Abstract
The Internet is not simply being used to search for information about disease and treatment. It is also being used by online disease-focused communities to organize their own experience base and to harness their own talent and insight in service to the cause of achieving better health outcomes. We describe how news of a possible effect of lithium on the course of Amyotrophic Lateral Sclerosis (ALS) was acquired by and diffused through an on-line community and led to participation in a patient-driven observational study of lithium and ALS. Our discussion suggests how the social web drives demand for patient-centered health informatics.
Introduction
Amyotrophic lateral sclerosis (ALS) is a rare neurodegenerative disease that begins with loss of voluntary motor function and progresses to the inability to communicate, swallow, or breathe unaided. There is no cure for ALS. There is one drug that is FDA approved but only improves outcomes marginally. The prognosis for a patient with ALS is three to five years. In the efforts to find support and knowledge to improve quality of life and outcomes, a large subset of ALS patients turn to the Internet.
The Internet serves both to facilitate the search for information on new medical developments and connect like-minded patients. Patients and caregivers organized around shared medical data are finding, collectively evaluating, and using information to inform treatment decisions and drive change in medical discovery and translation to treatment. In the early days of HIV/AIDS patients self-organized to mine scientific literature to gain detailed knowledge of disease, choose treatment, and demand research [1]. Under conditions of similar treatment uncertainty, advocates of children with rare genetic disorders, for example parents of children with GIST, have built online resources to not only gather medical knowledge but share details of experience, to locate a definitive diagnosis, raise funds and steer research [2]. This present study reports what can occur with an online tool that collects and presents structured, quantified patient-reported data.
In November 2007, a patient relayed an Italian news report about a promising result of a human trial to an online ALS community, PatientsLikeMe. This occurred ahead of the formal scientific peer review and replication process. The small trial suggested lithium may have a beneficial effect for patients with ALS. This paper reports on a unique course of events - how people with ALS, and their physicians, leveraged community, data sharing, and the Internet to accelerate the evaluation of a treatment and conduct a real time open investigation on the effects of Lithium on disease progression.
These events have important implications for the design, deployment, and support of interactive medical records.
Methods
PatientsLikeMe© is an online community in which patients with life-altering diseases share information about treatments and outcomes and use a forum to exchange information and support. Opened to the public in March 2006, the ALS Community was the first PatientsLikeMe site. Two years after launch, the community has over 3,200 total members including caregivers, researchers, and providers. There are over 1,750 patient users. Subsequently, PatientsLikeMe has developed communities in Multiple Sclerosis, Parkinson’s disease, HIV, and mood conditions. We perform a mixed methods qualitative and quantitative study of forum posts and treatments adopted by the ALS community after the first report of the Lithium trial in November 2007.
Personal health profiles
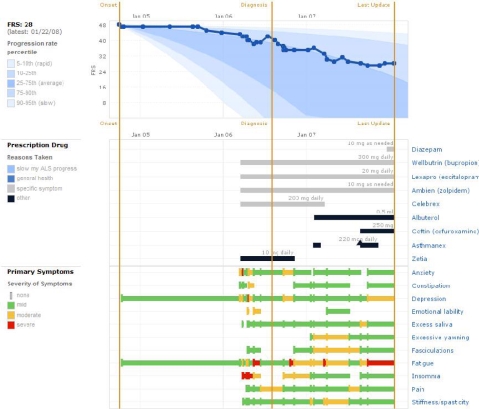
On PatientsLikeMe, patient-entered data are compiled and presented as a health history profile and shared within the site. The primary chart on the ALS site is a line graph of the individual’s functional level over time, superimposed onto a backdrop of population-level data (see Figure 1). Function is assessed through the clinically validated, self-administered form of the revised ALS functional rating scale (ALSFRS-R) [3]. Below the FRS chart are modified Gantt charts representing all the treatments taken and symptoms experienced. The profile is available for personal use and to be browsed and critiqued by other members.
Figure 1.
Charts comprising the personal profile.
Aggregate resources
Data are also aggregated from all individuals in the community to create community summaries of treatments and symptoms. Each element in these reports is hyperlinked to related items of interest e.g. other people on the treatment and related forum posts.
Social tools
Using search and browsing tools, members can locate other patients in similar circumstances and with shared medical experiences. Members discuss the profiles and reports, as well as general health concerns, through the Forum, private messages, and comments they post on one another’s profiles. The Forum is a threaded conversation available to every member of the community to pose questions, share research findings, share coping strategies, and so forth.
The study
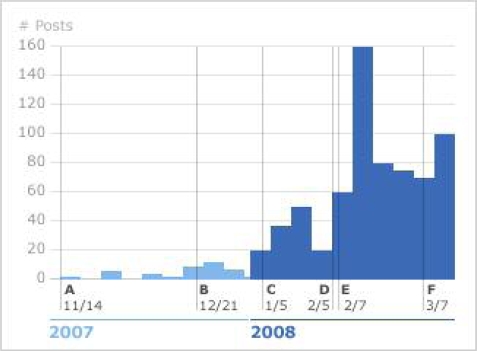
All Forum posts including the word “Lithium” were pulled from the site database. We plotted a frequency distribution of Lithium posts. We overlaid that plot with known significant events. We also observed changes in that frequency and used those observations to identify forum posts that appeared to spark those changes.
Results
Since November, when the study began, there have been 10,600 posts to the Forum included 687 containing the word “lithium”. 149 individuals contributed. 46% of these users posting once and 10% posted 13 or more times.
Time course of lithium conversation: The first post about the Italian lithium study appeared on the Forum on 11/14/2007 (Fig. 2, A). This post referenced an article in Italian from an online Italian newspaper, Dire Giovanni, written 6 days earlier. The article described findings presented at the 34th convention of the Lega Italiana per la lotta contro la malattia di Parkinson, le sindromi extrapiramidali e le demenze (LIMPE), which claimed that patients treated with lithium had not experienced significant progression of ALS symptoms during a 15-month trial, and had fared much better than a trial control group. The number of posts on lithium remained small through November and December, but there were several posts of note. On November 16, another member anticipated a negative response by drug companies because of the lack of commercial potential of this widely used drug. By the end of the month, users were aware of many of the details of the study. In a post on 11/27, a user indicated she knew that a paper would soon be published and reported the number of patients in the treatment group as 15 (the actual number is 16). This poster wanted to know whether people in the treatment group were still on the drug and what happened to them since the cessation of the trial.
Figure 2.
Frequency of Lithium Posts by week
At the beginning of December, a PatientsLikeMe researcher (Paul Wicks) was in attendance at 18th International ALS Symposium in Toronto, Canada. On December 5, a PatientsLikeMe user posted to the Forum requesting information about the informal conversation that took place at the symposium, noting that the proceedings indicated no formal presentation about the Italian study. In the week that followed, users noted other references to the study published in the Italian popular press, but the number of posts per week about lithium remained low.
The first reference to Lithium data recorded within PatientsLikeMe occurred on December 21 (B). In a post, a user noted that four patients on the site listed lithium as a treatment and asked for more information on the experience of those on the drug. A pivotal event occurred, on January 5, when a California caregiver, Karen Felzer, posted her intention to begin a “study” on lithium, noting her father’s recent diagnosis and intention to begin using lithium in the hopes of stopping his ALS progression (C). She, along with a Brazilian ALS patient, Humberto Macedo, had set up a website (http://alslithium.atspace.com/). Their site included a brief rationale for the use of lithium to treat ALS, based on a review of the Italian report and a spreadsheet to record patient information on functional status pre- and post-initiation of lithium therapy, lithium dosage, lithium blood levels, and annotations to record side-effects, benefits, and to enumerate other treatments. The site also included a simple chart showing the change in all patients’ functional status over time. Felzer advocated using PatientsLikeMe as a mechanism to determine FRS and asked people to add data to the spreadsheet. The following is her first post:
Hi everyone, I’m very enthusiastic about the news on the human ALS lithium trial coming out of Italy. I researched the literature and found that it has already been demonstrated that lithium decreases glutamate excitotoxicity, upregulates HSP-70 (Heat shock protein), down-regulates the neuron-killing caspase-3, and has many other beneficial neural-protective effects. Plus, it clearly crosses the blood-brain boundary! I am a skeptic by nature, and this is the first time I’ve been truly hopeful about any ALS treatment.
I posted an initial summary of my research, with references, and other information about taking and monitoring lithium for treatment of ALS at http://alslithium.atspace.com. Please take a look. I will be improving and editing the site over the next couple of weeks and welcome suggestions. I also want to keep careful track of the ALSFRS-R scores of everyone on lithium. I will do statistical analysis and post and publish promptly on what we find. But I need your help with this! If you already update your ALSFRS-R and other info on patientslikeme.com please just add to your profile your ALSFRS-R at the time that you started lithium, let me know your measured blood lithium level, and give me permission to use your data and to bug you at monthly intervals to update your ALSFRS-R.
Thank you so much!! Karen Felzer
The next week, the number of lithium posts jumped from 10 to 20. During the following two weeks, PatientsLikeMe researchers conferred with Felzer about her intentions and decided that a collaboration was in order.
To be as good as the spreadsheet, the site needed additional functionality including a way to record blood levels of lithium (which is how lithium dosing is monitored). PatientsLikeMe had the advantage of tighter control on data quality because outside of the site, patients could use slightly different questions to compute what is called the FRS.
In January, Felzer and Macedo maintained close contact with the members of their study, and postings to the PatientsLikeMe Forum documented growing interest in their efforts and the active tracking of lithium use both inside and outside the PatientsLikeMe system. Postings by PatientsLikeMe researchers during this time were limited to direct responses to users’ requests for comment on the news reports of the Italian study, Felzer’s attempt to organize a patient-driven observational study, and for links to basic information about lithium and its uses. The number of posts climbed for the next three weeks, then dropped again at the end of January.
During the first week of February, a refereed report of the Italian study was published in the Proceedings of the National Academy of Sciences[4] on-line edition and was quickly noted by PatientsLikeMe users. In light of our intention to collaborate with Felzer and Macedo, our research team posted a notice in the Forum on February 5 (See Figure 2, D), indicating our awareness of the publication of the Italian results, including a link to the freely downloadable journal article. Site researchers attempted to remain neutral on questions of efficacy and encouraged users to talk to their physicians. We stated: “Our goal is to, as much as possible, answer the question of whether lithium is an effective treatment in the real world by following the patients who decide to use the treatment.” We encouraged all users, both those on and off lithium, to continue to use our platform to measure their functional status, to track their treatments and symptoms, and share their experiences. A passionate conversation ensued in the Forum. Some members were incensed that the PatientsLikeMe researchers were not more positive about the study. Others advocated caution and restraint, the need to consult doctors and consider possible risks. One otherwise positive voice attempted to moderate with the post:
The study is what it is, whether you are a proponent or a critic makes no difference at the end of the day. Does it have its flaws? Sure. Does it have its redeeming points? Sure. It is a piece of evidence for people to use in their own judgments, nothing more, nothing less.
There was a short period of considerable discussion regarding the possible limitations of the Italian research, the difficulties associated with translating the results into treatment recommendations, and the need for further standard trials. Two days later (2/7), Humberto Macedo joined PatientsLikeMe and his first Forum posting announced “the Lithium Worldwide Survey” and reiterated the invitation for everyone using lithium to contribute their data to the Felzer-Macedo spreadsheet (E). As word of the publication of the Italian study spread and attention focused on the PatientsLikeMe response and the Felzer-Macedo “worldwide survey,” there was a sharp spike in Forum posts regarding lithium (160 that week). During this period the discussion quickly shifted from the quality of the Italian study and the hypothetical risks or benefits of treating ALS with lithium to ways to persuade your doctor to prescribe lithium, or alternatively finding a doctor willing to do so, how much lithium to take, how to monitor lithium blood levels, when to adjust dosages, possible side effects, and expectations or experience of positive benefits. There was also renewed criticism of the ALS Association (ALSA) and specialized treatment centers which have responded to patient requests for treatment with criticism of the Italian study and preliminary plans for new trials.
In this process, the patient community began to articulate its role not simply as consumers but as treatment subjects, reporters, analysts, and evaluators of knowledge. On February 19, one user collated all instances of user forum postings reporting benefits of lithium into a single forum post entitled: “Improvements reported by Lithium users,” in which he quoted eight members’ posts. Since that time, 2–3 members have added accounts to this thread daily. For PatientsLikeMe, it became necessary, and also an opportunity, to accelerate the development of tools to capture and evaluate new treatment information.
Response by PatientsLikeMe
During the month of February, PatientsLikeMe focused its attention on modifying and augmenting the system to improve data reporting and analysis relevant to the evaluation of Lithium. The PatientsLikeMe team turned its attention to the design of reporting mechanisms that would allow patients to explore for themselves the efficacy of the treatment based on the experience of site users. Our team included health services researchers, engineers, community support staff, and graphic designers. Our objective was to provide in a timely way methods for users to report relevant data for future analysis and preliminary tools for users to be able to filter and view their own data in the context of the total data reported by lithium users.
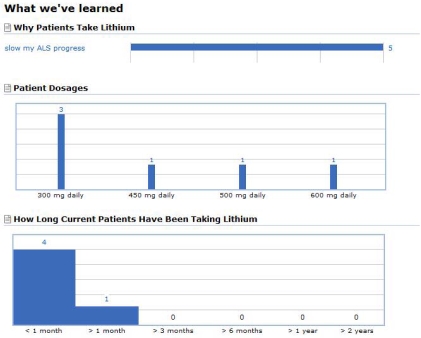
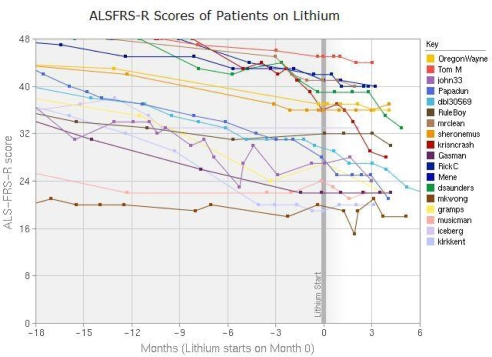
At that point, the site included a standard report on lithium. See Figure 3. To meet the new specialized need, we added functionality to record blood levels of lithium into the site, the ability to graph multiple people’s functional levels on the same axis, and mechanisms to filter the view by a variety of characteristics including: age class, onset type, functional level, gender, and treatment variation. We produced an interactive report that patients could use to navigate through the relevant data on the site. The new capability was formally announced on 3/7/08 (Fig. 2, F). See figure 4.
Figure 3.
Treatment report for Lithium before the redesign.
Figure 4.
Chart from the interactive Lithium treatment report after the redesign.
Members’ use of lithium
Prior to November and the announcement of the Italian study results, there was one patient in the PatientsLikeMe ALS community who reported taking lithium, and he did so for psychiatric purposes. Four months later there are 116 people on the drug. Members appear to be modeling their treatment regime on the Italian study. Most begin by taking the same initial dose then adjust their dose to achieve blood levels sought in that trial (target level = 0.4 mmol/l). We receive new lithium treatment reports daily.
Discussion
The ALS patient-driven natural experiment with lithium has implications for the design, deployment and support of personal health records (PHRs). Many institutions are beginning to embrace the Internet as a platform for improving patient engagement and support, but most PHRs only allow partial access to the electronic health record, scheduled appointments, prescriptions, test results, and link to “approved” health education materials. These consumer informatics exercises can become more patient-centered.
ALS patients have used the Internet to quickly shift from consuming to producing knowledge. We saw this within the PatientsLikeMe system, but found that it was happening outside our system when our users reported on the Felzer-Macedo Lithium Worldwide Survey. While the PatientsLikeMe system was used as it was intended (to share their treatment and symptoms) users were also reporting their data to the Felzer-Macedo project. They were clearly intrigued with the ability to see the experience of all patients together in real time. To make a successful collaboration with the Felzer-Macedo project, we needed to assess and respond to our users’ interests and needs. Our users brought new information to us quickly as well as bringing it to themselves, acted on it, and used it to help us develop tools that more effectively show them what they want to know. A patient-centered medical information system will not only meet patients’ desire for excellent care and shared decision making, but will address their craving for insight into the varieties of experiences associated with interventions rather than just showing what happens “on average”.
PatientsLikeMe users share their information openly with others. They are able to provide and see standardized information and qualitative information and engage in discussions around both types of information. In the case of lithium, social tools accelerated the sharing of information, allowed patients to organize themselves into a real-world “trial,” provided us with a reliable and steady stream of feedback about ways to enhance the use of our system, and alerted us to outside competition (albeit in this case friendly). As the data on use of lithium accumulates, it will allow the scientific and treatment communities to have a unique, real-time view on patient behaviors that would be otherwise unavailable. Surely, communities defined by affiliation with a hospital, provider network, or individual doctor’s office can be activated by issues as important to them as the lithium experiment is to the ALS community.
Conclusion
Our report shows that patients with few options will not wait for normal science to design studies, recruit patients, measure, analyze, and report. In the case of ALS and Lithium, patients are using the social web to organize their own experience base and to harness their own talent and insight in service of achieving better health outcomes. This platform provides the next generation of tools for the patients to assume the role of scientist. It is too early to tell with what effect, but the patient-driven lithium trial is happening. There is some concern that patient-led research of this type may not be reliable or credible, but these concerns exist in all scientific investigations. More work from within the community will be needed to create appropriate research and evaluation methods. In a world where patients finally own their health records and have more and growing options for sharing and using their data, there is little reason to believe that patient-driven trials will not occur more often across the disease spectrum.
Postscript
Since the time of submission of this manuscript (3/14/2008) the number of PatientsLikeMe patient users as increased to 2,200 (26%). The number of patients who are using or have used lithium is over 250.
There have been over 27,000 posts. 1,028 contain the word lithium. There are an additional 840 posts not containing the word lithium but which appear in a thread that contains lithium in the subject heading. 223 individuals have contributed with 79 (35%) posting once and 18% posting 13 or more times. About 125 patients have completed a side-effects survey; few have experienced any significant side effects.
Over this time, we have created and iterated upon a dedicated study page; it now includes additional filters, individual summaries of data completeness, and a lithium side-effects report.
References
- 1.Epstein S. Impure Science: AIDS, Activitism, and the Politics of Knowledge. Berkeley: UC Press; 1996. p. 466. [PubMed] [Google Scholar]
- 2.Solovitch S.The Citizen Scientists, in Wired 20012
- 3.Cedarbaum JM, et al. The ALSFRS-R: a revised ALS functional rating scale that incorporates assessments of respiratory function. BDNF ALS Study Group (Phase III) J Neurol Sci. 1999;169(1–2):13–21. doi: 10.1016/s0022-510x(99)00210-5. [DOI] [PubMed] [Google Scholar]
- 4.Fornai F, et al. Lithium delays progression of amyotrophic lateral sclerosis. Proc Natl Acad Sci U S A. 2008;105(6):2052–7. doi: 10.1073/pnas.0708022105. [DOI] [PMC free article] [PubMed] [Google Scholar]