Abstract
WHO-ART and MedDRA are the terminologies used in pharmacovigilance for coding of adverse drug reactions and statistical analysis. In previous work we showed that tools for automated signal detection and access to pharmacovigilance databases would benefit from terminological reasoning in order to provide improved groupings of terms describing the same medical condition. Such reasoning depends on formal definitions that are absent in both terminologies. A Categorial structure is defined as a minimal set of health care domain constraints which represents a biomedical terminology in a precise healthcare domain. Here we present a draft for a lite ontological model consisting in 19 semantic categories and 16 relations for the representation of adverse drug reactions. From this model we selected 8 semantic categories for the categorial structure. This study was restricted to WHO-ART and additional research is required in order to provide complete coverage of MedDRA.
Introduction
Pharmacovigilance is “the science and activities relating to the detection, assessment, understanding and prevention of adverse effects or any other drug-related problems” [1]. An adverse drug reaction (ADR) is defined as “An appreciably harmful or unpleasant reaction, resulting from an intervention related to the use of a medicinal product, which predicts hazard from future administration and warrants prevention or specific treatment, or alteration of the dosage regimen, or withdrawal of the product [2].” WHO-ART (World Health Organisation – Adverse Reaction Terminology) [3] and MedDRA (Medical Dictionary for Drug Regulatory Activities) [4] are the terminologies used in pharmacovigilance for coding of ADRs and data statistical analysis. They are declared as mandatory by the national and international regulatory authorities.
WHO-ART and MedDRA are built on the model of traditional terminologies like the International Classification of diseases. Therefore there are no formal definitions available to constrain the meaning of terms. We showed that terminological reasoning improves the performances of both data mining [5] and data access [6] in pharmacovigilance databases.
As a first step we explored the manual construction of an ontology of ADR terms [7]. Then we tried several approaches for knowledge extraction using natural language processing techniques and/or mapping to the UMLS. While using the UMLS we considered mappings to both: Snomed international [8] and SNOMED-CT [9]. Mapping to SNOMED international was based on the SNOMED axes. In order to constrain the definitions obtained from SNOMED-CT we filtered the kinds of relations that were the most relevant for the study domain. Whereas the manual approach was very precise and time consuming, the knowledge extraction, albeit on a poor ontological basis, was very relevant to lowering the development costs.
Before we continue the development of the formal definitions, we need to ensure a consistent ontological representation that not only takes into account the minimal requirements for the representation of ADRs but also has a simple structure in order to avoid unnecessary complexity and time consumption like in the manual approach.
The European Standard Body CEN TC 251 WG2 (Comité Européen de Normalisation Technical Committee 251 Working Group 2) and later the International Standard Organisation ISO TC 215 WG3 elaborated and developed a standard approach for biomedical terminology named Categorial structure [10].
In order to build a road towards standardisation the European Standard Body CEN has stated that it was not possible to convince the different European member states using different national languages to agree on a reference clinical terminology or to standardise a detailed language independent biomedical ontology. The two main supportive arguments were that European countries speak different natural languages and that different health care professionals within the same natural language do not convey the same meaning through a particular terminology. CEN has developed since 1990 the categorial structure as a step standardising only the terminologies model structure. Indeed the standards needed to facilitate developments in biomedical terminologies and not prevent the quickly evolving volume of terms used for different goals.
The CEN Categorial structure is defined as a minimal set of health care domain constraints to represent a biomedical terminology in a precise healthcare domain for example surgical procedures [11], terminologies of human anatomy [12], or properties in laboratory medicine [13]. These constraints consist of 1) a list of semantic categories; 2) the goal of the Categorial structure; 3) the list of semantic links between semantic categories authorised with their associated semantic categories; 4) the minimal constraints allowing the generation and validation of well formed terminological phrases.
This work was limited to the study of WHO-ART. Our objective was to describe a Categorial structure for ADRs. In the next section we describe our set of terms and methodology of development of a Categorial structure. In the third section we describe the semantic categories and relations we identified. Finally we discuss limits of this work and future perspectives.
Method
WHO-ART terms:
WHO-ART (version 2004) consists of 1,857 preferred terms organized in a hierarchical structure based on 32 system organ classes. We used 639 terms corresponding to the WHO-ART preferred terms used for coding case reports in our extract of the French Pharmacovigilance database (entered by the Pharmacovigilance regional centre at the Georges Pompidou hospital).
Building the Categorial structures:
The categorial structure proposes a frame for a lite ontological organisation to ensure standardisation of the knowledge representation of terminologies for pharmacovigilance. Having described the goal of the Categorial structure in the introduction, here we focus on the description of semantic categories, semantic links and constraints. The Categorial structure shall satisfy the above mentioned four criteria but can also add more constraints.
The starting point was the development of an ontological model to represent the domain of pharmacovigilance. From this model we identified the minimum semantic categories, relations, and constraints that represent the categorial structure. The semantic categories, links and constraints were identified in an iterative way. A first schema was built using previous experience in ontological modelling of ADRs [7]. Then we considered each WHO-ART term in our sample by describing the meaning of the terms according to the semantic categories and relations in the schema. We added new semantic categories, changed the relations until the schema allowed to represent every term in a consistent way.
Results
List of semantic categories:
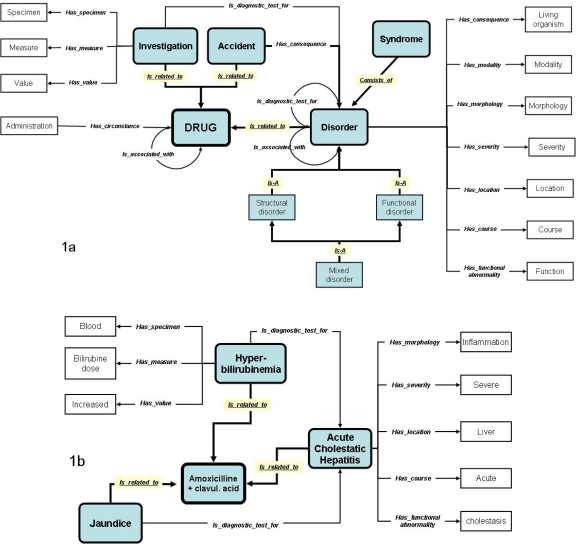
Figure 1a is our proposal for an ontological model of ADRs. The grey boxes and underlined relations correspond to the set of minimal constraints in the categorial structure. We identified 19 semantic categories (table 1) in the ontological model and the categorial structure consists of 8 semantic categories within this model. “Drug” is the central node within the schema. Considering ADR terminologies this drug is never specified because it would be impossible to describe every combination of drug and ADR. The “Administration”, for example “overdose” may be related to “Drug” with the Has_circumstances relation. The main categories are “Investigation (abnormal result of)” (e.g. SGPT increased, hypebilirubinemia etc.), “Accident” (falls, etc.), and “Disorder” (e.g Hepatitis, Anemia, Eruption, etc.). These are related to “Drug” with the Is_related_to link. One drug may be associated to a second drug (e.g. interactions) and a disorder may be related to a second disorder (e.g. Diabetic Nephropathy).
Figure 1.
(1a) Proposal for an ontological model of ADRs. Semantic categories within the categorial structure are displayed in grey color, and relations are underlined; (1b) Example of a pharmacovigilance case report with acute cholestatic hepatitis, hyperbilirubinemia, and jaundice.
Table 1.
Semantic categories
| Semantic category | Example |
|---|---|
| Accident | Fall |
| Administration | Overdose |
| Course | Chronic |
| Disorder | Hepatitis |
| Drug | Drug |
| Function (abnormal) | Tachycardia |
| Functional disorder | Hallucinations |
| Investigation | EEG abnormal |
| Living organism | Escherichia coli |
| Location | Liver |
| Measure | EEG |
| Mixed disorder | Cholestatic hepatitis |
| Modality | Site injection |
| Morphology | Inflammation |
| Severity | Severe |
| Specimen | Blood |
| Structural disorder | Gastric ulcer |
| Syndrome | Lyell’s syndrome |
| Value | Abnormal |
A disorder is related to several semantic categories: “Living organism” (when the drug is associated with some kind of immunosuppressive effect), “Modality” (for example site injection, local, or application site), “Morphology” (e.g. ulcer, haemorrhage), “Severity” (mild, severe, etc.), “Location” (cell, liver, etc.), “Course” (acute, chronic, etc.) and “Abnormal Function” (physical and mental). There are several kinds of disorders: “Structural disorder”, “Functional disorder”, and “Mixed disorder” (structural and functional). A “Syndrome” consists of several disorders (for example Lyell’s syndrome).
An “Accident” (e.g. a fall) may have a “Disorder” as a consequence (e.g. Fracture). The “Investigations” are related by the is_diagnostic_test_for link to “Disorder” and a “Disorder” may be a diagnostic test for another “Disorder”. “Investigations” are described in three semantic categories: “Specimen”, “Measure” and “Value”. For example “EEG abnormal” has_measure “EEG” and has_value “abnormal”.
Considering a given case report the drug is known but the resulting representation is a model of the case report and not a model of the ADR terminology. Figure 1b is an example of a case report that associates amoxicilline + clavulanic acid, and three ADRs: two disorders (acute cholestatic hepatitis and jaundice), and an investigation (hyperbilirubinemia).
List of semantic links:
We identified 16 semantic links: Consists_of, Has_Circumstances, Has_consequence, Has_course, Has_functional_abnormality, Has_location, Has_Measure, Has_Modality, Has_Morphology, Has_severity, Has_specimen, Has_value, Is associated_with, Is_A, Is_diagnostic_test_for, and Is_related_to.
Minimal constraints:
The set of minimal constraints was the following:
The ADR should be classified as a disorder, an accident, an investigation, or a syndrom.
A structural disorder is defined by at least one location and one morphology.
A functional disorder is defined by at least one abnormal function.
There are at least one semantic link is_related_to and one semantic category “Drug”.
Discussion
This work is a preliminary step towards the proposal of a Categorial structure for ADRs. Modelling ADRs is different from modelling other terminologies in more specific domains, for example, surgical procedures. There are several different semantic categories to represent - Disorders, Investigations, Accident, and so on.
Causal relation has a special meaning in pharmacovigilance. According to Meyboom et al. “causality assessment neither eliminates nor quantifies uncertainty but, at best, categorises it in a semi quantitative way” [14]. Some methods have been proposed for the imputation of the unexpected or toxic effects of drugs [15]. When considering an adverse effect, the circumstances of administration (e.g. an overdose) or modality (the lesion is close to the administration site) are important for the imputation of the ADR to the drug. This is a reason why it was important to introduce the Administration and Modality semantic categories in the model. However other criteria are important for imputation such as time relation but were not introduced in the model because they were not part of the WHO-ART concepts in our sample.
We decided to use the is_related_to link between “Drug” and the main categories (“Disorder”, “Investigation”, and “Accident”) rather than has_cause for two reasons:
One may never be sure of the causal relation between a drug and an adverse reaction.
There are several kinds of relations between a drug and an adverse reaction. For example a time relation when a patient has an anaphylactic shock within a couple of minutes after a drug injection; a statistical relation may be observed for example the risk of falling is higher in patients treated with certain drugs than in other patients [16].
Limitations:
We used a sample of WHO-ART terms used for coding case reports in a single regional pharmacovigilance centre. Some specific terms may be absent and require ad hoc representation. For example therapeutic ineffectiveness is a special kind of ADR [17]. We found several case reports of heparin that were coded with “thrombosis” rather than a term related to therapeutic ineffectiveness. It is not sure if the Modality semantic category can handle such finding or if a new semantic category is required. Therefore the model may evolve when new terms are considered and a complete evaluation is required to take the whole terminology into account. This evaluation should draw benefit from the classification of terms with a reasoner in order to check if the model is sound and consistent. We plan to consider semantic categories such as “Abnormal function” in order to differentiate between the physiologic function (e.g. cardiac rhythm) and the abnormality (e.g. tachycardia). Diseases and signs/symptoms are usually separated in other ontologies and this may be a requisite for the final model in order to ensure interoperability.
This work was limited to the study of WHO-ART and requires further research in order to provide additional features to represent the MedDRA terms. Contrary to WHO-ART, MedDRA includes specific system organ classes for investigations and social context whereas disorders and investigations are mixed in the same system organ classes in WHO-ART.
Some specific parts are not developed and may benefit from already published Categorial structures. For example there is a Categorial structure for “terminologies of human anatomy” that may complete the “Location” semantic category [12]. The Categorial structure on “Representation of dedicated kinds of property in laboratory medicine” may complete the “Investigation” semantic category [13].
Future Perspectives:
The 11th revision of the International Classification of Diseases (ICD) is a major effort of the World Health Organisation (WHO) in the terminological domain and will be based on a Categorial structure or similar model. WHO-ART, which is developed by a WHO centre, requires such model in order to ensure interoperability with the future version of ICD.
This work is part of the VigiTerms project that aims to build tools and methods for better signal detection in pharmacovigilance using knowledge engineering techniques. The PharmARTS tool allows searching case reports in a pharmacovigilance database according to semantic criteria [6]. It already contains formal definitions on ADRs extracted from SNOMED-CT that include information on finding site, morphology, function and living agent. The addition of semantic categories would increase the number and the performances of available queries in a pharmacovigilance database.
Acknowledgments
The VigiTerms project is supported by a grant from the French Agence Nationale pour la Recherche in the Technologies pour la Santé program.
REFERENCES
- 1.The Uppsala Monitoring Centre. The importance of Pharmacovigilance. WHO Technical report, 2002
- 2.Edwards IR, Aronson JK. Adverse Drug reactions: definitions, diagnosis, and management. Lancet. 2000;356:1255–59. doi: 10.1016/S0140-6736(00)02799-9. [DOI] [PubMed] [Google Scholar]
- 3.Uppsala Monitoring Center. The WHO-ART Adverse Reaction Terminology 2003http://www.umc-products.com/graphics/3149.pdf (last accessed Mar. 14, 2008).
- 4.Brown EG, Wood L, Wood S. The medical dictionary for regulatory activities (MedDRA) Drug Saf. 1999;20(2):109–17. doi: 10.2165/00002018-199920020-00002. [DOI] [PubMed] [Google Scholar]
- 5.Bousquet C, Henegar C, Lillo-Le Louët AL, Degoulet P, Jaulent MC. Implementation of automated signal generation in pharmacovigilance using a knowledge-based approach. Int J Med Inform. 2005;74(7–8):563–71. doi: 10.1016/j.ijmedinf.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 6.Alecu I, Bousquet C, Degoulet P, Jaulent MC.PharmARTS: terminology web services for drug safety data coding and retrieval Medinfo 200712Pt 1699–704. [PubMed] [Google Scholar]
- 7.Henegar C, Bousquet C, Lillo-Le Louët A, Degoulet P, Jaulent MC. Building an ontology of adverse drug reactions for automated signal generation in pharmacovigilance. Comput Biol Med. 2006;36(7–8):748–67. doi: 10.1016/j.compbiomed.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 8.Iavindrasana J, Bousquet C, Jaulent MC. Knowledge acquisition for computation of semantic distance between WHO-ART terms. Stud Health Technol Inform. 2006;124:839–44. [PubMed] [Google Scholar]
- 9.Alecu I, Bousquet C, Mougin F, Jaulent MC. Mapping of the WHO-ART terminology on Snomed CT to improve grouping of related adverse drug reactions. Stud Health Technol Inform. 2006;124:833–8. [PubMed] [Google Scholar]
- 10.Rodrigues JM, Kumar A, Bousquet C, Trombert B. Standards and Biomedical Terminologies: the CEN TC 251 and ISO TC 215 Categorial Structures. A step towards increased interoperability. Stud Health Technol Inform. 2008;136:857–62. [PubMed] [Google Scholar]
- 11.CEN AFNOR EN NF 1828:2002. Health informatics – Categorial Structure for classifications and coding systems of surgical procedures.
- 12.EN 15 521:2007. Health informatics –Categorial Structure for terminologies of human anatomy. [PubMed]
- 13.EN 1614: 2006, Health Informatics –Representation of dedicated kinds of property in laboratory medicine.
- 14.Meyboom RH, Hekster YA, Egberts AC, Gribnau FW, Edwards IR. Causal or casual? The role of causality assessment in pharmacovigilance. Drug Saf. 1997;17(6):374–89. doi: 10.2165/00002018-199717060-00004. [DOI] [PubMed] [Google Scholar]
- 15.Bégaud B, Evreux JC, Jouglard J, Lagier G. Imputation of the unexpected or toxic effects of drugs. Actualization of the method used in France. Therapie. 1985 Mar-Apr;40(2):111–8. [PubMed] [Google Scholar]
- 16.Souchet E, Lapeyre-Mestre M, Montastruc JL. Drug related falls: a study in the French Pharmacovigilance database. Pharmacoepidemiol Drug Saf. 2005;14(1):11–6. doi: 10.1002/pds.1038. [DOI] [PubMed] [Google Scholar]
- 17.Figueras A, Pedrós C, Valsecia M, Laporte JR. Therapeutic ineffectiveness: heads or tails? Drug Saf. 2002;25(7):485–7. doi: 10.2165/00002018-200225070-00002. [DOI] [PubMed] [Google Scholar]