Abstract
A home-monitoring program can be an important part of the follow-up care after lung transplantation surgery. We report mortality data from the home-monitoring program at University of Minnesota. The data from 246 lung recipients who participated in the home-monitoring program from 1992 to 2002 were analyzed. Subjects’ first year adherence rates were correlated with survival using a Cox proportional hazards model. The analysis showed a hazard ratio of 0.744, (95% CI 0.338–1.635). Kaplan-Meier survival analysis comparing the high adherence group (adherence rate > 75%) and the lower adherence group (adherence rate <= 75%) showed a tendency toward better survival, but again, it did not reach statistical significance (p=0.24). Competing risks analysis for causes of death showed a decreased risk ratio of 0.416 (95% CI 0.123–1.407) among pulmonary related mortality.
Introduction
Chronic disease management presents a major challenge to the current health care system. Home-monitoring, an important field of health informatics is gaining greater importance in chronic disease management. Poor adherence to home-monitoring frequently renders the technology ineffective. While adherence appears to be important, its impact on outcome must be evaluated. We provide an example of such a case among post lung-transplantation patients at the University of Minnesota.
Previously, studies have shown that home-monitoring provides early diagnostic information about rejection and infection of the lungs, which can prevent long-term transplant related complications.1 It also aids in detecting chronic rejection at an earlier time than the normal clinical follow-up.2 It was also associated with a shift from in-patient to out-patient care, resulting in potential cost savings for post-transplant care. 3
The current study presents the impact of post-lung transplant home-monitoring adherence on subjects’ survival.
Methods
Study Population:
Subjects were participants in the Lung Transplant Home Monitoring Program at the University of Minnesota. The study ran from 1992 to 2002, during which time there were 246 program participants, and the mortality data were collected until January 2007. All subjects provided written informed consent following the guidelines of the University of Minnesota Institutional Review Board. At the end of the study, subjects were able to switch to the University of Minnesota Medical Center, Fairview home spirometry program, which was the clinical successor of the research program
Home monitoring data:
Subjects were instructed to do daily spirometry measurements by performing three consecutive forced vital capacity (FVC) maneuvers using a home-based electronic spirometer/diary device (QRS Diagnostics, LLC, Minneapolis, MN USA). Daily data were stored in the device and downloaded weekly from the subjects’ home to the study data center using regular voice grade telephone lines. Forced vital capacity (FVC), forced expiratory volume in one second (FEV1), the peak expiratory flow rate (PEFR), and mid-range expiratory flow rates (FEF25–75) were determined from each FVC maneuver. Subjects also entered symptoms (frequency of coughing and wheezing, amount and color of sputum, dyspnea at rest after exercise, exercise time and activity, and level of stress and well-being) and vital signs (weight, pulse, temperature, and blood pressure) each day, using the device keypad. Symptoms and vital signs were stored daily and transmitted weekly with the spirometry data. The transmitted data were then stored in a database with one record per day of readings.
Adherence:
Weekly records of readings were used to determine adherence, where the subject was considered adherent for the week if at least one set of home monitoring data was sent for that week. The adherence rate was obtained by dividing the number of weeks with actual transmission by the maximum number of possible transmissions between the dates of the first and last readings. While our previous study subtracted the excused weeks from the total weeks of possible transmission, the data for excused weeks were not available from 2005 onward, and therefore it was not included. The adherence rate in the first year after transplantation was used in this analysis as it was the most influential adherence measure for the survival rate.
Survival Time:
Transplantation and mortality dates were obtained from a transplant database at University of Minnesota, and the survival time was defined as the time from transplantation to death. Cause of death was recorded according to UNOS heart-lung definitions, and other demographic data were obtained from the lung transplantation center at University of Minnesota. All the data were current as of January 23, 2007.
Statistical Analysis:
The raw data from home monitoring records were processed and analyzed using the SAS and R program. Survival plots were generated using Kaplan-Meier methods and the cumulative incidence plots were generated using the competing risk method. The Cox-proportional hazards model was used to analyze the association between adherence and mortality. Competing risks methodology was used to analyze the correlation between adherence and the cause specific death.
Results
Study Population:
A total of 262 post-lung transplant patients participated in the home monitoring program during the study period. Among these subjects, 16 were excluded from the analysis due to missing identification numbers (2), missing device reading information (8), and having incorrect or incompatible home monitoring readings, such as home-monitoring date preceding the transplantation dates (6). Thus, 246 patients’ records were included for analysis. Mean age was 49.3 (SD 11.8) and there were 146(59.4%) deaths on or before 1/23/2007. A slightly larger group of female subjects were noted and the majority of patients were Caucasian. The majority of underlying diseases leading to transplant were COPD, alpha-1-antitrypsin, cystic fibrosis (CF), and idiopathic pulmonary fibrosis (IPF).
Home Monitoring data:
A total of 132,822 daily readings of spirometry were collected from 1/27/1992 to 1/23/2007. The median duration of the monitoring (days between the first and the last reading) was 912.5 days (interquartile ranges 486 – 1760 days). The median number of the readings during the monitoring was 316.5 (interquartile ranges 108 –806).
Survival Analysis:
A Cox proportional hazard model showed a hazard ratio of 0.744 for the first year adherence with a 95% confidence interval of (0.338, 1.635). While the hazard ratio was less than one suggesting the negative association between the first year adherence and mortality, it did not reach statistical significance. Further analysis adjusting additional covariates was performed using the model (table 1). Age was positively associated with mortality, as was a pre-transplant diagnosis of IPF, comparing to having diagnosis of COPD.
Table 1.
Mortality analysis for all causes: primary predictor of adherence adjusting for age, gender, disease type and treatment type
| Hazard ratio | p-value | |
|---|---|---|
| First year adherence | 0.77 | 0.17 |
| Age (year) | 1.04 | 0.003 |
| Male | 0.78 | 0.17 |
| Disease (COPD as reference) | ||
| Alpha-1-antitrypsin | 0.75 | 0.31 |
| Primary pulm HTN | 1.55 | 0.35 |
| Eisenmenger's | 1.12 | 0.87 |
| IPF | 1.74 | 0.052 |
| CF | 0.81 | 0.65 |
| Rejection | 1.06 | 0.89 |
| Other | 1.25 | 0.72 |
| Treatment (bilateral as reference) | ||
| R lung | 1.58 | 0.11 |
| L lung | 1.10 | 0.76 |
| Heart/lung | 0.98 | 0.97 |
| Others | 0.84 | 0.87 |
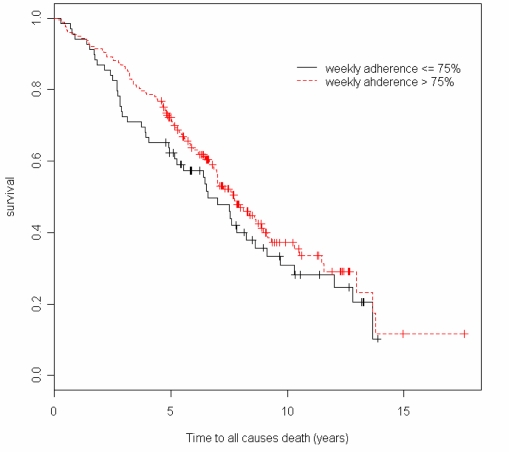
For the Kaplan-Meier plot, subjects’ were initially divided into quartiles, but since there were few subjects below a 75% adherence rate (28.5%), the lower three quartile groups (<=75%) were combined together and were compared with the highest quartile group (>75%). As seen in Figure 1, the higher adherence group had a better survival rate than the lower adherence group, but it did not reach statistical significance (p=0.24).
Figure 1.
Kaplan-Meier Plot of two adherence groups (Log-rank test p=0.24).
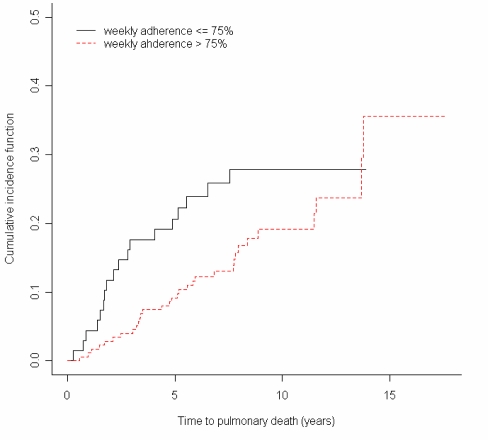
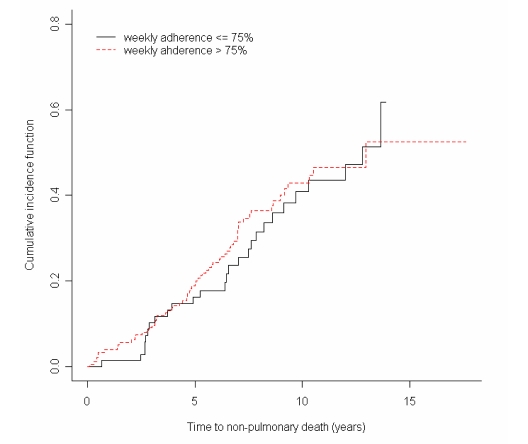
In order to determine the effect of home monitoring on pulmonary related death, competing risks analysis was performed4. The result showed a risk ratio of 0.416(95% CI 0.123–1.407) among pulmonary related mortality and a risk ratio of 1.347 (95% CI 0.508–3.572) for the non-pulmonary related mortality. The results suggest that risk was reduced with improved adherence for the subjects who subsequently died from pulmonary-related causes.
Cumulative incidence graphs5 demonstrating this difference based on cause of death are seen in figures 2 and 3, which shows a greater difference between groups in the pulmonary related mortality; both groups were very similar for non-pulmonary related mortality.
Figure 2.
Cumulative incidence function of pulmonary death in low and high adherence groups. (p = 0.17)
Figure 3.
Cumulative incidence function of NON-pulmonary death in low and high adherence groups. (p = 0.70)
Discussion
The adherence to home monitoring in the early years of the post-transplant period resulted in the trend towards improved survival. The competing risk regression analysis showed that the benefit came largely in the group that subsequently died from pulmonary-related causes. This was to be expected as monitoring pulmonary function would be most helpful in alerting a disease condition that has direct bearing on the lung. It probably was not as helpful in selecting out the development of other conditions with oncologic, cardiac, or non-pulmonary infectious causes that led to mortality.
One of the limitations of this study was the inclusion of the period in which “excused weeks” were not reported. The excused weeks were the weeks that patients had legitimate reasons not to perform the home monitoring tasks, such as going on a trip, hospitalization, etc. For this reason, we did not subtract the excused weeks from non-adherent period, and it may have underestimated the true adherence rates over all. While this is the case, when the subset of data during the periods with available excused weeks data was analyzed separately, the result did not differ substantially because the excused weeks occurred evenly among the groups.
Conclusion
This study shows that home monitoring for the post-lung transplantation patients has a positive impact on their survival, but it did not reach statistical significance. Further analysis of mortality causes showed a trend in greater reduction of the pulmonary related mortality, but this also did not reach statistical significance.
Acknowledgments
This research was funded in part by NLM Grant no. T15LM-07041(Training in Medical Informatics) and NIH grant no. R01NR02128.
References
- 1.Wagner FM, Weber A, Park JW, et al. New telemetric system for daily pulmonary function surveillance of lung transplant recipients. Ann Thorac Surg. 1999;68:2033–2038. doi: 10.1016/s0003-4975(99)01140-6. [DOI] [PubMed] [Google Scholar]
- 2.Finkelstein SM, Snyder M, Edin-Stibbe C, et al. Staging of bronchiolitis obliterans syndrome using home spirometry. Chest. 1999;116:120–126. doi: 10.1378/chest.116.1.120. [DOI] [PubMed] [Google Scholar]
- 3.Adam TJ, Finkelstein SM, Parente ST, Hertz MI. Cost analysis of home monitoring in lung transplant recipients. Int J Technol Assess Health Care. 2007 Spring;23:216–222. doi: 10.1017/S0266462307070080. [DOI] [PubMed] [Google Scholar]
- 4.Fine JP, Gray RJ. A proportional hazards model for the sub-distribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 5.Gray RJ. A class of k-sample tests for comparing the cumulative incidence of a competing risk. Annals of Statistics. 1988;16:1141–1154. [Google Scholar]
- 6.Dew MA, Dimartini AF, De Vito Dabbs A, et al. Adherence to the medical regimen during the first two years after lung transplantation. Transplantation. 2008;85:193–202. doi: 10.1097/TP.0b013e318160135f. [DOI] [PMC free article] [PubMed] [Google Scholar]