentials: computer-automated threshold-tracking procedure using an objective detection criterion. Ear Hear. 1987 Jun;8(3):151–156. [PubMed] [Google Scholar]
- Woods T. C., Helsel L. O., Swaminathan B., Bibb W. F., Pinner R. W., Gellin B. G., Collin S. F., Waterman S. H., Reeves M. W., Brenner D. J. Characterization of Neisseria meningitidis serogroup C by multilocus enzyme electrophoresis and ribosomal DNA restriction profiles (ribotyping). J Clin Microbiol. 1992 Jan;30(1):132–137. doi: 10.1128/jcm.30.1.132-137.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]