Abstract
To investigate the possibility of the involvement of an oxidative stress induction in the mechanism of the cytotoxic effect of quinolone antibiotics, we examined the viability of human fibroblast cells exposed to ciprofloxacin (CPFX), and measured the levels of lipid peroxidation (LP), glutathione (GSH), and the activities of the antioxidant enzymes catalase (CAT), superoxide dismutase (SOD), and glutathione peroxidase (GPX). The data showed that the effect of CPFX on the viability of cells, as determined by neutral red uptake assay, was time-dependent, and the dose-response relation was biphasic. Cytotoxicity was not observed in the concentration range 5–150 mg/l CPFX when the cells were incubated for 24 h. In contrast, lower concentrations (5 and 12.5 mg/l) of CPFX increased the cell growth in all incubation periods tested. Marked decreases in the viability of fibroblasts were observed at concentrations 50 and 75 mg/l, and ≥50 mg/l, following 48 and 72 h exposure, respectively (p < 0.05). However, when the cells were exposed to > 75 mg/l CPFX for 48 h, no cytotoxicity was observed. By exposing fibroblast cultures to 75 mg/l CPFX for 48 h, an induction of LP enhancement and a marked decrease in intracellular GSH were observed. Vitamin E pretreatment of the cells lowered the level of LP, increased the total GSH content, and provided significant protection against CPFX-induced cytotoxicity. The biphasic effect of CPFX possibly resulted from the complex dose-dependent relationships between reactive oxygen species (ROS), cell proliferation, and cell viability. It was previously reported, in fact, for several cell models that ROS exert a biphasic effect on cell growth. Furthermore, cultured fibroblasts release their own free radicals, and the inhibition of endogenous ROS inhibits the fibroblast cell proliferation, whereas the effect of exogenous ROS is biphasic.
Keywords: biphasic response, ciprofloxacin, cytotoxicity, fibroblast cell cultures, hormesis, reactive oxygen species
INTRODUCTION
Hormesis is described as characterizing the dose-response continuum as stimulatory at low doses and inhibitory at high doses, and leading to the biphasic, hormetic dose-response curve (Calabrese and Baldwin, 2001). Our recent studies on fluoroquinolone antibiotic ciprofloxacin (CPFX) in human fibroblast cell cultures resulted in a biphasic response that appeared to be a good example for the hormesis concept (Gürbay et al., 2002).
Our interest in CPFX was started with a point of view that its adverse effects, particularly phototoxicity and epileptogenic seizures, might be related to an oxidative stress induction. Considering the occurrence of oxidative radical reactions (at a continuous rate and usually in association with cellular electron transfer chains and certain enzyme activities), the concept of the existence of a physiological steady-state level of reactive oxygen species (ROS) in mammalian tissues is well accepted (Boveris and Cadenas, 1997). The consequences of xenobiotically induced disturbances in that delicate balance are of great importance. Therefore, we investigated earlier the possibility of oxidative stress induction by CPFX in vivo and observed significant enhancement of lipid peroxidation (LP) and alteration of glutathione redox status (GSSG%) in cerebral and hepatic tissues of rats (Hincal and Taskin, 1995). Furthermore, vitamin E or allopurinol pretreatment was found to be significantly protective. Several investigators also reported that the phototoxic effects of quinolones were related to the generation of ROS, particularly singlet oxygen and superoxide anion, in the presence of UVA irradiation in vivo and in vitro (Wagai and Tawara, 1991, 1992a, 1992b; Umezawa et al., 1997).
In the present study, our aim was to elucidate the involvement of oxidative stress and LP induction in the mechanism of action of CPFX in mammalian cells in the absence of UVA irradiation.
MATERIALS AND METHODS
Chemicals
Penicillin, streptomycin, and kanamycin were purchased from Boehringer-Mannheim (Mannheim, Germany), RPMI 1640 medium and fetal calf serum (FCS) from Biological Industries (Israel), sodium bicarbonate 7.5% and Puck’s saline A from Gibco (Scotland), fungizone from Squibb (Princeton, NJ, USA) and Tris from Merck (Darmstad, Germany). CPFX was a gift from Deva Laboratory (Istanbul, Turkey). The other chemicals, including α-tocopherol (vitamin E), were purchased from Sigma (Saint Louis, MO, USA).
Cell Culture
Primary cell lines of fibroblasts were prepared after skin biopsy from voluntary healthy adults aged 20–40 years. Dilacerated skin samples were incubated in calf-serum-rich culture medium [RPMI 1640 medium, supplemented with 10% FCS, L-glutamine (1.8 mM), penicillin (180 U/ml), streptomycin (180 mg/l), kanamycin (56 mg/l), and fungizone (0.9 mg/l)] at 37°C, in a humid atmosphere containing 5% CO2 and 95% air (Forma, Scientific incubator) in 75 cm2 plastic culture flasks (Nunc, Denmark) by the method of Horn (1976). The cells were re-fed two times a week with fresh medium, split every seventh day, and the cells of 5 and 10 subcultures were used for the experiments.
Cytotoxicity Assay
Before exposure to CPFX, the cells were incubated for 24 h to allow adherence and initiation of proliferation. CPFX was then added to the medium in eight different doses to achieve final concentrations ranging from 5 to 150 mg/l, and the cells were incubated for 24, 48, or 72 h. Each concentration of drug was tested in triplicate. All CPFX solutions were freshly prepared and protected from light (covered by aluminum foil). During all treatment procedures, all culture flasks and petri dishes were protected in the same way.
Neutral red (NR) uptake assay for the determination of potential cytotoxicity of CPFX was performed by the method of Borenfreund and Puerner (1985). The accumulation of NR in lysosomes of viable cells was determined after an extraction process. The absorbance value of samples was read at 540 nm and was corrected by subtracting the mean absorbance value of cell-free controls. Results were expressed as the percent value (percent survival) of the mean absorbance of drug-free control cells.
To examine the protective effect of vitamin E, the cells were preincubated with 50 μM vitamin E for 4 h and then exposed to 75 mg/l CPFX for 48 h. The viability of the cells was evaluated by the NR uptake assay as described.
Determination of LP, Total GSH Level, and Antioxidant Enzyme Activities
After incubation with 75 mg/l CPFX for 48 h, with or without 50 μM vitamin E pretreatment, the cells were trypsinized, resuspended in fresh media, and pelleted by centrifugation. Pellet was washed three times with isotonic Tris-HCl (0.2 mM, pH 7.4) buffer, then resuspended in hypotonic Tris-HCl buffer, pH 7.3, and grounded in a potter homogenizer at 80g for 5 min. The lysate was used for the determination of LP, the total intracellular glutathione (GSH) content, and the activities of antioxidant enzymes.
LP was measured fluorometically as thiobarbituric acid reactive substances (TBARS) using a Perkin-Elmer model LS 50 fluorometer by the method of Richard el al. (1992).
For the measurement of the total GSH content, the lysate was diluted with metaphosphoric acid (6%) [(lysate: metaphosphoric acid (5:l,v/v)], centrifuged at 1500g, 4°C, for 10 min, and supernatant was used for the total GSH determination by the method of Akerboom and Sies (1981) as we described earlier (Emonet et al., 1997).
After further centrifugation of the cell lysate at 1500g, 4°C, for 10 min, superoxide dismutases (SODs), glutathione peroxidase (GPX), and catalase (CAT) activities were determined in the supernatant. The activities of total SOD, Mn SOD, and CuZn SOD were assessed by monitoring auto-oxidation of pyrogallol according to a procedure adapted to the cells (Parat et al., 1998). The GPX activity was assessed by using the method of Gunzler et al. (1974). The CAT activity was quantitated using the method of Aebi (1984) by following the decomposition of hydrogen peroxide (H2O2) at 240 nm.
All results were expressed as values normalized to the cell protein content, and protein concentrations in the homogenate (total protein) were determined by the procedure described by Shopsis and Mackay (1984) or in the supernatant (soluble protein) according to the method of Lowry et al. (1951).
Statistics
The two-tailed Mann–Whithney U-test was employed to calculate statistical significance between control and treated groups.
RESULTS
Effect of CPFX on the Survival of Normal Human Fibroblast Cells
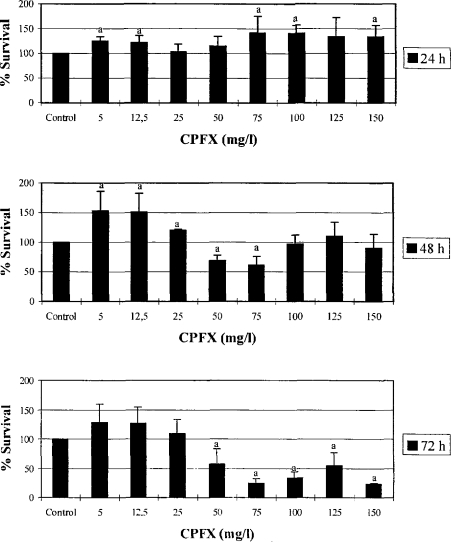
The effect of CPFX on the viability of normal human fibroblast cells, as determined by the NR assay, is illustrated in Figure 1. The data show that the effect of CPFX on the viability of cells was time-dependent, and the dose-response relation was biphasic. Cytotoxicity was not observed in the concentration range of 5–150 mg/l CPFX when the cells were incubated for 24 h. In contrast, lower concentrations (5 and 12.5 mg/l) of CPFX increased the cell survival in all incubation periods tested. Marked decreases in the viability of fibroblasts were observed at concentrations of 50 and 75 mg/l, and ≥50 mg/l, following 48 and 72 h exposure, respectively (p < 0.05). When the cells were exposed to > 75 mg/l CPFX for 48 h, no cytotoxicity was observed. However, at the same concentration range following the 72 h incubation period, viability of the cells decreased compared to controls and 48 h.
Figure 1.
The effect of CPFX on cell survival in fibroblast cultures in 24, 48, or 72 h. Viability was determined by NR uptake assay and expressed as the percent value of the control. Values are the mean ± SD of three separate experiments performed in triplicate. a p < 0.05 vs. control.
Effect of Vitamin E on the Cytotoxic Effect of CPFX
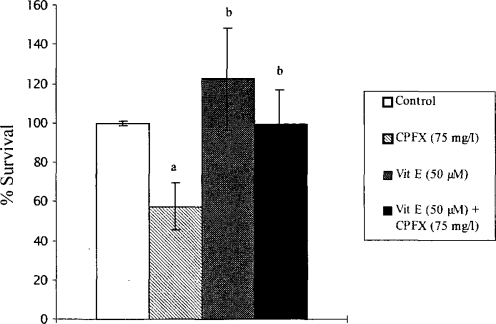
As illustrated in Figure 2, when vitamin E-pretreated fibroblasts were exposed to 75 mg/l CPFX for 48 h, the cell survival significantly increased compared to CPFX-treated cells (p < 0.001).
Figure 2.
Protective effect of vitamin E against CPFX-induced cytotoxicity. Fibroblasts were preincubated with 50 μM vitamin E (4 h) at 37°C before the addition of 75 mg/l CPFX. After a 48-h incubation, survival was determined via NR method and expressed as the percent value of the control. Values are the means ± SD of three separate experiments performed in triplicate. a p < 0.01 vs. control; b p < 0.001 vs. CPFX.
Effects of CPFX and CPFX Plus Vitamin E on LP, Total GSH Level, and Antioxidant Enzyme Activities
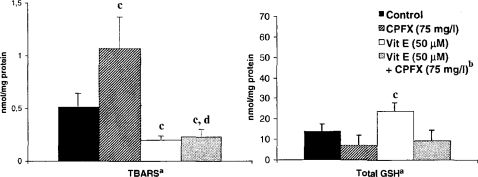
By exposing fibroblast cultures to 75 mg/l CPFX for 48 h, an induction of LP enhancement and a marked decrease in intracellular GSH were observed (Fig. 3). Vitamin E pretreatment lowered the basal TBARS level of the cells and provided complete protection against the effect of CPFX. However, while vitamin E pretreatment significantly increased the total GSH content, it was not able to maintain the same level in the presence of CPFX and provided only a moderate increase compared to the cells exposed to CPFX alone.
Figure 3.
The effect of CPFX and vitamin E on TBARS and total GSH levels in normal human fibroblast cells. aThe results were presented as mean ± SD (n = 3). Statistical comparisons were made using the Mann-Whitney U-test. bThe cells were preincubated with 50 μM vitamin E (4 h) and afterward exposed to 75 mg/l of CPFX for 48 h. c p < 0.05 vs. control; d p < 0.05 vs. CPFX.
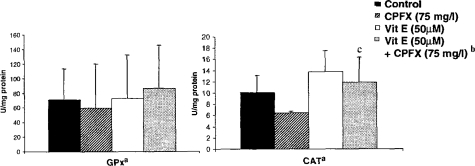
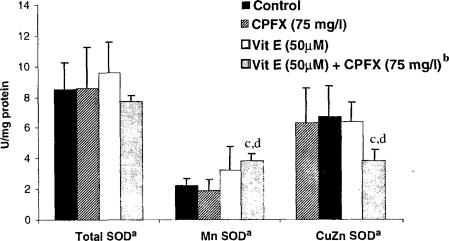
As shown in Figure 4, GPX activity was not altered significantly with any of the pretreatment schemes, and very high variations were noticed. Activity of CAT decreased with CPFX treatment, but not significantly, and remained nearly at the same level with vitamin E pretreatment. Total SOD changed neither in the presence of CPFX nor with the vitamin E pretreatment (Figure 5). Treatment with CPFX or vitamin E alone did not cause significant changes in the activities of Mn SOD or CuZn SOD. However, a significant increase in the activity of Mn SOD and a significant decrease in the activity of CuZn SOD were observed with CPFX plus vitamin E treatment.
Figure 4.
The effect of CPFX and vitamin E on CAT and GPX enzyme activities in normal human fibroblast cells. aThe results were presented as mean ± SD (n = 3). Statistical comparisons were made using the Mann-Whitney U-test. bThe cells were preincubated with 50 μM Vitamin E (4 h) and afterward exposed to 75 mg/l of CPFX for 48 h. c p < 0.05 vs. CPFX.
Figure 5.
The effect of CPFX and vitamin E on total CuZn, and Mn SOD levels in normal human fibroblast cells. aThe results were presented as mean ± SD (n = 3). Statistical comparisons were made using the Mann–Whitney U-test. bThe cells were preincubated with 50 μM Vitamin E (4 h) and afterward exposed to 75 mg/l of CPFX for 48 h. c p < 0.05 vs. control; d p < 0.05 vs. CPFX.
DISCUSSION
Quinolones are potent antimicrobials with a broad spectrum of activity and are effective against a wide range of infections caused by gram-negative and gram-negative bacteria, including Bacillus anthracis (Wolfson and Hooper, 1985; Blondeau, 1999). They exert their bactericidal effect by inhibiting the bacterial DNA gyrase, a type II topoisomerase (Gellert, 1981; Gootz et al., 1990). However, the influence of quinolones on mammalian DNA topoisomerases is several orders of magnitude weaker than prokaryotic topoisomerase II (Wolfson and Hooper, 1985; Hussy et al., 1986). They have the advantage of possessing a relatively good pharmacokinetic profile and bioavailability, and ease of oral dosing (Blondeau, 1999). In addition, compared to other commonly used antimicrobial agents, quinolones are considered relatively well tolerated. However, clinical experience has indicated that they have some undesirable adverse effects including cutaneous reactions like phototoxicity, juvenile cartilage toxicity, and, although the incidence is very low, adverse central nervous system reactions including epileptogenic convulsions (Wolfson and Hooper, 1985; Grayson, 1999; Stahlman and Lode, 1999). The mechanism underlying these adverse effects is still unknown. On the other hand, the capacity of quinolones to inhibit cell growth and cell functions in various cell lines in vitro was well documented (Forsgren et al., 1987; Oomori et al., 1988; Nordmann et al., 1989; Lawrence et al, 1993, 1996). Inhibition of the cell cycle and cell size progression in mitogen-stimulated human lymphocytes was reported (Forsgren et al., 1987), and CPFX was shown to have cytotoxic effects on both murine and human carcinoma cells in vitro (Zehavi-Willner and Shalit, 1992). Interfering de novo pyrimidine synthesis (Forsgren et al., 1987) or mitochondrial enzymes involved in energy metabolism (Lawrence et al, 1993) were suggested as the underlying mechanisms. However, in none of these publications was a biphasic dose-response curve reported. Results were presented to be dose-dependent; thus, with high concentrations of quinolone antibiotics, including CPFX, growth of various cultured mammalian cells was inhibited (Hussy et al., 1986; Forsgren et al., 1987; Oomori et al., 1988; Lawrence et al., 1993, 1996). Cytotoxicity started at concentrations over 20 mg/l, more often in the range 40–80 mg/l, and complete growth inhibition was frequently observed at concentrations over 100 mg/l (Hussy et al., 1986; Nordmann et al., 1989).
In the present study, CPFX inhibited the proliferation of normal human fibroblast cells at a similar dose range as previously described. The inhibitory effect was observed at concentrations of 50 and 75 mg/l, and ≥50 mg/l, when cells were incubated for 48 or 72 h, respectively. However, the effect of CPFX on the viability of cells was time-dependent, and the dose-response relation was biphasic. Low doses of CPFX appeared to be stimulatory at all incubation periods, whereas cytotoxicity was not observed in the whole concentration range tested (5–150 mg/l) during the 24 h of incubation. This delayed effect was pointed out earlier by Lawrence et al. (1996) and was described as a usual feature of the CPFX-induced growth inhibition. Depending on the type of cells, the lag period was 2–4 days and corresponded approximately to 3–4 cell doublings. They further observed a time-dependent decrease in cellular content of mtDNA prior to inhibition of cell proliferation with CPFX and related the observed cytotoxicity to the disturbances in the mitochondria. Their conclusion was that the inhibition of mammalian cell proliferation by CPFX is related to selective depletion of mtDNA through an interference with mitochondrial topoisomerase type II–like activity. Although we did not measure mtDNA damage in this study, this mechanism might also be an explanation for the lag period we observed.
The biphasic effect observed in the present study may also result from the complex dose-response relationship among ROS, cell proliferation, and cell viability. ROS are involved in cellular processes as diverse as proliferation, and cell death (Pervaiz and Clement, 2002). A growing body of evidence suggests that ROS, such as superoxide anions and hydrogen peroxide, function as intracellular second messengers involved in cellular signaling and, thus, can influence the growth as well as the death of the cell (Finkel, 1998; Clement and Pervaiz, 1999). In fact, it is well described by several cell models, including fibroblasts, that ROS exert a biphasic effect on cell growth (Murrell et al., 1990; Los et al., 1995; Schafer et al., 1996). As in the case of our previous study with paraquat on HeLa cells (Seve et al., 1999), lower doses increase proliferation and higher doses lead to cell death by apoptosis or necrosis (Buttke and Dandstrom, 1994). In addition, cultured fibroblasts were reported to release their own free radicals (Murell et al., 1990). Their proliferation was inhibited when endogenous ROS were inhibited, whereas the effect of exogenous ROS was biphasic. The data obtained in the present study also showed the occurrence of oxidative stress in fibroblast cell proliferation. Exposure of fibroblast cultures to 75 mg/l CPFX for 48 h induced a significant level of LP and a marked decrease in intracellular GSH. These observations were further supported by the data obtained with vitamin E pretreatment of cells and were in good agreement with our previous in vivo results obtained in CPFX-treated rats (Hincal and Taskin, 1995).
Considering the aforementioned facts and arguments, the overall interpretation of the results of the present study might be as follows. If fibroblasts need a certain level of ROS to proliferate, and if CPFX not only interferes with mtDNA replication, but also introduces ROS into the system, there might be a certain point at which a sensitive balance is established. Cell doubling time of fibroblasts is 22 h (Baudhuin, 1975), hence, when fibroblasts are exposed to 75 mg/l CPFX for 48 h, two full cell doublings may occur and the extent of dilution in the cellular content of mtDNA might reach a critical point that is not sufficient to sustain the cell growth. However, the level of ROS induced by higher concentrations (≥100 mg/l) of CPFX might compensate for the effect of such a level of loss in mtDNA and, hence, the cells continue to grow. In fact, when exposure time was increased to 72 h, the cell viability and growth was affected starling with doses over 25 mg/l and continued to decrease in a dose-dependent manner. Therefore, more than one biochemical effect, such as oxygen radicals, and mitochondrial loss might be responsive at different doses for the cytotoxicity of CPFX, and the juxtaposition of these mechanisms might result in the biphasic aspect of the survival curves. Nevertheless, further studies combining mtDNA and ROS measurements at different time points and concentrations are needed for making a precise conclusion.
Free radical production due to exogenous chemicals occurs either during the direct redox cycling of the compound or metabolization by cytochrome P450. For CPFX, it is possible that the generation of ROS may occur during its oxidative metabolism (Sörgel, 1989). In fact, in our recent study performed with hepatic rat microsomes, we observed the induction of free radical production by CPFX, which was inhibited completely by a cytochrome P450 inhibitor, SKF525A (Gürbay et al., 2001).
The results we obtained for antioxidant enzymes were not conclusive, and it appears that the effect of CPFX at this dose level and time period was not enough to produce any significant changes in the antioxidant enzyme activities.
In conclusion, CPFX exerted a hormetic-like dose-response in normal human fibroblast cell cultures. The biphasic effect of CPFX possibly resulted from the complex dose-dependent relationships among ROS, cell proliferation, and cell viability, as well as a selective depletion of mtDNA through an interference with mitochondrial topoisomerase type II–like activity.
Footnotes
Arnold Journals is acknowledged for kindly providing the permission to reprint data that was originally published in Human and Experimental Toxicology.
REFERENCES
- Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–125. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- Akerboom TPM, Sies H. Assay of glutathione, glutathione disulfide, and glutathione mixed disulfides in biological samples. Methods Enzymol. 1981;77:373–382. doi: 10.1016/s0076-6879(81)77050-2. [DOI] [PubMed] [Google Scholar]
- Baudhuin P, editor. Cytologie et Biologie Cellulaire. Editions Acco Louvain; 1975. pp. 120–123. [Google Scholar]
- Blondeau JM. Expanded activity and utility of the new fluoroquinolones: A review. Clin Ther. 1999;21:3–40. doi: 10.1016/s0149-2918(00)88266-1. [DOI] [PubMed] [Google Scholar]
- Borenfreund E, Puerner JA. Toxicity determined in vitro by morphological alterations and neutral red absorption. Toxicol Lett. 1985;24:119–124. doi: 10.1016/0378-4274(85)90046-3. [DOI] [PubMed] [Google Scholar]
- Boveris A, Cadenas E.1997Cellular sources and steady-state levels of reactive oxygen species Oxygen, Gene Expression, and Cellular FunctionLung Biology in Health and Disease Series,105Clerk LB, Maassaro DJ.Marcel Dekker; New York: 1–25. [Google Scholar]
- Buttke T, Dandstrom P. Oxidative stress as a mediator of apoptosis. Immunol Today. 1994;15:7–10. doi: 10.1016/0167-5699(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Calabrese EJ, Baldwin LA. Hormesis: U-shaped dose responses and their centrality in toxicology. Trends Pharmacol Sci. 2001;22:285–291. doi: 10.1016/s0165-6147(00)01719-3. [DOI] [PubMed] [Google Scholar]
- Clement MV, Pervaiz S. Reactive oxygen intermediates regulate cellular response to apoptotic stimuli: An hypothesis. Free Radical Res. 1999;30:247–252. doi: 10.1080/10715769900300271. [DOI] [PubMed] [Google Scholar]
- Emonet N, Leccia MT, Favier A, Beani JC, Richard MJ. Thiols and selenium: Protective effect on human skin fibroblasts exposed to UVA irradiation. J Photochem Photobiol. 1997;40:469–474. doi: 10.1016/s1011-1344(97)00041-9. [DOI] [PubMed] [Google Scholar]
- Finkel T. Oxygen radicals and signaling. Curr Opin Cell Biol. 1998;10:248–253. doi: 10.1016/s0955-0674(98)80147-6. [DOI] [PubMed] [Google Scholar]
- Forsgren A, Bredberg A, Pardee AB, Schlossman SF, Tedder TF. Effects of ciprofloxacin on eukaryotic pyrimidine nucleotide biosynthesis and cell growth. Antimicrob Agent Chemother. 1987;31:774–779. doi: 10.1128/aac.31.5.774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gellert M. DNA topoisomerases. Annu Rev Biochem. 1981;50:879–910. doi: 10.1146/annurev.bi.50.070181.004311. [DOI] [PubMed] [Google Scholar]
- Gootz TD, Barret JF, Holden HE, Ray VA, McGuirk PR. Selective toxicity: The activity of 4-quinolones against eukaryotic DNA topoisomerase. In: Crumplin G, editor. The 4-Quinolones: Antibacterial Agents In Vitro. Springer-Verlag; London: 1990. pp. 159–171. [Google Scholar]
- Grayson ML. Ciprofloxacin. In: Kucesrs A, Crowe SM, Grayson ML, Hoy JF, editors. The Use of Antibiotics: A Clinical Review of Antibacterial, Antifungal, and Antiviral Drugs. Avon, The Bath Press; 1999. pp. 981–1060. [Google Scholar]
- Gunzler WA, Kremers H, Flohe L. An improved test procedure for glutathione peroxidase in blood. Z Klin Chew Klin Biochem. 1974;12:444–448. doi: 10.1515/cclm.1974.12.10.444. [DOI] [PubMed] [Google Scholar]
- Gürbay A, Gonthier B, Daveloose D, Favier A, Hincal F. Microsomal metabolism of ciprofloxacin generates free radicals. Free Radical Biol Med. 2001;30:1118–1121. doi: 10.1016/s0891-5849(01)00508-1. [DOI] [PubMed] [Google Scholar]
- Gürbay A, Garrel C, Osman M, Richard M-J, Favier A, Hincal F. Cytotoxicity in ciprofloxacin-treated human fibroblast cells and protection by vitamin E. Hum Exper Toxicol. 2002;21:635–641. doi: 10.1191/0960327102ht305oa. [DOI] [PubMed] [Google Scholar]
- Hincal F, Taskin T.1995The mechanism of convulsions induced by ciprofloxacin may involve the generation of free radicals and the activation of excitatory amino acid receptors Abstracts of the International Congress of Toxicology—VII Seattle, WA, Abst. 99, p. 27 [Google Scholar]
- Horn N. Copper incorporation studies on cultured cells for prenatal diagnosis of Menke’s disease. Lancet. 1976;1:156–158. doi: 10.1016/s0140-6736(76)91543-9. [DOI] [PubMed] [Google Scholar]
- Hussy P, Maass G, Tummler B, Grosse F, Schomburg U. Effect of 4-quinolones and novobiocin on calf thymus DNA polymerase A primase complex, topoisomerases I and II, and growth of mammalian lymphoblasts. Antimicrob Agent Chemother. 1986;29:1073–1078. doi: 10.1128/aac.29.6.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence JW, Darkin-Rattray S, Xie F, Neims AH, Rowe TC. 4-Quinolones cause a selective loss of mitochondrial DNA from mouse L1210 leukemia cells. J Cell Biochem. 1993;51:165–174. doi: 10.1002/jcb.240510208. [DOI] [PubMed] [Google Scholar]
- Lawrence JW, Claire DC, Weisis V, Rowe TC. Delayed cytotoxicity and cleavage of mitochondrial DNA in ciprofloxacin-treated mammalian cells. Mol Pharmacol. 1996;50:1178–1188. [PubMed] [Google Scholar]
- Los M, Droge W, Strieker K, Baeuerle P, Schulze-Osthoff K. Hydrogen peroxide as a potent activator of T lymphocyte functions. Eur J Immunol. 1995;25:159–165. doi: 10.1002/eji.1830250127. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Murrell GA, Francis MJO, Bromley L. Modulation of fibroblast proliferation by oxygen free radicals. Biochem J. 1990;265:659–665. doi: 10.1042/bj2650659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordmann P, Pechinot A, Kazmierczak A. Cytotoxicity and uptake of pefloxacin, ciprofloxacin, and ofloxacin in primary cultures of rat hepatocytes. J Antimicrob Chemother. 1989;24:355–363. doi: 10.1093/jac/24.3.355. [DOI] [PubMed] [Google Scholar]
- Oomori Y, Yashue T, Aoyama H, Hirai K, Suzue S, Yokota T. Effects of fleroxacin on HeLa cell functions and topoisomerase II. J Antimicrob Chemother. 1988;22(Suppl. D):91–97. doi: 10.1093/jac/22.supplement_d.91. [DOI] [PubMed] [Google Scholar]
- Parat M-O, Richard MJ, Beani JC, Favier A. Involvement of zinc in cellular oxidant/antioxidant balance. Biol Trace Elem Res. 1988;60:187–204. doi: 10.1007/BF02784439. [DOI] [PubMed] [Google Scholar]
- Pervaiz S, Clement MV. A permissive apoptotic environment: Function of a decrease in intracellular superoxide anion and cytosolic acidification. Biochem Biophys Res Commun. 2002;290:1145–1150. doi: 10.1006/bbrc.2001.6274. [DOI] [PubMed] [Google Scholar]
- Richard M-J, Portal B, Meo J, Coudray C, Hadjian A, Favier A. Thiobarbituric acid reactant determination in plasma and lipoprotein fractions. Evaluation of Sobioda MDA-kit. Clin Chem. 1992;38:704–709. [PubMed] [Google Scholar]
- Seve M, Favier A, Osman M, Vaitaitis G, McCord JM, Flores SC. The human immunodeficiency virus-1 tat protein increases sensitivity to zinc chelator-induced apoptosis and changes Spl DNA binding in HeLa cells. Arch Biochem Biophys. 1999;351:165–172. doi: 10.1006/abbi.1998.0942. [DOI] [PubMed] [Google Scholar]
- Shafer D, Hamm-Kunzelmann B, Hermfisse U, Brand K. Differences in DNA-binding efficiency of Spl to aldolase and pyruvate kinase promoter correlate with altered redox states in resting and proliferating rat thymocytes. FEBS Lett. 1996;391:35–38. doi: 10.1016/0014-5793(96)00701-6. [DOI] [PubMed] [Google Scholar]
- Shopsis C, Mackay GJ. Sem-automated assay for cell culture. Anal Biochem. 1984;140:104–107. doi: 10.1016/0003-2697(84)90139-8. [DOI] [PubMed] [Google Scholar]
- Sörgel F. Metabolism of gyrase inhibitors. Rev Infect Dis. 1989;11(Suppl):S1119–S1129. doi: 10.1093/clinids/11.supplement_5.s1119. [DOI] [PubMed] [Google Scholar]
- Stahlman R, Lode H. Toxicity of quinolones. Drugs. 1999;58(Suppl):37–42. doi: 10.2165/00003495-199958002-00007. [DOI] [PubMed] [Google Scholar]
- Umezawa N, Arakane K, Ryu A, Mashiko S, Hirobe M, Nagano T. Participation of reactive oxygen species in phototoxicity induced by quinolone antibacterial agents. Arch Biochem Biophys. 1997;342:275–281. doi: 10.1006/abbi.1997.0124. [DOI] [PubMed] [Google Scholar]
- Wagai N, Tawara K. Quinolone antibacterial–agent-induced cutaneous phototoxicity: Ear swelling reactions in Balb/c mice. Toxicol Lett. 1991;58:215–223. doi: 10.1016/0378-4274(91)90176-7. [DOI] [PubMed] [Google Scholar]
- Wagai N, Tawara K. Possible reasons for difference in phototoxic potential of 5-quinolone antibacterial agents: Generation of toxic oxygen. Free Radical Res Commun. 1992a;17:387–398. doi: 10.3109/10715769209083143. [DOI] [PubMed] [Google Scholar]
- Wagai N, Tawara K. Possible direct role of reactive oxygens in the cause of cutaneous phototoxicity induced by five quinolones in mice. Arch Toxicol. 1992b;66:392–397. doi: 10.1007/BF02035128. [DOI] [PubMed] [Google Scholar]
- Wolfson JS, Hooper DC. The fluoroquinolones: Structures, mechanisms of action and resistance, and spectra of activity in vitro. Antimicrob Agent Chemother. 1985;28:581–586. doi: 10.1128/aac.28.4.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zehavi-Willner T, Shalit I. The inhibitory effect of ciprofloxacin on proliferation of murine bladder carcinoma cell line. J Antimicrob Chemother. 1992;29:323–328. doi: 10.1093/jac/29.3.323. [DOI] [PubMed] [Google Scholar]