Abstract
In this paper we examine frequently performed clinical research activities with the objective of identifying aspects of workflow that could be amenable to informatics-based re-engineering. This paper is part of a series of studies under the NIH Roadmap initiative, which examines workflow of clinical research in community practices. We describe three common work activities, detailing the main actors involved, the tools used and the challenges faced. These activities illustrate inefficiencies in the clinical research workflow which include: a) lack of supporting tools to perform routine work activities, b) redundancy, low reuse of data and poor interoperability between systems and c) the fragmented and distributed nature of the workflow. We identify opportunities for re-engineering at both a micro (activity) and macro level (organization).
Keywords: Clinical research, community practice, direct observations, interviews, workflow
Introduction
Informatics solutions have been envisioned to form a critical role in transforming a predominantly paper-based clinical research (CR) enterprise [1]. Over the last 3 years, Columbia University’s InterTrial project, which is funded under the National Institutes of Health (NIH) sponsored Roadmap Initiative, has focused its determination of re-engineering strategies and interventions in community practices, which are becoming the prevailing setting for clinical research (CR) [2]. Understanding workflow is important in developing solutions that are grounded in the end users’ needs and the organizational and social environment [3]. Numerous examples exist of software systems that have not been adopted because of their misfit in users’ workflow [4].
To ground our re-engineering solutions, we have performed a series of empirical studies (using qualitative and quantitative methods) to investigate stakeholder roles, organizational structures, workflow, information needs and communication patterns within CR. Our studies have focused primarily on the key agent in CR, the Clinical Research Coordinator (CRC) who performs the bulk of CR work. We developed a basic workflow model of CR in community practice settings, which focused on the activities of a CRC [5]. It included elements such as the tasks (e.g., documentation, recruitment) and activities (e.g., scheduling a patient visit) performed as part of research, the tools (e.g., phone, paper forms, artifacts) used to accomplish these activities and the status of these actions (e.g., completed, incomplete). We used this model in a time-motion study (TMS) to determine the workflow of a CRC. The TMS provided useful insights into the research workflow, highlighting such aspects as, the paper-driven nature of work, the occurrence of multiple interruptions and the identification of oft-repeated and time-consuming activities. Our second study, based on direct observations of the workflow [6], revealed the nature of information communication (e.g., back and forth dialogs are common; the same message may be communicated in multiple copies and formats) and the extent of use of Information Technology (IT) by the CRC. In this paper, we build on the previously reported work, which reported the snapshots or general aspects of workflow. Here we present step-by-step details of the oft-performed work processes. The process details illustrate how the current systems and workflow are not entirely sufficient to manage day-to-day work for the CRC and consequently highlight the need and areas for informatics-based interventions to support CR workflow.
Methods
The details of activities described in this paper are primarily based on findings from direct observations and interviews, with supplementary information from surveys and the TMS. Direct observation and interviews can provide an opportunity to understand the nuances of a phenomenon [7]. We interviewed the CRCs and asked them detailed questions about certain work activities that are routinely performed (identified in our survey and TMS). We also used direct observations to shadow CRCs and to record their work activities, information needs and other aspects of workflow. This technique is helpful in validating interview results and discovering unstated findings, since most respondents cannot precisely recall their work habits in an interview or survey [7]. The observations and interviews were conducted (by SK) between May and September of 2006 in three community practices (three day visits at each site) within the Columbia University Clinical Trials Network. A larger study within 6 other sites (which were visited over the course of 2005–06) provides supplemental data. IRB approval was obtained for all the studies. For the purposes of this study, a community practice is defined as a physician office providing outpatient clinical care. The interviews were audio taped and observations were recorded in field notes (along with sketches of the work processes), which were later transcribed and analyzed for important findings. The interviews were coded using grounded theory and the codes were determined through an iterative process including by a team of three. Detailed process diagrams were validated by two domain experts in clinical trials and with informatics experts within the research group. The diagrams were also presented to several CRCs invited to our annual InterTrial luncheon. For each activity (e.g., scheduling a patient visit), we describe the tools used, the actors involved, the information needed to complete the activity, the challenges faced and any best practices to make the activity easier to perform. The steps in an activity are identified as action verbs (such as determined, dialed, noted), defined here as observable units of work. What we describe is a representative description of each activity, which generalizes over various exceptions and variations observed across sites and protocols for the same activity. We also note that the activity steps may not always occur in the sequence that we describe and within a short time span; our observations have routinely determined that the CRCs workflow is highly interrupted and work may be left in midstream and taken up much later. For convenience, we will adopt a composite perspective of a fictitious CRC named Sarah and refer to a fictitious patient, John.
Findings
Scheduling a Patient Visit
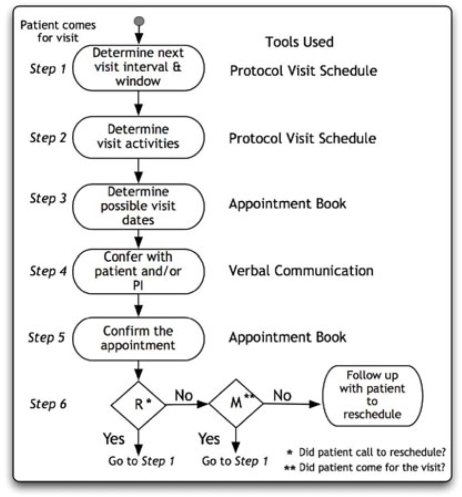
Scheduling a patient visit is one of the most frequently performed activities by the CR staff [5]. Scheduling a visit is dependent on the specific protocol for each study and each visit has to follow a set interval and must take place within a time window. Assume that John is enrolled in a trial and visits Sarah for his third protocol-specified visit. Upon completion of the visit, Sarah has to schedule the next visit for John. She maintains an appointment book (a tool), which is shared among other staff in the clinic for managing patient appointments. Before she selects an appropriate date for the visit, Sarah needs to determine (step 1) the visit interval and window, for which she refers to the protocol visit schedule (a tool, which she keeps in the study binder). The schedule states the sequence (and intervals) of visits for an enrolled patient, and Sarah uses this to determine the interval between the third and the fourth visit (4 weeks, for example). Next, she also takes note (step 2) of the activities that need to be done for the visit. She realizes that on the fourth visit, John needs to be examined by the principal investigator (PI) and also submit a blood sample. She then consults (step 3) her office calendar (a tool) to choose a date (she finds one just 2 days beyond the one month) when the PI would be available for John’s fourth visit a month from now.
She then confers with John (step 4) if the date she has in mind would be suitable to him (patient preference is priority for Sarah and she works the dates based on John’s preference) and only when he confirms his availability does she commit (step 5) the date and time in her appointment book for John’s fourth visit.
As can be seen from the above, there are several steps and tools involved in one activity. Though it is Sarah who usually schedules visits (she may share steps with a receptionist, if the site has one), she does need to confer with the PI occasionally, as his calendar frequently changes, and Sarah double checks before confirming the appointment. Sarah also needs to know the trial interval, window and activities that need to be performed during a visit, the PI’s and patient’s preferences before identifying a satisfactory visit date. Determining the activities to be performed during a patient visit influences the choice of possible dates. For example, some visits only require brief follow-up some require detailed examination by the PI and some others may require extensive blood work. Such requirements necessitate that Sarah chose a date and time that is sufficient to perform the necessary activities. The process has several challenges. Finding a day four weeks in advance that is not a holiday and is acceptable to everyone is not trivial. The process becomes even more complicated when John calls Sarah to reschedule (or fails to show up for) an appointment (step 6). Rescheduling may force Sarah to repeat the above steps to find an acceptable date for John, within the allowed window. Visits outside the window require approval by the sponsor and more paperwork. Sarah has learned that the best strategy to schedule research visits is to combine them with a clinic visit, if applicable (many of her trial participants are patients at the clinic too). Sarah usually schedules a patient in the beginning of a visit window so that if they miss the appointment, she can still reschedule within the window.
Dispensing Medication
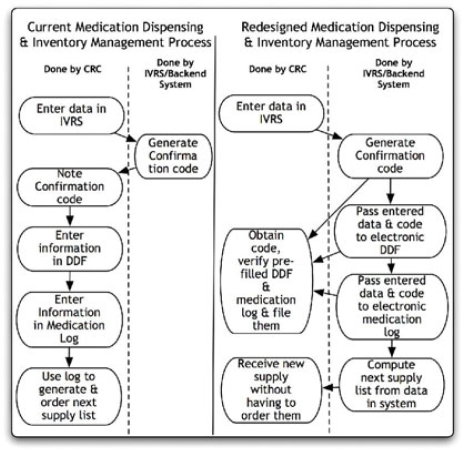
Dispensing medication and managing the medication inventory is another routine task that Sarah often performs. To begin, Sarah connects (step 1) to an Interactive Voice Response System (IVRS) (a tool) by dialing a toll-free number from a list of numbers (a tool) she maintains. The system requires her to key-in certain patient-specific identifiers in a series of interactions. On completion, the system provides her a confirmation number, which she notes (step 2) on a piece of paper (a tool). This number corresponds to the medication box that needs to be dispensed to the patient and confirms that the patient has come for the visit. Sarah finds (step 3) the medication box with the exact code from her medicine closet, enters the date of dispensing on the box and photocopies (step 4, a tool) a bar code label stuck on the medication box. She then enters (step 5) the confirmation code and sticks the label in the drug dispensing form (DDF) (a tool) for the patient (Figure 2 shows some steps). She files (step 6) the photocopy in the patient binder (tool) as an additional record of the dispensing activity. Sarah then provides (step 7) the medication to John along with dosing instructions. Later, she enters (step 8) the confirmation code, medication box barcode and related information in a medication log (tool) that is used to track the medication inventory in a study and to order new drug supplies. At some point, she has to have the DDF and medication log entries signed by the PI (step 9).
Figure 2.
Comparison of current and redesigned Medication Dispensing and Inventory Management Process
Once again, we see that an activity involves several steps, interaction with different tools and requires different pieces of information to be managed. This activity is almost always done by the CRC, with the PI only acting in step 9. Sarah has to contend with a number of problems in the medication dispensing process. The confirmation code she receives is transmitted to her in multiple formats (e.g., mail, fax, or email), an aspect of workflow reported in our earlier study [6]. She has to maintain the record of all communications in the patient’s trial binder to comply with audit requirements. On occasion, the confirmation code provided doesn’t match any remaining medication boxes she has in her inventory (e.g., the supplies were either delayed or not shipped in the first place), in which case Sarah can’t dispense medication to John. As a best practice, she updates both the DDF and the log at the same time of dispensing the medication so as to keep all records in agreement.
Completing Case Report Forms
The process of filling out a case report form (CRF), which is used to document information regarding a patient’s study-specific status, is a good illustration of a work process that is burdensome and entails considerable redundant data entry. Before a visit takes place, Sarah actually keeps (step 1) the appropriate CRFs ready to be filled the day of the patient visit. The patient visit is (in most studies) first recorded (step 2) in source documents (tools) such as a patient chart. The CRF is usually transcribed (step 3) from the source document, though sometimes Sarah may directly record the patient information in them. Since the PI may complete the source documents, Sarah may need to consult (step 4) the PI while filling out the CRF. Some trials support eCRFs (electronic) that are filled using trial-specific software (tool). Even if the trial has an eCRF, many CRCs like Sarah prefer to note information in a paper CRF and then transcribe that in the eCRF. Upon completion of data entry, an eCRF is transmitted (step 5) via an Internet connection (a tool), as most eCRFs are provided within standalone desktop applications. In cases where the CRF is paper-based, Sarah files (step 5) the document in the patient binder (a tool) and sends (step 6) a copy to the sponsor (or may wait until the CRF is audited by the sponsors monitor) through fax and/or mail (tool).
There are noteworthy challenges in the CRF completion process. Some CRFs are long and require Sarah to ask the patient many questions. In addition, the CRFs often require filling out the same fields many times (e.g., site ID, patient ID number and date), adding to the process burden [5,6]. Despite meticulous attention, transcribing from the patient source document to the CRF can result in both transcription and omission errors. Such errors lead to laborious query resolutions when the sponsor sends back questions related to the data errors. In this regard, Sarah notes that eCRFs are much better than paper CRFs; they prompt her for corrections when she types in implausible values for a field or when she fails to fill a required field. Additionally, she notes that eCRFs save her from extra typing because they pre-populate certain fields like patient IDs, date, etc. Queries are also generated when the coordinator uses an out-of-date version of the CRF.
As a best practice, Sarah tries to fill a CRF on the day of the patient visit because the information obtained from the patient is fresh in her mind.
Discussion
Understanding clinical research workflow in community practice settings is critical in re-engineering efforts. In a series of studies we have elucidated CR workflow in varying details and in this paper, we report, in detail, on three commonly performed research activities. Our examples illustrate certain broad themes about the workflow, of which we briefly pointed out issues with information communication. We now discuss the fragmented and distributed nature of the workflow. The investigations reveal that even relatively simple activities require many steps, tools and complex information requirements. As reported in our earlier work, paper-based tools are the most ubiquitous [5], and have well known drawbacks for information management [6]. Importantly, research activities require coordination between distributed and diverse resources. The dispersed nature of required information and tools make the work process complex and error-prone. The use of several tools requires multiple transfers of data. For example, transferring a phone number from an email to a sticky note. Each such transmission interface may introduce errors. Sarah must ensure that she reads and interprets every data element correctly across the interfaces and mediums. The distributed information resources (numerous lists, sticky notes, checklist documents, etc.) highlight the need for a system that allows Sarah to have access to information in one place with the ability to easily update the information.
The other major theme discovered in our study is that the CRCs are ill equipped with tools to support their day-to-day work. Even if tools (technological or otherwise) exist they are not well designed to make the CRC’s work easier. For example, determining patient visit dates to satisfy the protocol and other constraints can be a chore. A computerized scheduling system could reduce the burden of scheduling by calculating the allowed days for the next patient visit given the constraints, even if it would not make the process entirely automated. It is conceivable that, as a patient is randomized, their visit dates can be calculated by a computer program as per the protocol and updated as every visit proceeds (propagating any changes to the schedule forward). Similarly, the lack of an electronic tool to manage medication logs and inventory is also evident in our example. We mentioned earlier that Sarah has to obtain a confirmation code by keying certain data elements (patient identifiers) before every medication box is dispensed. She enters (Figure 2) the same information into a patient-specific DDF and in a cumulative medication logbook for the trial. A redesigned workflow could populate the confirmation data into a constantly updated electronic DDF for every patient and into an electronic sheet that serves as a medication inventory log, both of which could be made available via the web or secure email. Sarah would only need to verify that the information in each of these forms is accurate, and print them for signature by the PI.
Another theme evident in our studies is the redundant data entry, lack of reuse of information and poor interoperability across work processes/systems, which are significant barriers to achieving greater efficiency within CR workflow. Redundant data entry is rampant in our examples. Within the CRFs, several data fields are entered repeatedly on each page. Additionally, the CRFs are transcribed from source documents and eCRFs from paper CRFs; downstream data input is rife for potential human errors. Redundant data entry also occurs when the same data fields are entered in the medication confirmation system, DDF and medication logbooks. As shown in Figure 2, even though the medication confirmation system has all the data regarding medication dispensing, these data are not reused to compute the next medication order list, an activity that Sarah has to perform manually by comparing her medication logs to her inventory.
Our findings demonstrate that the CR workflow processes are not designed keeping the end-user, the CRC in perspective. They represent a top-down vision from the sponsor’s perspective, with little consideration of how the CRCs would accomplish the CR work. The reality of performing redundant tasks (such as managing multiple copies of confirmation codes) and the lack of basic work-supporting tools for mundane activities (such as determining a visit date), result in significant cognitive overload and time investment. Simple solutions could reduce some of the burden or prevent errors (like missing values in a CRF, which generate queries) from occurring in the first place. Small process missteps cascade into larger downstream system inefficiencies. We note that redundancy provides checks and balances, but these checks can be obtained without bogging down the overall system considerably.
To fulfill the acute needs of a work-supporting information system, the InterTrial project has designed a software application called WorkWeb. WorkWeb combines software features found in project management tools with some features of protocol-tracking systems. WorkWeb focuses on daily tasks performed in clinical research rather than electronic capture of clinical data (as in typical clinical trials management systems). The system is integrated with a Wiki platform, enabling users to collaboratively edit web pages, share documents and participate in online discussion forums [8]. WorkWeb also includes a number of features such as integrated scheduling, to-do list management and research services tracking. Users can create personal or group calendars, which are integrated with to-do lists. In addition, the CRC can track future and completed patient visits. This information is reused to populate the CRC’s schedule and to generate quarterly reports on the payments due for the services provided [9].
Not all problems identified in the CR workflow, however, have technical solutions. Our findings reveal several fundamental inefficiencies in the way research related work processes are defined and drafted, which leads to redundancy and lack of interoperability. Such inefficiencies would require concerted effort by all research stakeholders to simplify and standardize the components of an activity and the definition and labeling of common research work processes. Building such standards, which are agreed upon by major stakeholders, such as the sponsors, could greatly reduce the number of different reporting forms that have common data elements but differ in structure (e.g., two trials having different adverse event notification forms). Standards to denote work processes would improve the possibility of integrating different processes to foster interoperability. Importantly, there is a need to involve the end-user, the CRCs, actively in the discussion when creating a new protocol workflow. Such actions could lead to large efficiency gains.
Conclusion
Understanding workflow is critical to the design of the high quality IT solutions and their ultimate adoption. The InterTrial project has, through numerous studies, elucidated some basic aspects of the CR workflow in community practices. These findings reveal the need for both micro (activity) level and macro (organizational) level changes. We identified several potential areas for micro-interventions where simple solutions can provide incremental gains in efficiency. However, larger macro changes that are championed by all stakeholders are required to achieve fundamental re-engineering and efficiency gains.
Figure 1.
Steps in Scheduling a Patient Visit
Acknowledgments
We thank our funding agency, the NHLBI (HHSN268200455208C) and the community practices and CRCs for their time and support.
References
- [1].Zerhouni E. The NIH roadmap. Science. 2003 Oct 3;302(5642):63–72. doi: 10.1126/science.1091867. [DOI] [PubMed] [Google Scholar]
- [2].Sung NS, Crowley WF, Jr, Genel M, et al. Central challenges facing the national clinical research enterprise. JAMA. 2003;289:1278–87. doi: 10.1001/jama.289.10.1278. [DOI] [PubMed] [Google Scholar]
- [3].Kukafka R, Johnson SB, Linfante A, Allegrante JP. Grounding a new IT implementation framework in behavioral science: a systematic analysis of the literature on IT use. J Biomed Inform. 2003;36(3):218–27. doi: 10.1016/j.jbi.2003.09.002. [DOI] [PubMed] [Google Scholar]
- [4].Ash J, Bates D. Factors and forces affecting EHR system adoption: 2004 ACMI discussion. JAMIA. 2005;12:8–12. doi: 10.1197/jamia.M1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Khan SA, Payne PRO, Kukafka R, Bigger JT, Johnson SB. Modeling clinical trials workflow in community practice settings. Proc AMIA. 2006 [PMC free article] [PubMed] [Google Scholar]
- [6].Khan SA, Kukafka R, Payne PR, Johnson S, Bigger T. A day in the life of a clinical research coordinator. Proceedings of the MEDINFO. 2007 [PubMed] [Google Scholar]
- [7].Creswell JW. Research Design. Qualitative, Quantitative and Mixed Methods Approaches. Sage Publications. 2003 [Google Scholar]
- [8].Khan SA, Florenz M, Kukafka R, Bigger T, Johnson S. Workweb: Enhancing Collaboration and Communication in Community Based Clinical Research through innovative use of Wikis. Proceedings of the MEDINFO. 2007 [Google Scholar]
- [9].Bigger JT, Busacca LV, Florenz MK, Slavik WM, Steinman RC, Johnson SB. Re-engineering clinical research: a scalable model integrating financial management with workflow in community clinical research networks. Presented at the National Leadership Forum. May 31, 2006.