Abstract
Background:
Trigger tools are an important development in the identification and reduction of adverse drug events (ADEs). Most previously published triggers are simple, consisting of one or two conditions. Simple logic may lead to alerts for conditions not caused by a drug or already treated by the provider.
Methods:
We created a knowledge-encoding tool to develop outpatient ADE triggers to more specifically identify harm caused by a drug and which require further clinical intervention. The tool presented the user with data on similar triggers from the literature and a series of fields to facilitate the creation of algorithms based on epidemiological principles.
Results:
Using this tool, we created 23 triggers that addressed 55 high-harm outpatient drugs and ADEs.
Conclusion:
Informatics tools can facilitate the design of clinically rich triggers. More investigation is needed to determine whether the performance characteristics of clinically rich triggers are better than those of simple triggers.
Background
Adverse drug event detection continues to be an important objective of patient safety research. Improvements in the ability to accurately identify adverse drug events (ADEs) include computerized triggers or algorithms that use electronic patient data to identify patterns consistent with a possible ADE1. The concurrent or real-time evaluation of triggered alerts has been used to guide clinical interventions to prevent emerging ADEs or mitigate actual ADEs2–4. These action-oriented triggers have been popular with clinicians at several sites5, 6.
A typical trigger consists of one or two logical steps such as a lab value threshold or the combination of a lab value threshold and an active prescription2. These types of triggers have moderate positive predictive value on the order of 0.23% to 0.59%6–9.
When investigating a triggered alert a clinician applies additional criteria to make a determination of iatrogenic harm10. Some of these criteria include alternative explanations, reasonable timing, and indications that the team has addressed the problem. Searching for information relevant to these criteria takes extra attention and time of the clinician. We hypothesized that it would be possible to create a new type of computerized trigger that was rich in clinical knowledge and more efficient at identifying opportunities to intervene in ADEs.
This paper reports on one aspect of an AHRQ contract to develop action-oriented ADE triggers for the outpatient setting. Few triggers target outpatient ADEs, despite high prevalence rates and excess utilization from preventable emergency room visits and hospitalizations11–13. Instead of merely adapting ADE triggers developed for the inpatient setting, we chose to test the concept of encoding clinically rich triggers for outpatient ADEs. We describe our process of developing an informatics tool to encode clinical logic into trigger development. We also present the outpatient ADE triggers resulting from this process.
Methods
The outpatient ADE trigger development project was funded by the Agency for Healthcare Research and Quality (AHRQ). A team of researchers from the Veterans Health Administration (VA), Boston Medical Center (BMC) and Intermountain Healthcare developed a set of triggers to detect ambulatory adverse events (AEs)14. The AHRQ study capitalizes on the availability of electronic ambulatory data at each institution, as well as the clinical and trigger development expertise of the research team. While the goal of the project is to develop AE triggers for the outpatient setting, this paper focuses only on the ADE triggers.
The AHRQ project began with a review of the literature on triggers designed to detect AEs, including ADEs, in the inpatient and outpatient setting15. The review captured all of the triggers identified in the literature, and highlighted types and causes of ADEs associated with significant harm or high frequency in the outpatient setting. The project team’s clinical experts used the literature on triggers and prevalence of causal agents and ADEs to determine gaps in trigger development and to prioritize outpatient trigger design. Priority was given to triggers that could easily be translated into the outpatient setting, and to the development of triggers where gaps existed in the literature.
A knowledge-encoding tool was created using Microsoft (MS) Access to incorporate results from the trigger literature along with clinical and trigger development expertise into a set of fields to build rules. Trigger information, including established rules, test characteristics (sensitivity, specificity, and positive predictive value (PPV)), ADE prevalence, and harm were collected systematically from the literature into the literature form. A separate form linked to the literature form captured clinical input to develop trigger rules. The structure of the database supported broad analysis of existing research and highlighted areas for future trigger development.
The knowledge-encoding tool was designed to enable trigger developers to build off advancements in the field. The literature form included a field for action-oriented logic, purposefully crafted logic to maximize the identification of imminent or actual iatrogenic ADEs in which the clinical team could intervene to prevent or ameliorate the event. The form also captured information on whether the trigger was scoped to address any ADE versus a specific ADE.
Several fields were included to fully articulate existing triggers in the literature: ADE(s) targeted; cause of the ADE; and data sources used by the trigger. In addition to literature on triggers, the knowledge-encoding tool had a form to input literature on significant causes of patient harm. Areas that were previously unaddressed by triggers were easily discerned by comparing prevalence information for ADE causes and events and triggers from the literature.
The knowledge-encoding tool used a separate MS Access form, the clinical input form, to develop outpatient trigger rules. The clinical input form was designed to capture and code clinical knowledge based on epidemiological principles of ADE causality16.
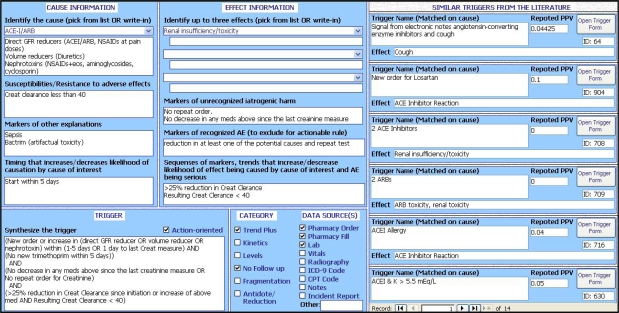
Clinicians using the knowledge-encoding tool had access to the information captured from the literature to specifically take into consideration prior research when compiling triggers. The clinical input form mapped to results from the literature based on AE cause. When the clinician picked the ADE cause, similar triggers documented in the literature form appeared on the right hand side of the screen. Information on existing triggers included the effect associated with the trigger, the trigger rule and the average PPV (see Figure 1).
Figure 1.
Screenshot of the knowledge-encoding tool’s clinical input form and results for similar triggers in the literature
The elements required to design a trigger using the clinical input form include the effect, the cause (there can be many effects and/or many causes), and the algorithm (see Table 1). The creator must also specify the data source(s) that support the algorithm. More complex triggers also present information on timing and how different data sources can be used to identify specific effects.
Table 1.
Description of the clinical input form fields
| Field Name | Explanation |
|---|---|
| Effect | The ADE targeted. Linked to ADE targeted on the literature form and includes a write-in option. Can select up to three effects |
| Cause | Cause of the ADE. Linked to causes on the literature form and includes a write-in option. Can select only one cause |
| Synthesize the Trigger | The outline of the trigger rule. May not incorporate all the detail captured in the clinical input form |
| Interventionist Trigger | Check box to indicate trigger uses action-oriented logic and can be acted on by clinicians to prevent or ameliorate the event |
| Category | Type of information from patient data, e.g. weight gain, classified as a trend |
| Data Source | Forms of patient data used in the algorithm such as laboratory orders |
| Susceptibilities/Resistance to AE | Patients at high or low risk for the ADE, e.g. patients with chronic kidney disease at high risk for opiod ADE |
| Markers of Other Explanations | Patient information that would lead to similar effects based on causes that are not adverse |
| Timing that Impacts Likelihood of Causation | Patient information occurring prior to effect that increases or decreases risk of ADE from specific ADE cause, such as initiating new drug regimen |
| Markers of Unrecognized Iatrogenic Harm | Patient information that shows evidence of an ADE but does not document a medical intervention |
| Markers of Recognized Harm | Patient information consistent with a medical reaction to an ADE, such as administration of an antidote |
| Sequences of Markers | Sequences of markers or trends in patient information that increase or decrease the likelihood that effect results from cause or that ADE is serious |
Since the knowledge-encoding tool described the data source and the average PPV of similar triggers from the literature, upon development of outpatient triggers, researchers on the AHRQ project could prioritize the triggers based on how well they lined up with electronic data within each health system and how likely they were to perform well. This list of ADE triggers, developed through the knowledge-encoding tool, was presented to a Delphi panel of trigger experts to identify final triggers for testing.
The final list of ADE triggers to test was determined through a modified Delphi approach17. A panel of eleven trigger experts participated in three rounds of consensus development conducted through email. Panelists ranked triggers on utility for patient or system-level interventions based on a scale of 1–9 (1 is most useful). The research team chose to test ADE triggers with patient or system-level utility rankings of three or lower by at least nine of the panelists based on Round 3 results.
Results
Three members of the research team populated the literature section of the database with more than 900 AE triggers from 110 research articles. Results of the literature review are available in a separate manuscript15. Clinician researchers developed 23 outpatient ADE triggers using the clinical input form. The triggers addressed 55 prevalent and harmful outpatient drugs and ADEs.
The knowledge-encoding tool allowed clinicians to supply detailed information on the causal agents and effects associated with a particular ADE trigger. However, the trigger rule did not always incorporate all the detail encoded in the tool. The example in Table 2 shows which fields populated in the knowledge-encoding tool were also included in the trigger rule. Delphi panelists rated the 23 ADE triggers and six triggers met our criteria for baseline assessment (see Table 3). Each of the six triggers includes action-oriented logic.
Table 2.
Example of the construction of an outpatient ADE triggers using the knowledge-encoding tool
| ADE Trigger Description | Criteria Used in Trigger Design | Encoded In Tool | Incorporated In Rule |
|---|---|---|---|
|
Action-oriented Logic | ✓ | ✓ |
| Susceptibilities/ Resistance to AE | ✓ | ||
| Markers of Other Explanations | |||
| Timing that Impacts Likelihood of Causation | |||
| Markers of Unrecognized Iatrogenic Harm | ✓ | ✓ | |
| Markers of Recognized Harm | ✓ | ✓ | |
| Sequences of Markers that Impact the Likelihood that Effect Results from Specific Cause or that AE is Serious | ✓ | ✓ |
Table 3.
List of outpatient ADE triggers eligible for baseline assessment
| Trigger | Trigger Logic |
|---|---|
| Creat | [New order or increase in (direct GFR reducer OR volume reducer OR nephrotoxin) within (1–5 days OR 1 day to last creatinine measure)
AND (No new trimethoprim within 5 days)] AND (No decrease in any meds above since the last creatinine measure OR no repeat order for creatinine) AND (>25% reduction in creatinine clearance since initiation or increase of above med AND resulting creatinine clearance < 50) |
| Klow | Use of potassium reducer
AND [K <3.0 OR (K < 3.5 AND K decreased by >15%) versus previous measurement] AND (No new potassium raiser OR decreased potassium reducer) within 5 days of triggering potassium result |
| Khigh | [(K+>5.5 and up by >10% since last measurement)
OR (K+>6.0)] AND (Potassium raiser active OR Potassium reducer discontinued 1 day to 4 weeks days prior) AND No new potassium reducer OR decrease in potassium raiser within 5 days of triggering result |
| BMT | (On bone-marrow-toxic drug with a course more than 2 weeks AND No chemotherapy within 2 weeks)
AND [(WBCs<2,500 AND decrease from before course by more than 2,000) OR (WBCs<2,000 AND decrease from before course by more than 1,000) OR (Platelets<50k AND decrease by 75k within 1 week)] AND (no repeat CBC OR no decrease in drug) within 5 days of triggering result |
| Warf | [Started on warfarin within 14 days AND (INR>3.0 AND INR increased by 1 within 2 days) AND no repeat INR within 2 days]
OR (Started in warfarin longer than 14 days prior AND INR>4 AND no repeat INR within 2 weeks) OR (INR>6 AND no repeat INR within 2 days) Note: INR stands for International Normalized Ratio |
| Gork | Active prescription of sedative hypnotic including anticholinergic
AND Subsequent diagnosis of (dementia, fall, delirium) |
Discussion
The goal of trigger development is to efficiently and effectively detect AEs to prevent patient harm in real-time and to aid in system-wide surveillance that can lead to process improvement1. The incorporation of clinical logic into triggers designed to work with electronic patient data may reduce the time needed to follow up on activated triggers, while keeping false negatives to a minimum.
One of the strengths of the knowledge-encoding tool is that the input fields for developing the trigger reflect the logic behind the causality assessment of the ADE. As the clinician described the causes and effects, they could see similar triggers from the literature on the right side of the screen. The inclusion of this information supports the consistency requirement in the Bradford-Hill criteria for causality18. The knowledge-encoding tool also employed fields related to specificity and temporality, which are both critical to establishing causality. Clinicians using the tool processed the research evidence and applied experience to judge the various scenarios in which a patient’s information would be consistent or inconsistent with the causal pathway between agent and effect.
Research and clinical experience enable chart reviewers to discern causality when identifying AEs. In addition to increasing the accuracy of the triggers, the incorporation of clinical logic may also increase the reliability of trigger evaluation. Chart review reliability is typically poor but improves with increased detail in the abstraction process19. The knowledge-encoding tool increases the specificity of the trigger thereby eliminating some of the decision-making required by reviewers and improving the reliability of ADE identification.
The clinical input obtained through the knowledge-encoding tool also allows users to understand how the trigger can be applied to improve patient safety. The field on action-oriented logic and the description of how to identify an iatrogenic event leads to triggers that can be used to identify immediate patient harm and intervene as appropriate. Non-interventionist triggers can be applied retrospectively to support system-wide efforts to improve processes of care.
The construction of triggers is limited by the extent to which patient data is available electronically. While the epidemiological perspective may be well understood, the data may not be available to apply this type of reasoning in trigger rules. Specifically, electronic patient notes prove quite difficult to search without human chart review. Advancements in natural language processing and other text-searching tools increase the capacity of trigger rules to include electronic notes in trigger detection3, 20. In the future, triggers may be designed to detect a wider range of AEs using detailed notes data.
A limitation of this research is that the outpatient triggers developed with the knowledge-encoding tool have not been tested. The next stage of the AHRQ trigger project is a baseline assessment of the six ADE triggers using structured chart review by trained pharmacist abstractors. A sample of trigger positive and trigger negative cases from VA, BMC and Intermountain Healthcare patient data will be reviewed. Rates of ADEs detected as well as specificity and sensitivity will be measured. Trigger algorithms will be modified to obtain optimal sensitivity and specificity in identifying ADEs.
Future research should explore how triggers can capture additional information related to the epidemiology of ADE development. This information can be used to optimize the balance between specificity and sensitivity. As triggers become more effective in detecting ADEs, researchers should also consider other factors affecting system-wide trigger adoption, including data availability and cost of implementation.
References
- 1.Resar RK, Rozich JD, Classen D. Methodology and rationale for the measurement of harm with trigger tools. Qual Saf Health Care. 2003 Dec;12(Suppl 2):ii39–45. doi: 10.1136/qhc.12.suppl_2.ii39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Resar RK, Rozich JD, Simmonds T, Haraden CR. A trigger tool to identify adverse events in the intensive care unit. Jt Comm J Qual Patient Saf. 2006 Oct;32(10):585–590. doi: 10.1016/s1553-7250(06)32076-4. [DOI] [PubMed] [Google Scholar]
- 3.Penz JF, Wilcox AB, Hurdle JF. Automated identification of adverse events related to central venous catheters. J Biomed Inform. 2007 Apr;40(2):174–182. doi: 10.1016/j.jbi.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 4.Sharek PJ, Horbar JD, Mason W, et al. Adverse events in the neonatal intensive care unit: development, testing, and findings of an NICU-focused trigger tool to identify harm in North American NICUs. Pediatrics. 2006 Oct;118(4):1332–1340. doi: 10.1542/peds.2006-0565. [DOI] [PubMed] [Google Scholar]
- 5.Classen DC, Pestotnik SL, Evans RS, Burke JP. Computerized surveillance of adverse drug events in hospital patients. Jama. 1991 Nov 27;266(20):2847–2851. [PubMed] [Google Scholar]
- 6.Kilbridge PM, Alexander L, Ahmad A. Implementation of a system for computerized adverse drug event surveillance and intervention at an academic medical center. J Clin Outcomes Manage. 2006;13(2):94–100. [Google Scholar]
- 7.Kilbridge PM, Campbell UC, Cozart HB, Mojarrad MG. Automated surveillance for adverse drug events at a community hospital and an academic medical center. J Am Med Inform Assoc. 2006 Jul-Aug;13(4):372–377. doi: 10.1197/jamia.M2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nebeker JR, Yarnold PR, Soltysik RC, et al. Developing indicators of inpatient adverse drug events through nonlinear analysis using administrative data. Med Care. 2007 Oct;45(10 Supl 2):S81–88. doi: 10.1097/MLR.0b013e3180616c2c. [DOI] [PubMed] [Google Scholar]
- 9.Szekendi MK, Sullivan C, Bobb A, et al. Active surveillance using electronic triggers to detect adverse events in hospitalized patients. Qual Saf Health Care. 2006 Jun;15(3):184–190. doi: 10.1136/qshc.2005.014589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Phansalkar S, Hoffman JM, Nebeker JR, Hurdle JF. Pharmacists versus nonpharmacists in adverse drug event detection: a meta-analysis and systematic review. Am J Health Syst Pharm. 2007 Apr 15;64(8):842–849. doi: 10.2146/ajhp060335. [DOI] [PubMed] [Google Scholar]
- 11.Budnitz DS, Pollock DA, Weidenbach KN, Mendelsohn AB, Schroeder TJ, Annest JL. National surveillance of emergency department visits for outpatient adverse drug events. Jama. 2006 Oct 18;296(15):1858–1866. doi: 10.1001/jama.296.15.1858. [DOI] [PubMed] [Google Scholar]
- 12.Cantor MN, Feldman HJ, Triola MM. Using trigger phrases to detect adverse drug reactions in ambulatory care notes. Qual Saf Health Care. 2007 Apr;16(2):132–134. doi: 10.1136/qshc.2006.020073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thomsen LA, Winterstein AG, Sondergaard B, Haugbolle LS, Melander A. Systematic review of the incidence and characteristics of preventable adverse drug events in ambulatory care. Ann Pharmacother. 2007 Sep;41(9):1411–1426. doi: 10.1345/aph.1H658. [DOI] [PubMed] [Google Scholar]
- 14.Rosen AK, Nebeker JR, Shimada S, et al. AHRQ; 2007. Development and Use of Ambulatory Adverse Event Trigger Tools. [Google Scholar]
- 15.Mull HJ, Shimada S, Nebeker JR, Rosen AK. A Review of the Trigger Literature: Adverse Events Targeted and Gaps in Detection (in press) Rockville: Triggers and Targeted Injury Detection Systems (TIDS) Expert Meeting, Agency for Healthcare Research and Quality AHRQ; 2008. [Google Scholar]
- 16.Edwards IR, Aronson JK. Adverse drug reactions: definitions, diagnosis, and management. Lancet. 2000 Oct 7;356(9237):1255–1259. doi: 10.1016/S0140-6736(00)02799-9. [DOI] [PubMed] [Google Scholar]
- 17.Jones J, Hunter D. Consensus methods for medical and health services research. Bmj. 1995 Aug 5;311(7001):376–380. doi: 10.1136/bmj.311.7001.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hill AB. The Environment and Disease: Association or Causation? Proc R Soc Med. 1965 May;58:295–300. doi: 10.1177/003591576505800503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu L, Ashton CM. Chart review. A need for reappraisal. Eval Health Prof. 1997 Jun;20(2):146–163. doi: 10.1177/016327879702000203. [DOI] [PubMed] [Google Scholar]
- 20.Bates DW, Evans RS, Murff H, Stetson PD, Pizziferri L, Hripcsak G. Detecting adverse events using information technology. J Am Med Inform Assoc. 2003 Mar-Apr;10(2):115–128. doi: 10.1197/jamia.M1074. [DOI] [PMC free article] [PubMed] [Google Scholar]