Abstract
Curcumin, a major constituent of the spice turmeric, is a nutriceutical compound reported to possess therapeutic properties against a variety of diseases ranging from cancer to cystic fibrosis. In whole-cell patch-clamp experiments on bovine adrenal zona fasciculate (AZF) cells, curcumin reversibly inhibited the Kv1.4 K+ current with an IC50 of 4.4 µM and a Hill coefficient of 2.32. Inhibition by curcumin was significantly enhanced by repeated depolarization; however, this agent did not alter the voltage-dependence of steady-state inactivation. Kv1.4 is the first voltage-gated ion channel demonstrated to be inhibited by curcumin. Furthermore, these results identify curcumin as one of the most potent antagonists of these K+ channels identified thus far. It remains to be seen whether any of the therapeutic actions of curcumin might originate with its ability to inhibit Kv1.4 or other voltage-gated K+ channel.
Keywords: Curcumin, Potassium channel, Kv1.4
Curcumin (diferuloylmethane) is a phenolic compound isolated from the rhizome of Curcuma longa (turmeric), commonly used as a spice in some cultures where daily intake may reach several grams per day. Curcumin is also a nutriceutical agent reported to have therapeutic activity against a variety of diseases including cancer, Alzheimer’s disease, and cystic fibrosis [1–5]. At the molecular level, curcumin produces a range of effects, some of which may underlie its putative therapeutic actions [6]. The anti-cancer actions of curcumin may be due to its irreversible inhibition of amino-peptidase N1, an enzyme that plays a key role in tumor invasion and angiogenesis [1]. Curcumin induces apoptosis in mammary tumor cells through an action on P53 expression [2]. The potential effectiveness of curcumin in the prevention of Alzheimer’s disease may originate with its ability to inhibit formation of amyloid β oligomers and fibrils through direct binding to β amyloid species [3]. Well-known antioxidant and anti-inflammatory actions of curcumin may underlie its ability to induce heme oxygenase, a protein that provides efficient protection against oxidative stress [7].
More recently, curcumin has been shown to correct cystic fibrosis defects through its actions on the cystic fibrosis transmembrane conductance Cl-channel [4]. In cystic fibrosis, the cystic fibrosis transmembrane conductance regulator (CFTR) is retained in the endoplasmic reticulum (ER), where it is targeted for degradation. Curcumin induces the functional appearance of CFTR in the plasma membrane of airway cells, possibly through inhibition of the Ca2+-ATPase of the ER, allowing for the transport of this channel to the plasma membrane [4,5,8]. Additionally, curcumin has recently been reported to directly stimulate the activity of the CFTR Cl-channel by prolonging channel open time and reducing channel closed time [9].
In addition to the CFTR chloride channel, curcumin has been shown to inhibit the inositol 1,4,5-triphosphate receptor calcium channel of porcine cerebellar microsomes [10]. Until now, curcumin has not been reported to modulate the activity of voltage-gated ion channels. In the present study, we found that curcumin potently and reversibly inhibits the voltage-gated, rapidly inactivating Kv1.4 K+ channels expressed by bovine adrenal zona fasciculate cells [11].
Materials and methods
Tissue culture media, antibiotics, fibronectin, and fetal bovine sera (FBS) were obtained from Invitrogen (Carlsbad, CA). Coverslips were from Bellco (Vineland, NJ). Phosphate-buffered saline (PBS), enzymes, 1,2-bis-(2-aminophenoxy)ethane-N,N,N′,N′′-tetraacetic acid (BAPTA), cyclic AMP, and ATP were from Sigma (St. Louis). Curcumin was purchased from Biomol (Plymouth Meeting, PA).
Isolation and culture of AZF cells
Bovine adrenal glands were obtained from steers (age 2–3 years) at a local slaughterhouse. Isolated AZF cells were obtained and prepared as previously described [12]. After isolation, cells were either resuspended in DMEM/F12 (1:1) with 10% FBS, 100 U/ml penicillin, 0.1 mg/ml streptomycin, and the antioxidants 1 µM tocopherol, 20 nM selenite, and 100 µM ascorbic acid (DMEM/F12+), and plated for immediate use or resuspended in FBS/5% DMSO, divided into 1 ml aliquots, and stored in liquid nitrogen for future use. For patch-clamp experiments, cells were plated in DMEM/F12+ in 35 mm dishes containing 9 mm2 glass coverslips. Coverslips were treated with fibronectin (10 µg/ml) at 37 °C for 30 min and then rinsed with warm, sterile PBS immediately before adding cells. Cells were maintained at 37 °C in a humidified atmosphere of 95% air-5% CO2.
Recording conditions and electronics
AZF cells were used for experiments 2–12 h after plating. Typically, cells with diameters <15 µm and capacitances of 8–15 pF were selected. Coverslips were transferred from 35 mm culture dishes to the recording chamber (volume, 1.5 ml) which was perfused by gravity at a rate of 3–5 ml/min. Patch electrodes with resistances of 1.0–2.0MΩ were fabricated from Corning 0100 glass (WPI, Sarasota, FL) using a Brown-Flaming model P-97 microelectrode puller (Sutter Instruments, Novato, CA). K+ currents were recorded at room temperature (22–25 °C) following the procedure of Hamill et al. [13] with a List-EPC 7 patch-clamp amplifier (Axon Instruments, Burlingame, CA).
Pulse generation and data acquisition were done using a personal computer and PCLAMP software with TL-1 interface (Axon Instruments). Currents were digitized at 2–20 KHz after filtering with an eight-pole Bessel filter (Frequency Devices, Haverhill, MA). Linear leak and capacity currents were subtracted from current records using scaled hyperpolarizing steps of one-third to one-fourth amplitude. Data were analyzed and plotted using PCLAMP 9 (Clampfit) and SigmaPlot (ver 8.0).
Recording solutions
For recording whole-cell K+ currents, the standard pipette solution consisted of (in mM): 120 KCl, 2MgCl2, 1 CaCl2, 10 Hepes, 11 BAPTA, 0.2 GTP, and 2 MgATP with pH titrated to 7.2 using KOH. With this composition, free [Ca2+] was determined to be 2.3 × 10−8 M using the “Bound and Determined” program [14]. The development of the noninactivating bTREK K+ current that is present in these cells was completely eliminated by including 100 µM cAMP in the pipette solution. cAMP selectively inhibits the bTREK K+ current and does not alter Kv1.4 [15]. Pipette solutions were filtered through 0.22 μ cellulose acetate filters. The external solution consisted of (in mM): 140 NaCl, 5 KCl, 2 CaCl2, 2 MgCl2, 10 Hepes, and 5 glucose, with pH adjusted to 7.3 using NaOH.
Results
Bovine AZF cells express two distinctive K+ currents. These include a voltage-gated, rapidly inactivating Kv1.4 K+ current and a background, two-pore domain bTREK-1 K+ current [16,17]. Kv1.4 can be isolated in whole-cell recordings by including 100 µM cAMP in the recording pipette to selectively block the bTREK-1 current [11].
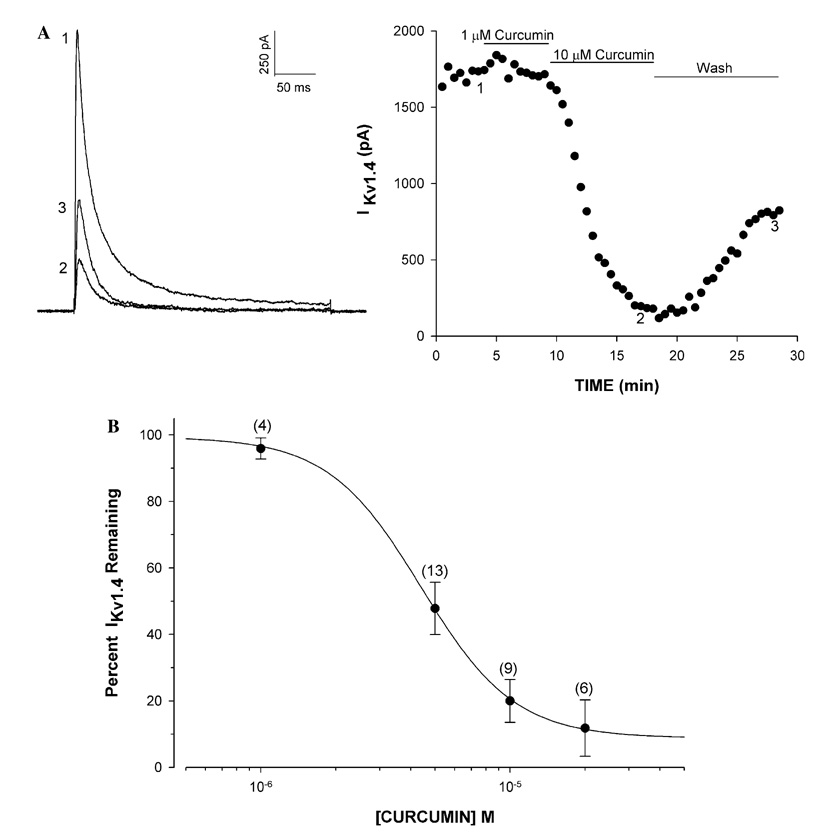
The Kv1.4 K+ current in AZF cells was potently blocked by curcumin with an IC50 of 4.4 × 10−6 ± 0.13 µM and a Hill coefficient of 2.32 ± 0.24 (Fig. 1A and B). At a concentration of 10 µM, inhibition by curcumin reached a steady-state within 10 min and was slowly reversible upon washing (Fig. 1A).
Fig. 1.
Concentration-dependent inhibition of Kv1.4 K+ current by curcumin in bovine AZF cells. Kv1.4 K+ current was activated from a holding potential of −80 mV by voltage steps to +20 mV, applied at 30 s intervals. After recording currents in standard saline, cells were superfused with curcumin at various concentrations. (A) K+ current traces and corresponding plot of Kv1.4 peak amplitude against time for cell superfused with curcumin at the indicated concentrations. Numbers on traces correspond to currents recorded at times indicated on graph at right. (B) Inhibition curve for curcumin constructed from experiments as in (A). Percentage of unblocked current is plotted against curcumin concentration. Data are fit with the equation: I/Imax × 100 = 1/1 + (B/IC50)x where B is curcumin concentration, IC50 is the concentration that reduces Kv1.4 by 50%, and x is the Hill slope.
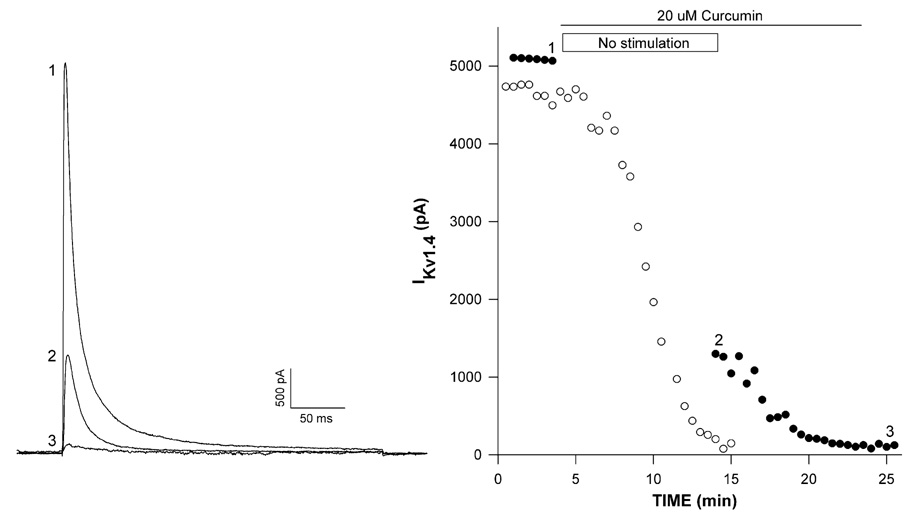
Some antagonists preferentially bind to and block channels that are in the conformations corresponding to open or inactivated, rather than the closed state. Block by these agents is often use-dependent and enhanced by repeated depolarization [18–20]. Inhibition of Kv1.4 by curcumin displayed limited use-dependence. In the experiment illustrated in Fig. 2, K+ currents were recorded in standard saline before superfusing curcumin (20 µM) with (●) or without (○) a 10 min stimulation-free period. With uninterrupted stimulation, Kv1.4 K+ current was inhibited nearly completely after a 10 min exposure to curcumin (Fig. 2, right panel). By comparison, when superfusion of curcumin was accompanied by a 10 min pulse-free period, inhibition had reached maximum value of 74.8% upon resumption of depolarizing steps (Fig. 2, trace 2). When voltage steps were applied for an additional 5 min, inhibition reached a steady-state value of 97.6%. Overall, in a total of four experiments, curcumin inhibited Kv1.4 by 68.4 ± 6.5% in the absence of stimulation, while inhibition increased to 96.3 ± 3.9% (n = 4) when voltage steps were applied at 30 s intervals for 5 min.
Fig. 2.
Use-dependent block of Kv1.4 K+ current by curcumin. Kv1.4 K+ currents were activated after recording currents at 30 s intervals from a holding potential of −80 mV by voltage steps to +20 mV. After recording currents in standard saline, cells were superfused with 20 µM curcumin with or without a 10 min pulse-free period. Kv1.4 K+ current traces (left panel) and corresponding plot (right panel) of peak amplitudes against time (closed circles) for a cell superfused with curcumin for 10 min with no stimulation. Numbers on traces correspond to currents recorded at indicated times on graph at right. Open circles on plot show time-dependent inhibition by curcumin with uninterrupted stimulation.
Inhibition of Kv1.4 by curcumin could occur through a direct occlusion of the pore, or by an allosteric mechanism whereby preferential binding of this agent to the inactivated state of the channel produces a hyperpolarizing shift in the steady-state availability such that channels inactivate at more negative potentials [19,20]. The voltage-dependent steady-state inactivation of the Kv1.4 K+ currents was assessed in the absence and presence of curcumin by applying 10 s conditioning pulses to various potentials between −80 and 0 mV, followed by activating steps to +20 mV. Normalized K+ currents were averaged and the mean values were fit with a smooth curve according to the Boltzmann relationship I/Imax = 1/[1+exp (υ − υ1/2/K)] where Imax is the current activated from a holding potential of −80 mV, υ1/2 is the voltage where 1/2 of the channels are in the open configuration, and K is the slope factor. Curcumin failed to significantly shift the voltage-dependence of Kv1.4 inactivation. In control saline, Kv1.4 inactivated with a υ1/2 of −51.7 ± 1.5 mV (n = 6), compared to −49.8 ± 2.7 mV (n = 3) in the presence of 5 µM curcumin.
Discussion
The findings of this study identify curcumin as one of the most potent organic antagonists of A-type voltage-gated K+ currents yet described. Kv1.4 K+ channels are widely distributed in excitable cells of mammalian tissues, including the brain and heart [21–23]. At the cellular level in neurons, rapidly inactivating K+ channels function pivotally in regulating action potential waveform and firing frequency [18,24]. At the subcellular level in neurons, Kv1.4 K+ channels are located at presynaptic nerve terminals where they may function in the regulation of transmitter release [25,26]. Consequently, the pharmacology of rapidly inactivating K+ channels has been a subject of interest.
A summary of the pharmacology of Kv1.4 K+ channels is provided in Table 1. Among the classic antagonists of voltage-gated K+ channels, curcumin is more than 25 and 1000 times as potent as 4-aminopyridine and TEA, respectively, as inhibitors of these channels. Curcumin is also significantly more potent than each of the organic antagonists identified as Kv1.4 blockers to date. The inhibition curve for curcumin was steep, with a Hill coefficient of 2.321, indicating that multiple curcumin molecules interact with a single Kv1.4 channel.
Table 1.
Pharmacology of Kv1.4 K+ channels
Block of Kv1.4 by curcumin exhibited partial use-dependence and did not shift the voltage-dependence of steady-state inactivation. These results suggest that curcumin may bind with slightly higher affinity to open, rather than inactivated, K+ channels and thus its potency increases under conditions of repeated depolarization where Kv1.4 channels spend a larger fraction of time in the open conformation [19].
Curcumin is consumed in large quantities in the diet of certain individuals, yet side effects in humans have rarely been reported. In a phase 1 clinical trial using curcumin as a chemoprotective agent for patients with pre-malignant lesions, curcumin was not toxic to humans in amounts up to 8000 mg/day [27]. However, peak serum levels in these patients were only 1.77 µM, a value several fold lower than our measured IC50 for inhibition of Kv1.4 K+ current. It is conceivable that, if curcumin were consumed or administered in even higher amounts as a therapeutic or prophylactic agent, serum concentrations could be reached where block of Kv1.4 and perhaps other ion channels could occur. In this regard, the potency of curcumin as an inhibitor of Kv1.4 is similar to that reported for stimulation of the CFTR chloride channel, and for inhibition of the IP3 receptor Ca2+ channel [9,10].
It is not known if any of the many therapeutic actions attributed to curcumin could occur through interaction with voltage-gated K+ channels such as Kv1.4. However, K+ channels including Kv1.4 regulate the proliferation of various cells, including oligodendrocyte progenitors and vascular smooth muscle cells, and pituitary adenomas [8,28–30]. Block of these K+ channels could contribute to the anti-neoplastic actions of curcumin.
Acknowledgments
This work was supported in part by National Institutes of Health Grant R01-DK47875 (to J.J.E.) and in part by the National Science Foundation under Agreement 0112050 (S.D.).
References
- 1.Shim JS, Kim JH, Cho HY, Yum YN, Kim SH, Park HJ, Shim BS, Choi SH, Kwon HJ. Irreversible inhibition of CD13/aminopeptidase N by the antiangiogenic agent curcumin. Chem. Biol. 2003;10:695–704. doi: 10.1016/s1074-5521(03)00169-8. [DOI] [PubMed] [Google Scholar]
- 2.Choudhuri T, Pal S, Das T, Sa G. Curcumin selectively induces apoptosis in deregulated cyclin D1-expressed cells at G2 phase of cell cycle in a p53-dependent manner. J. Biol. Chem. 2005;280:20059–20068. doi: 10.1074/jbc.M410670200. [DOI] [PubMed] [Google Scholar]
- 3.Yang F, Lim GP, Begum AN, Ubeda OJ, Simmons MR, Ambegaokar SS, Chen PP, Kayed R, Glabe CG, Frautschy SA, Cole GM. Curcumin inhibits formation of amyloid beta oligomers and fibrils, binds plaques, and reduces amyloid in vivo. J. Biol. Chem. 2005;280:5892–5901. doi: 10.1074/jbc.M404751200. [DOI] [PubMed] [Google Scholar]
- 4.Egan ME, Pearson M, Weiner SA, Rajendran V, Rubin D, Glockner-Pagel J, Canny S, Du K, Lukacs GL, Caplan MJ. Curcumin, a major constituent of turmeric, corrects cystic fibrosis defects. Science. 2004;304:600–602. doi: 10.1126/science.1093941. [DOI] [PubMed] [Google Scholar]
- 5.Zeitlin P. Can curcumin cure cystic fibrosis? N. Engl. J. Med. 2004;351:606–608. doi: 10.1056/NEJMcibr041584. [DOI] [PubMed] [Google Scholar]
- 6.Maheshwari RK, Singh AK, Gaddipati J, Srimal RC. Multiple biological activities of curcumin: a short review. Life Sci. 2006;78:2081–2087. doi: 10.1016/j.lfs.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 7.Scapagnini G, Foresti R, Calabrese V, Giuffrida Stella AM, Green CJ, Motterlini R. Caffeic acid phenethyl ester and curcumin: a novel class of heme oxygenase-1 inducers. Mol. Pharmacol. 2002;61:554–561. doi: 10.1124/mol.61.3.554. [DOI] [PubMed] [Google Scholar]
- 8.Czarnecki A, Dufy-Barbe L, Huet S, Odessa MF, Bresson-Bepoldin L. Potassium channel expression level is dependent on the proliferation state in the GH3 pituitary cell line. Am. J. Physiol. Cell physiol. 2003;284:C1054–C1064. doi: 10.1152/ajpcell.00446.2002. [DOI] [PubMed] [Google Scholar]
- 9.Berger AL, Randak CO, Ostedgaard LS, Karp PH, Vermeer DW, Welsh MJ. Curcumin stimulates cystic fibrosis transmembrane conductance regulator Cl-channel activity. J. Biol. Chem. 2005;280:5221–5226. doi: 10.1074/jbc.M412972200. [DOI] [PubMed] [Google Scholar]
- 10.Dyer JL, Khan SZ, Bilmen JG, Hawtin SR, Wheatley M, Javed Mu, Michelangeli F. Curcumin: a new cell-permeant inhibitor of the inositol 1,4,5-triphosphate receptor. Cell Calcium. 2002;31:45–52. doi: 10.1054/ceca.2001.0259. [DOI] [PubMed] [Google Scholar]
- 11.Enyeart JA, Xu L, Enyeart JJ. A bovine adrenocortical Kv1.4 K+ channel whose expression is potently inhibited by ACTH. J. Biol. Chem. 2000;275:34640–34649. doi: 10.1074/jbc.M004214200. [DOI] [PubMed] [Google Scholar]
- 12.Enyeart JJ, Gomora JC, Xu L, Enyeart JA. Adenosine triphosphate activates a noninactivating K+ current in adrenal cortical cells through nonhydrolytic binding. J. Gen. Physiol. 1997;110:679–692. doi: 10.1085/jgp.110.6.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch clamp techniques for high resolution current recording from cells and cell-free membrane patches. Pfügers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- 14.Brooks SP, Storey KB. Bound and determined: a computer program for making buffers of defined ion concentrations. Anal. Biochem. 1992;201:119–126. doi: 10.1016/0003-2697(92)90183-8. [DOI] [PubMed] [Google Scholar]
- 15.Enyeart JJ, Mlinar B, Enyeart JA. Adrenocorticotropic hormone and cAMP inhibit noninactivating K+ current in adrenocortical cells by an A-kinase-independent mechanism requiring ATP hydrolysis. J. Gen. Physiol. 1996;108:251–264. doi: 10.1085/jgp.108.4.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mlinar B, Biagi BA, Enyeart JJ. A novel K+ current inhibited by ACTH and Angiotensin II in adrenal cortical cells. J. Biol. Chem. 1993;268(No 12):8640–8644. [PubMed] [Google Scholar]
- 17.Enyeart JJ, Xu L, Danthi S, Enyeart JA. An ACTH- and ATP-regulated background K+ channel in adrenocortical cells is TREK-1. J. Biol. Chem. 2002;277:49186–49199. doi: 10.1074/jbc.M207233200. [DOI] [PubMed] [Google Scholar]
- 18.Hille B. Ion Channels of Excitable Membranes. Sinauer Associates; 2001. [Google Scholar]
- 19.Hondeghem LM, Katzung BG. Time and voltage-dependent interactions of antiarrhythmic drugs with cardiac sodium channelsm. Biochem. Biophys. Acta. 1977;472:373–398. doi: 10.1016/0304-4157(77)90003-x. [DOI] [PubMed] [Google Scholar]
- 20.Bean B, Cohen CJ, Tsien RW. Lidocaine block of cardiac sodium channelsm. J. Gen. Physiol. 1983;81:613–642. doi: 10.1085/jgp.81.5.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lujan R, de Cabo de la Vega C, Dominguez del Toro E, Ballesta JJ, Criado M, Juiz JM. Immunohistochemical localization of the voltage-gated potassium channel subunit Kv1.4 in the central nervous system of the adult rat. J. Chem. Neuroanat. 2003;26:209–224. doi: 10.1016/j.jchemneu.2003.07.006. [DOI] [PubMed] [Google Scholar]
- 22.Wymore RS, Negulescu D, Kinoshita K, Kalman K, Aiyar J, Gutman GA, Chandy KG. Characterization of the transcription unit of mouse Kv1.4, a voltage-gated potassium channel gene. J. Biol. Chem. 1996;271:15629–15634. doi: 10.1074/jbc.271.26.15629. [DOI] [PubMed] [Google Scholar]
- 23.Brahmajothi MV, Campbell DL, Rasmusson RL, Morales MJ, Trimmer JS, Nerbonne JM, Strauss HC. Distinct transient outward potassium current (Ito) phenotypes and distribution of fast-inactivating potassium channel alpha subunits in ferret left ventricular myocytes. J. Gen. Physiol. 1999;113:581–600. doi: 10.1085/jgp.113.4.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Neher E. Two fast transient current components during voltage clamp on snail neurons. J. Gen. Physiol. 1971;58:36–53. doi: 10.1085/jgp.58.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sheng M, Tsaur ML, Jan YN, Jan LY. Subcellular segregation of two A-type K+ channel proteins in rat central neurons. Neuron. 1992;9:271–284. doi: 10.1016/0896-6273(92)90166-b. [DOI] [PubMed] [Google Scholar]
- 26.Cooper EC, Milroy A, Jan YN, Jan LY, Lowenstein DH. Presynaptic localization of Kv1.4-containing A-type potassium channels near excitatory synapses in the hippocampus. J. Neurosci. 1998;18:965–974. doi: 10.1523/JNEUROSCI.18-03-00965.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheng AL, Hsu CH, Lin JK, Hsu MM, Ho YF, Shen TS, Ko JY, Lin JT, Lin BR, Ming-Shiang W, Yu HS, Jee SH, Chen GS, Chen TM, Chen CA, Lai MK, Pu YS, Pan MH, Wang YJ, Tsai CC, Hsieh CY. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res. 2001;21:2895–2900. [PubMed] [Google Scholar]
- 28.Vautier F, Belachew S, Chittajallu R, Gallo V. Shaker-type potassium channel subunits differentially control oligodendrocyte progenitor proliferation. Glia. 2004;48:337–345. doi: 10.1002/glia.20088. [DOI] [PubMed] [Google Scholar]
- 29.Ivanov A, Gerznaich V, Ivnaova S, Denhaese R, Tsymbalyuk O, Simard JM. Adenylate cyclase 5 and KCa1.1 channel are required for EGFR up-regulation of PCNA in native contractile rat basilar artery smooth muscle. J. Physiol. 2006;570:73–84. doi: 10.1113/jphysiol.2005.100883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cheong A, Bingham AJ, Li J, Kumar B, Sukumar P, Munsch C, Buckley NJ, Neylon CB, Porter KE, Beech DJ, Wood IC. Downregulated REST transcription factor is a switch enabling critical potassium channel expression and cell proliferation. Mol. Cell. 2005;20:45–52. doi: 10.1016/j.molcel.2005.08.030. [DOI] [PubMed] [Google Scholar]
- 31.Zhang H, Zhu B, Yao JA, Tseng GN. Differential effects of S6 mutations on binding of quinidine and 4-aminopyridine to rat isoform of Kv1.4: common site but different factors in determining blockers’ binding affinity. J. Pharmacol. Exp. Ther. 1998;287:332–343. [PubMed] [Google Scholar]
- 32.Choi BH, Choi JS, Ahn HS, Kim MJ, Rhie DJ, Yoon SH, Min DS, Jo YH, Kim MS, Hahn SJ. Fluoxetine blocks cloned neuronal A-type K+ channels Kv1.4. Neuroreport. 2003;14:2451–2455. doi: 10.1097/00001756-200312190-00032. [DOI] [PubMed] [Google Scholar]
- 33.Herrera D, Mamarbachi A, Simoes M, Parent L, Sauve R, Wang Z, Nattel S. A single residue in the S6 transmembrane domain governs the differential flecainide sensitivity of voltage-gated potassium channels. Mol. Pharmacol. 2005;68:305–316. doi: 10.1124/mol.104.009506. [DOI] [PubMed] [Google Scholar]
- 34.Yamagishi T, Ishii K, Taira N. Antiarrhythmic and bradycardic drugs inhibit currents of cloned K+ channels, Kv1.2 and Kv1.4. Eur. J. Pharmacol. 1995;281:151–159. doi: 10.1016/0014-2999(95)00240-l. [DOI] [PubMed] [Google Scholar]
- 35.Mlinar B, Enyeart JJ. Voltage-gated transient currents in bovine adrenal fasciculata cells II: A-type K+ current. J. Gen. Physiol. 1993;102:239–255. doi: 10.1085/jgp.102.2.239. [DOI] [PMC free article] [PubMed] [Google Scholar]