Abstract
Corneal avascularity—the absence of blood vessels in the cornea—is required for optical clarity and optimal vision, and has led to the cornea being widely used for validating pro- and anti-angiogenic therapeutic strategies for many disorders1-4. But the molecular underpinnings of the avascular phenotype have until now remained obscure5-10 and are all the more remarkable given the presence in the cornea of vascular endothelial growth factor (VEGF)-A, a potent stimulator of angiogenesis, and the proximity of the cornea to vascularized tissues. Here we show that the cornea expresses soluble VEGF receptor-1 (sVEGFR-1; also known as sflt-1) and that suppression of this endogenous VEGF-A trap11 by neutralizing antibodies, RNA interference or Cre-lox-mediated gene disruption abolishes corneal avascularity in mice. The spontaneously vascularized corneas of corn1 and Pax6+/− mice12,13 and Pax6+/− patients with aniridia14 are deficient in sflt-1, and recombinant sflt-1 administration restores corneal avascularity in corn1 and Pax6+/− mice. Manatees, the only known creatures uniformly to have vascularized corneas15, do not express sflt-1, whereas the avascular corneas of dugongs, also members of the order Sirenia, elephants, the closest extant terrestrial phylogenetic relatives of manatees, and other marine mammals (dolphins and whales) contain sflt-1, indicating that it has a crucial, evolutionarily conserved role. The recognition that sflt-1 is essential for preserving the avascular ambit of the cornea can rationally guide its use as a platform for angiogenic modulators, supports its use in treating neovascular diseases, and might provide insight into the immunological privilege of the cornea.
Although the absence of blood vessels in the cornea was known to the ancients, such as Susruta1 and Galen2, millennia ago, it was only in the last century that angiostatic substances were postulated to underpin corneal avascularity3. Because of its avascularity and ease of accessibility, the cornea has been a proving ground for anti-angiogenic strategies for more than 30 years4. However, themolecular foundations of corneal avascularity remain unclear. In the last decade, many anti-angiogenic molecules such as angiostatin, endostatin, interleukin-1 receptor antagonist, pigment epithelium-derived factor, and thrombospondins have been found in the cornea (reviewed in ref. 5), leading to recognition of their actions as tumour suppressors, atherosclerotic plaque growth inhibitors, or modulators of wound healing. But none of these molecules is required for corneal avascularity, because mice deficient in any of them retain normal corneal phenotypes6-10. This has led to a view of multiply redundant mechanisms of corneal avascularity.
The search for inhibitors of angiogenesis to treat atherosclerosis, cancer, diabetic kidney and retina damage, macular degeneration, and rheumatoid arthritis often relies on testing in the cornea for initial efficacy, owing to the absence of blood vessels in the cornea despite it being surrounded by the highly vascular conjunctiva (Fig. 1a). The cornea is ideal for understanding the ability of tissues to demarcate vascular ingrowth and identifying the efficacy of therapies against known angiogenic stimuli.
Figure 1. Avascular cornea contains sflt-1 bound to VEGF-A.
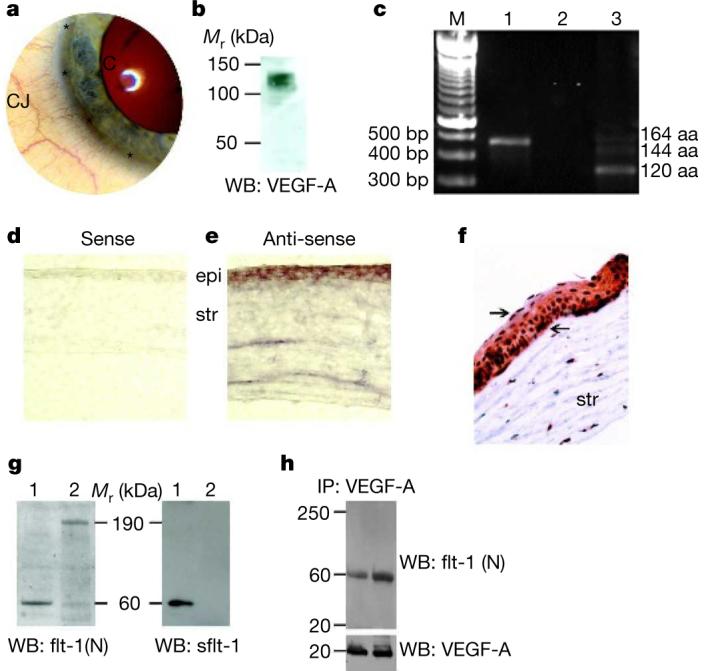
a, Photo of human eye demonstrates abrupt termination of blood vessels in the conjunctiva (CJ) at its border with the cornea (C), the limbus (asterisks). b, Representative non-reducing western blot of mouse cornea reveals immunoreactive bands of VEGF-A at 100–130 kDa corresponding to bound forms, and negligible immunoreactivity at 45–50 kDa corresponding to the free form. n = 5. c, sflt-1 (lane 1) and VEGF-A (lane 3) transcripts in mouse cornea identified by representative polymerase chain reaction with reverse transcription (RT–PCR). Lane 2 is water (template-negative) control. n = 5. bp, base pairs; aa, amino acids. d, e, sflt-1 mRNA detected by in situ hybridization in mouse corneal epithelium (epi) and stroma (str). Antisense RNA probes show purple-brown reactivity. Sense RNA probes show negligible reactivity. f, Immunolocalization (brown) of sflt-1 protein in mouse cornea. g, Representative reducing western blots (WB) with an antibody against the amino (N) terminus of flt-1 that recognizes both mbflt-1 and sflt-1 and an antibody against the unique C terminus of sflt-1 show that mouse cornea (1) contains primarily sflt-1 (60 kDa) whereas the conjunctiva (2) contains mainly mbflt-1 (190 kDa). h, Representative western blot of two independent mouse cornea samples immunoprecipitated (IP) with anti-VEGF-A antibody and immunoblotted (WB) with a biotinylated antibody against the N terminus of flt-1 that recognizes both mbflt-1 and sflt-1 shows that VEGF-A interacts with sflt-1 (60 kDa). Subsequent immunoblot with a biotinylated anti-VEGF-A antibody confirms the pull-down of VEGF-A by the immunoprecipitating antibody. n = 6.
In our studies we found, surprisingly, that the cornea contained VEGF-A, but nearly all of it was bound (Fig. 1b). To understand the paradoxical presence of this potent pro-angiogenic molecule in an avascular tissue, we hypothesized that it was counterbalanced by the expression of sflt-1, an alternatively spliced, secreted isoform of the cell-surface receptor membrane-bound flt-1 (mbflt-1). sflt-1 lacks the transmembrane (tm) and tyrosine kinase (tk) domains of mbflt-1 and can act as a ‘manacle’ for VEGF-A (ref. 11). We found that sflt-1 mRNA and protein exist in the cornea (Fig. 1c-g, Supplementary Fig. 1); by contrast, mbflt-1 was found in the conjunctiva but not in the cornea (Fig. 1g). Consistent with its proposed function as a trap for secreted VEGF-A, sflt-1 was present extracellularly (Supplementary Fig. 2). We used immunoprecipitation to confirm that sflt-1 and VEGF-A interact in vivo (Fig. 1h) and corroborated it by immunostaining (Supplementary Fig. 3).
We used three independent strategies to test whether sflt-1 preserved corneal avascularity in mice. First, we injected a neutralizing antibody against flt-1 into the cornea, with fellow eyes receiving isotype control antibodies. Eyes that were treated with blocking antibody consistently developed corneal vascularization from the limbus within 1 day, whereas those treated with control antibody did not (n = 14, P < 0.001) (Supplementary Fig. 4a, b). Corneas treated with anti-flt-1 antibodies contained more free VEGF-A than did control-treated corneas (Supplementary Fig. 4c), indicating that sequestration of VEGF-A by sflt-1 maintains corneal avascularity. We confirmed this by showing that concomitantly treating corneas with neutralizing anti-VEGF-A antibodies, but not with isotype-control antibodies, prevented corneal vascularization induced by the anti-flt-1 antibody (n = 8, P < 0.029). Because the anti-flt-1 antibody would theoretically block ligand-binding by both mbflt-1 and sflt-1 (although the former is undetectable in the cornea), we tested this antibody in flt-1 tyrosine kinase−/− (flt-1 tk−/−) mice, which are deficient in signalling induced by ligation of the flt-1 receptor16. The anti-flt-1 antibody, but not control antibodies, also induced corneal vascularization in flt-1 tk−/− eyes (n = 8, P = 0.029), indicating that the vascular phenotype resulted from suppression of sflt-1 function and not from interference with flt-1 signalling. Subconjunctival injection of anti-flt-1 antibodies, which eliminates the confounding effect of corneal trauma, also elicited corneal vascularization (n = 10, P = 0.008; Supplementary Fig. 4d, e).
The second strategy was genomic deletion: we suppressed sflt-1 by conditional Cre-lox-mediated gene ablation because flt-1 deletion is lethal17. Injections into the cornea of a plasmid18 that encoded Cre recombinase (pCre), but not of pNull, induced corneal vascularization in flt-1loxP/loxP mice (n = 10; P < 0.001) within 2 days (Supplementary Fig. 5a,b). Expression of Cre was accompanied by significantly reduced sflt-1 and increased free VEGF-A (Supplementary Fig. 5c, d). Neither plasmid could induce corneal vascularization in wild-type mice (n = 8). To avoid injection trauma, we delivered a cell-permeable, enzymatically active Cre containing a nuclear localization sequence (NLS–Cre; refs 19, 20) to the cornea by topical eye drops (Fig. 2a–d). NLS–Cre, but not NLS–β-galactosidase, induced corneal vascularization in flt-1loxP/loxP mice (n = 11; P < 0.001) within 2 days (Fig. 2e, f). Neither NLS–enzyme induced corneal vascularization in wild-type mice (n = 8; Fig. 2g, h).
Figure 2. Topical enzymatically active Cre recombinase abolishes corneal avascularity in flt-1loxP/loxP mice.
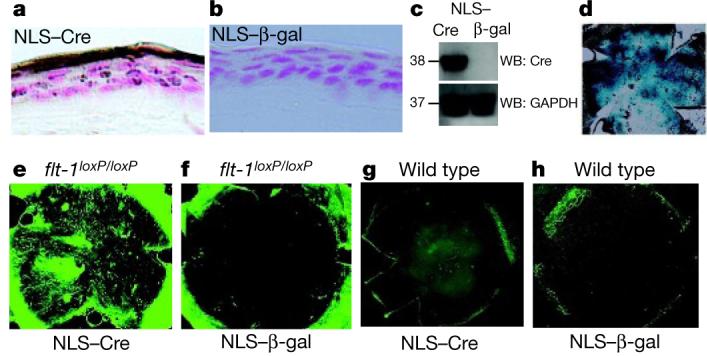
NLS–Cre but not NLS–β-galactosidase induces Cre expression (brown) in cornea within 1 h of eye drop application as shown by immunolocalization in cell nuclei stained red (a, b), and by representative reducing western blot (WB; c). d, X-gal staining of corneal flat mount of ROSA26R lacZ reporter mouse confirms expression of β-galactosidase (blue) 2 days after Cre expression. e, f, Representative corneal flat mounts show CD31+ (green)/LYVE-1− blood vessels in flt-1loxP/loxP mouse corneas 14 days after treatment with NLS–Cre eye drops (e) but not with NLS–β-galactosidase (f). No corneal vascularization occurs in wild-type mice after topical NLS–Cre (g) or NLS–β-galactosidase (h). n = 4 (a, b, d), n = 6 (c), n = 8–11 (e–h).
The final strategy specifically knocked down sflt-1 using RNA interference (RNAi). We injected into the cornea a plasmid that expressed a short hairpin RNA (shRNA) targeted against a sequence in the unique carboxyl-terminus region of sflt-1 (pshRNA–sflt-1). The control was a plasmid that expressed an shRNA targeted against a sequence in the unique C-terminus region of mbflt-1 but that is not present in sflt-1 (pshRNA–mbflt-1). pshRNA–sflt-1, but not pshRNA–mbflt-1, substantially reduced sflt-1 mRNA and protein, indicating that knockdown was achieved through RNAi (Fig. 3a, b), and increased free VEGF-A (Fig. 3c), corroborating the thesis that sflt-1 sequesters VEGF-A to maintain physiological avascularity. Corneal vascularization was induced by pshRNA-sflt-1, but not pshRNA-mbflt-1, within 3 days of injection (n = 36, P < 0.0001) (Fig. 3d–f). pshRNA–sflt-1 also induced corneal vascularization in mice that had been systemically depleted of macrophages and neutrophils by treatment with clodronate liposomes and anti-Gr-1 antibodies (Supplementary Fig. 6a–c), indicating that corneal vascularization was not induced by infiltration of inflammatory cells and their delivery of VEGF-A. Furthermore, pshRNA–sflt-1 did not elevate VEGF-A mRNA (Supplementary Fig. 6d).
Figure 3. Knockdown of sflt-1 mRNA abolishes corneal avascularity.
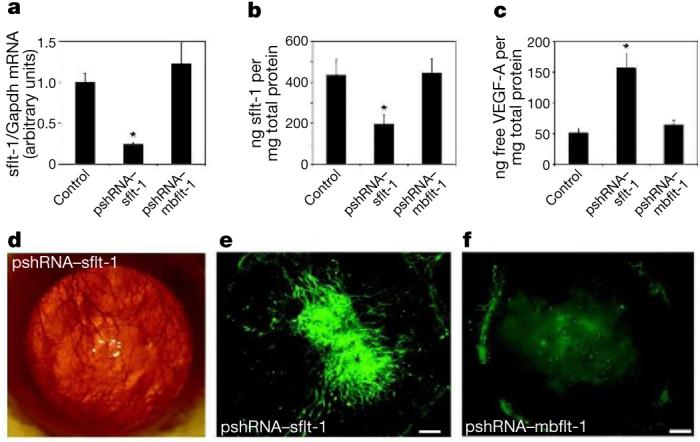
a–c, Real-time RT–PCR reveals reduced sflt-1 mRNA (a) and enzyme-linked immunosorbent assay (ELISA) reveals reduced sflt-1 protein (b) and increased free VEGF-A protein (c) in wild-type mouse corneas 3 days after injection of pshRNA–sflt-1 but not pshRNA–mbflt-1. Asterisk denotes P < 0.05, Bonferroni corrected Mann–Whitney U-test. n = 8–12. Error bars depict s.e.m. d–f, pshRNA–sflt-1 but not pshRNA–mbflt-1 induces corneal vascularization in wild-type mice. n = 36. Photo of eye (d) and corneal flat mounts showing CD31+ (green, vascular endothelial cells) LYVE-1− (lymphatic vessel endothelial hyaluronan receptor) blood vessels 14 days after injection (e, f). Scale bars, 500 μm.
Like sflt-1, the transmembrane domain of flt-1 (flt-1-TM) also can trap VEGF-A, at least during development21. Like wild-type mice, flt-1 tk−/− mice (n > 60), which retain expression of sflt-1 and flt-1-TM, have avascular corneas. Corneal vascularization was induced by pshRNA–sflt-1, but not pshRNA–mbflt-1, in flt-1 tk−/− mice (n = 8; P = 0.029) just as in the wild type, indicating that sflt-1 and not flt-1-TM is required for corneal avascularity.
Apart from VEGF-A, sflt-1 also binds VEGF-B and placenta growth factor (PlGF). Expression of these ligands in mouse corneas was much less than that of VEGF-A (data not shown). Moreover, pshRNA–sflt-1, but not pshRNA–mbflt-1, induced corneal vascularization in both Vegfb−/− (n = 8, P = 0.029) and Plgf−/− (n = 16; P < 0.0001) mice, supporting the contention that corneal vascularization results from desequestration of VEGF-A from sflt-1. Direct evidence for this assertion was obtained by showing that corneal vascularization induced by pshRNA–sflt-1 in wild-type mice was prevented by a neutralizing anti-VEGF-A antibody but not by isotype-control antibodies (n = 10; P = 0.008).
Interferon (IFN)-mediated responses can allow pshRNAs to inhibit gene expression nonspecifically; however, pshRNA–sflt-1 induced corneal vascularization in mice deficient in IFN(alpha, beta and omega)receptor-1 (Ifnar1−/−) or IFN(gamma) (Ifng−/−) (n = 8) just as in wild-type mice, indicating that corneal vascularization was not attributable to IFN response effectors. To investigate whether other off-target effects might be responsible for corneal vascularization induced by pshRNA–sflt-1, we created p2shRNA–sflt-1, which was targeted against a different sequence in the unique C-terminus region of sflt-1. Corneal injection of p2shRNA-sflt-1 also induced corneal vascularization in wild-type mice (n = 10; Supplementary Fig. 7a), making it unlikely that off-target effects, which are sequence-specific and not target-specific, were responsible for loss of corneal avascularity.
To confirm that corneal vascularization induced by pshRNA–sflt-1 was mechanistically linked to sflt-1 knockdown, we developed a plasmid coding for a ‘hardened-target’ version of sflt-1 (psflt-1*). This contained seven translationally silent wobble position mutations that rendered expressed sflt-1 refractory to pshRNA–sflt-1. psflt-1*, but not psflt-1, prevented suppression of sflt-1 and the development of corneal vascularization in eyes that had been treated with pshRNA–sflt-1 (n = 10, P = 0.008; Supplementary Fig. 7b). This functional control definitively established that the angiogenic phenotype was due to RNAi-mediated knockdown of sflt-1. Genetic, transcriptional and protein-targeting suppression of sflt-1 all induced corneal vascularization, showing that sflt-1 is the pre-eminent molecular defender of corneal avascularity.
The cornea remains avascular even in states of hypoxia such as those induced by eyelid closure during sleep or coma, and in a variety of ischaemic and occlusive disease states. We examined VEGF-A and sflt-1 levels in the corneas of mice exposed to 8% O2 (comparable to corneal hypoxia during sleep) for 24 h. Despite profound hypoxia, these corneas remained avascular (n = 20). Although hypoxia can increase VEGF-A production, free VEGF-A was not significantly elevated in hypoxic corneas (11 ± 23% greater than non-hypoxic corneas; n = 9; P = 0.78). This was attributed to an increase of 86 ± 34% in sflt-1 in hypoxic corneas (n = 17; P = 0.05), consistent with the presence of a hypoxia-responsive element in the flt-1 gene22. These data confer an important protective role upon sflt-1 in maintaining corneal avascularity during physiological hypoxia. By contrast, elevation of VEGF-A without concomitant induction of sflt-1, which was modelled by injection of recombinant VEGF-A, was reversed by administration of recombinant sflt-1/Fc but not of isotype control IgG/Fc (Supplementary Fig. 8), confirming its specificity.
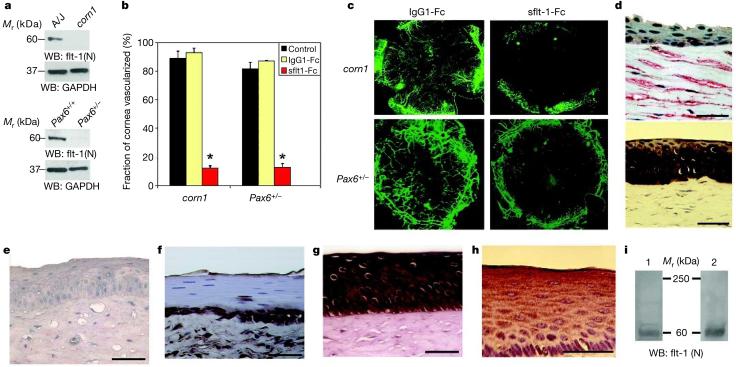
We examined the spontaneously vascularized corneas of corn1 and Pax6+/− mice12,13 for the presence of sflt-1. Corneas of corn1 and Pax6+/− mice, unlike those of their background strains, were deficient in sflt-1 (Fig. 4a). Although it is unknown why these mice do not express sflt-1 in the cornea, it is notable that both strains have abnormalities in their corneal epithelium13,23, the predominant source of sflt-1. sflt-1/Fc injection significantly reduced the area of corneal vascularization24,25 in corn1 and Pax6+/− mice compared with both IgG/Fc treated and untreated corneas (Fig. 4b, c), both implying that sflt-1 has a significant role in maintaining corneal homeostasis and suggesting that it might be possible to rescue corneal vascularization in a clinical setting. Although mutations in destrin, the protein that is altered in corn1 mice, have not been reported in humans, Pax6 mutations are present in patients with aniridia, who also have vascularized corneas14. Interestingly, the corneas of patients with aniridia (n = 5) were deficient in sflt-1 compared with normal human corneas (n = 7; Fig. 4d).
Figure 4. Spontaneously vascularized corneas lacking sflt-1 are rescued by sflt-1 administration.
a, Representative reducing western blots (WB) reveal deficiency of sflt-1 in corneas of corn1 and Pax6+/− mice compared with background strain A/J and Pax6+/+ mice. n = 10–12. b, c, Administration of sflt-1-Fc inhibits corneal vascularization in corn1 and Pax6+/− mice compared with administration of IgG1-Fc (by 87 ± 2% in corn1; n = 12; P = 0.01; by 85 ± 3% in Pax6+/−; n = 10; P = 0.03) and with control untreated mice (by 87 ± 2% in corn1; n = 12; P = 0.01; by 84 ± 3% in Pax6+/−; n = 10; P = 0.03). Significance by Bonferroni corrected Mann–Whitney U-test. Error bars depict s.e.m. (b). Representative flat mounts show CD31+ (green)/LYVE-1− corneal blood vessels (c). d, Immunostaining reveals deficiency of sflt-1 (brown) in the cornea of a 32-yr-old woman (top) with aniridia-associated vascularization, revealed by vascular cell adhesion molecule-1 (VCAM-1) staining (red) compared to the avascular cornea (lack of VCAM-1 staining) of a 38-yr-old man without aniridia (bottom). e–i, Marked deficiency of sflt-1 (reddish brown) staining in cornea of Antillean manatee (e) compared with dugong (f), African elephant (g) and beaked whale (h). Nuclear counterstain is blue (d–h). i, Representative reducing western blots with an antibody against the N terminus of flt-1 reveal presence of sflt-1 (60 kDa) and absence of mbflt-1 (190 kDa) in corneas of bottlenose dolphin (1) and Asian elephant (2); n = 4. Scale bars, 200 μm.
Florida manatees (Trichechus manatus latirostris) are the only organisms that have been reported uniformly to have spontaneously vascularized corneas15. We also observed this phenotype in the Antillean manatee (Trichechus manatus manatus; unpublished data). Interestingly, neither manatee expressed sflt-1 in the cornea, whereas the avascular corneas of dugongs (Dugong dugon), which also belong to the order Sirenia, and of Asian (Elephas maximus) and African (Loxodanta africana) elephants, the closest extant terrestrial phylogenetic relatives of manatees, did express sflt-1 in the cornea (Fig. 4e–g). The avascular corneas of other marine mammals such as dolphins (bottlenose: Tursiops truncatus; Risso's: Grampus griseus) and whales (Cuvier's beaked: Ziphius cavirostris; fin: Balaenoptera physalus; melon-headed: Peponocephala electra) also contained sflt-1 (Fig. 4h, i). The correlation between sflt-1 expression and corneal avascularity in diverse mammals supports the idea that sflt-1 has an evolutionarily conserved role in conferring corneal avascularity. Unlike dolphin and elephant corneas (Fig. 4i), manatee corneas expressed mbflt-1 (Supplementary Fig. 9), indicating that a splicing switch might account for their vascularized state. The teleological basis of the vascularized manatee cornea is intriguing. The absence of corneal sflt-1 and potentially suboptimal vision might result from a non-deleterious mutation in manatees, as they live primarily in turbid waters. Unlike dugongs, which are strictly marine, manatees are believed to be physiologically dependent on fresh water, and corneal vascularization could protect against, or perhaps result from, irritations due to this hypotonic environment.
The presence of many anti-angiogenic molecules in the cornea indicates that avascularity, which is essential for optical transparency and clear vision, might be maintained by multiply redundant mechanisms. Therefore the finding that neutralization or knockdown of sflt-1 alone abolishes corneal avascularity is surprising, but consistent with the presence of VEGF-A in the normal cornea. We speculate that VEGF-A is produced and held in a sequestered state by the cornea as a readily available store because this exposed tissue is susceptible to injuries that might require an angiogenic response. Alternatively, it might be a vestigial residue of an evolutionary requirement to provide blood to the eye that later required biochemical compensation in the form of sflt-1 expression to support improved vision.
The utilization of sflt-1 to regulate the bioavailability of VEGF-A is conserved in other systems such as cyclic vascularization26 and embryonic sprouting27, and disturbances in this regulation underlie preeclampsia28. Our findings unveil a new role for sflt-1 in the evolutionary establishment of optimal vision resulting from and requiring optical clarity. Apart from trapping VEGF-A, sflt-1 can heterodimerize with mbflt-1 and VEGFR-2 (ref. 11). Although neither mbflt-1 nor VEGFR-2 is expressed in the normal cornea (Fig. 1g, Supplementary Fig. 10), such heterodimerization might modulate pathological vascularization of the cornea. Other mechanisms of regulating VEGF-A bioavailability, such as matrix metalloproteinase-induced release, have been identified in a model of tumour angiogenesis29.
The cornea has long been used as a readout platform to assay anti-angiogenic therapy in oncology, cardiovascular biology and other fields. The recognition that sflt-1 is dominant in maintaining corneal avascularity directly affects the degree to which this tissue can be generalized in individual models. Our data also provide insights into the relative immunological privilege of the cornea, as corneal avascularity is crucial to the high success rate of corneal allografts30. These findings also support the use of sflt-1 in preventing or treating neovascularization. Furthermore, they illuminate its potential as a therapeutic target in conditions where inducing angiogenesis in an sflt-1-rich microenvironment might be beneficial, for example, preeclampsia, wound healing, stroke and heart disease.
METHODS
Imaging
In vivo images were captured by CCD camera (Nikon) under a dissecting microscope. Blood vessels were defined by positive labelling with FITC-conjugated rat antibody against mouse CD31 (1:333; BD Pharmingen) and negative labelling with rabbit antibody against mouse LYVE-1 (1:333; Abcam) on corneal flat mounts, as previously reported24,25.
Injections
Neutralizing goat antibody (10 μg) against mouse flt-1 (R&D Systems), isotype control goat IgG (10 μg; Jackson Immunoresearch), shRNAs (4 μg) against mbflt-1 or sflt-1, psflt-1 (4 μg), psflt-1* (4 μg), pCre (4 μg; gift of R.K. Nordeen, University of Colorado), pNull (4 μg), rmVEGF-A164 (20–500 ng; R&D Systems), sflt-1/Fc (5 μg; R&D Systems), or isotype control IgG1/Fc (5 μg; Jackson Immunoresearch) were injected (2 μl) into the cornea with a 33-gauge needle, as previously reported18. The efficiency of corneal transfection by naked plasmid of pGFP (gift of X. Li, University of Kentucky) or placZ (gift of B.T. Spear, University of Kentucky) exceeded 70%, as gauged by flow cytometry and X-gal staining (Supplementary Fig. 11). We performed tail-vein injection of clodronate liposomes (200 μl) and intraperitoneal injection of anti-Gr-1 antibodies (200 μg; eBioscience) on each of the two days before and after corneal injection of pshRNA–sflt-1 to deplete peripheral monocytes/macrophages and neutrophils.
Supplementary Material
Acknowledgements
We thank the various aquaria, zoos and wildlife rehabilitation centres that donated tissues for comparative studies; R. Groom, S. Joshi, M. Kellogg, R. King, C. K. Lau, P. Lewis, N. Mezei, K.K. Smith and L. Xu for technical assistance; R. J. Kryscio for statistical guidance; and R. Mohan, S. Bondada, M. W. Fannon, L. Mazzaro, Y. Nozaki, P. A. Pearson, L. Peichl, A. M. Rao, G. S. Rao and K. Ambati for discussions. J.A. was supported by the NEI/NIH, the Lew R. Wasserman Merit Award (Research to Prevent Blindness), the Dennis W. Jahnigen Career Development Award (American Geriatrics Society, John A. Hartford Foundation, Atlantic Philanthropies), the Macula Vision Research Foundation, the International Retinal Research Foundation, the E. Matilda Ziegler Foundation for the Blind, the Dr E. Vernon Smith and Eloise C. Smith Macular Degeneration Endowed Chair, a physician–scientist award from University of Kentucky, and a departmental challenge grant from Research to Prevent Blindness; M.N. by ARVO/Japan National Society for the Prevention of Blindness; E.S. by Fight For Sight; R.J.C.A by Research to Prevent Blindness; J.Z.B. and B.R. by the NIDCD/NIH; A.T. by a Japan Young Scientist Award; B.K.A. by a VA Career Development Award, the Knights-Templar Eye Foundation and Fight for Sight; N.S by ARVO/Alcon; S.I. by the NEI/NIH; J.M.C. by the Wellcome Trust and the Birth Defects Foundation; T.S.K by the NEI/NIH and the NIAMS/NIH; S.B. by the Muscular Dystrophy Association; and S.D.F. by the AIRC (Italian Association for Cancer Research).
Footnotes
Supplementary Information is linked to the online version of the paper at www.nature.com/nature.
The authors declare competing financial interests: details accompany the paper on www.nature.com/nature.
References
- 1.Sharma PV. Susruta-Samhita. Chaukhambha Visvabharati; Varanasi, India: 2001. [Google Scholar]
- 2.Magnus H. Ophthalmology of the Ancients. J. P. Wayenborgh; Oostende, Belgium: 1999. [Google Scholar]
- 3.Meyer K, Chaffee E. The mucopolysaccharide acid of the cornea and its enzymatic hydrolysis. Am. J. Ophthalmol. 1940;23:1320–1325. [Google Scholar]
- 4.Gimbrone MA, Jr, Cotran RS, Leapman SB, Folkman J. Tumor growth and neovascularization: an experimental model using the rabbit cornea. J. Natl Cancer Inst. 1974;52:413–427. doi: 10.1093/jnci/52.2.413. [DOI] [PubMed] [Google Scholar]
- 5.Chang JH, Gabison EE, Kato T, Azar DT. Corneal neovascularization. Curr. Opin. Ophthalmol. 2001;12:242–249. doi: 10.1097/00055735-200108000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Wiegand SJ, et al. Genetic modulation of pigment epithelium-derived factor (PEDF) expression does not alter normal or pathological angiogenesis in the eye, or tumor growth. Invest. Ophthalmol. Vis. Sci. 2004;45 abstr. 1884. [Google Scholar]
- 7.Cursiefen C, et al. Roles of thrombospondin-1 and -2 in regulating corneal and iris angiogenesis. Invest. Ophthalmol. Vis. Sci. 2004;45:1117–1124. doi: 10.1167/iovs.03-0940. [DOI] [PubMed] [Google Scholar]
- 8.Bugge T, Flick M, Daugherty C, Degen J. Plasminogen deficiency causes severe thrombosis but is compatible with development and reproduction. Genes Dev. 1995;9:794–807. doi: 10.1101/gad.9.7.794. [DOI] [PubMed] [Google Scholar]
- 9.Fukai N, et al. Lack of collagen XVIII/endostatin results in eye abnormalities. EMBO J. 2002;21:1535–1544. doi: 10.1093/emboj/21.7.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hirsch E, Irikura VM, Paul SM, Hirsh D. Functions of interleukin 1 receptor antagonist in gene knockout and overproducing mice. Proc. Natl Acad. Sci. USA. 1996;93:11008–11013. doi: 10.1073/pnas.93.20.11008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kendall RL, Thomas KA. Inhibition of vascular endothelial cell growth factor activity by an endogenously encoded soluble receptor. Proc. Natl Acad. Sci. USA. 1993;90:10705–10709. doi: 10.1073/pnas.90.22.10705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith R, et al. Corn1: a mouse model for corneal surface disease and neovascularization. Invest. Ophthalmol. Vis. Sci. 1996;37:397–404. [PubMed] [Google Scholar]
- 13.Ramaesh T, et al. Corneal abnormalities in Pax6+/− small eye mice mimic human aniridia-related keratopathy. Invest. Ophthalmol. Vis. Sci. 2003;44:1871–1878. doi: 10.1167/iovs.02-0576. [DOI] [PubMed] [Google Scholar]
- 14.Jordan T, et al. The human PAX6 gene is mutated in two patients with aniridia. Nature Genet. 1992;1:328–332. doi: 10.1038/ng0892-328. [DOI] [PubMed] [Google Scholar]
- 15.Harper JY, Samuelson DA, Reep RL. Corneal vascularization in the Florida manatee (Trichechus manatus latirostris) and three-dimensional reconstruction of vessels. Vet. Ophthalmol. 2005;8:89–99. doi: 10.1111/j.1463-5224.2005.00351.x. [DOI] [PubMed] [Google Scholar]
- 16.Hiratsuka S, Minowa O, Kuno J, Noda T, Shibuya M. Flt-1 lacking the tyrosine kinase domain is sufficient for normal development and angiogenesis in mice. Proc. Natl Acad. Sci. USA. 1998;95:9349–9354. doi: 10.1073/pnas.95.16.9349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fong GH, Rossant J, Gertsenstein M, Breitman ML. Role of the Flt-1 receptor tyrosine kinase in regulating the assembly of vascular endothelium. Nature. 1995;376:66–70. doi: 10.1038/376066a0. [DOI] [PubMed] [Google Scholar]
- 18.Stechschulte SU, et al. Rapid ocular angiogenic control via naked DNA delivery to cornea. Invest. Ophthalmol. Vis. Sci. 2001;42:1975–1979. [PubMed] [Google Scholar]
- 19.Lin Q, Jo D, Gebre-Amlak KD, Ruley HE. Enhanced cell-permeant Cre protein for site-specific recombination in cultured cells. BMC Biotechnol. 2004;4:25. doi: 10.1186/1472-6750-4-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jo D, et al. Epigenetic regulation of gene structure and function with a cell-permeable Cre recombinase. Nature Biotechnol. 2001;19:929–933. doi: 10.1038/nbt1001-929. [DOI] [PubMed] [Google Scholar]
- 21.Hiratsuka S, et al. Membrane fixation of vascular endothelial growth factor receptor 1 ligand-binding domain is important for vasculogenesis and angiogenesis in mice. Mol. Cell. Biol. 2005;25:346–354. doi: 10.1128/MCB.25.1.346-354.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gerber HP, Condorelli F, Park J, Ferrara N. Differential transcriptional regulation of the two vascular endothelial growth factor receptor genes. Flt-1, but not Flk-1/KDR, is up-regulated by hypoxia. J. Biol. Chem. 1997;272:23659–23667. doi: 10.1074/jbc.272.38.23659. [DOI] [PubMed] [Google Scholar]
- 23.Ikeda S, et al. Aberrant actin cytoskeleton leads to accelerated proliferation of corneal epithelial cells in mice deficient for destrin (actin depolymerizing factor) Hum. Mol. Genet. 2003;12:1029–1036. doi: 10.1093/hmg/ddg112. [DOI] [PubMed] [Google Scholar]
- 24.Ambati BK, et al. Sustained inhibition of corneal neovascularization by genetic ablation of CCR5. Invest. Ophthalmol. Vis. Sci. 2003;44:590–593. doi: 10.1167/iovs.02-0685. [DOI] [PubMed] [Google Scholar]
- 25.Ambati BK, Joussen AM, Kuziel WA, Adamis AP, Ambati J. Inhibition of corneal neovascularization by genetic ablation of CCR2. Cornea. 2003;22:465–467. doi: 10.1097/00003226-200307000-00013. [DOI] [PubMed] [Google Scholar]
- 26.Graubert MD, Ortega MA, Kessel B, Mortola JF, Iruela-Arispe ML. Vascular repair after menstruation involves regulation of vascular endothelial growth factor-receptor phosphorylation by sFLT-1. Am. J. Pathol. 2001;158:1399–1410. doi: 10.1016/s0002-9440(10)64091-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kearney JB, Kappas NC, Ellerstrom C, DiPaola FW, Bautch VL. The VEGF receptor flt-1 (VEGFR-1) is a positive modulator of vascular sprout formation and branching morphogenesis. Blood. 2004;103:4527–4535. doi: 10.1182/blood-2003-07-2315. [DOI] [PubMed] [Google Scholar]
- 28.Levine RJ, et al. Circulating angiogenic factors and the risk of preeclampsia. N. Engl. J. Med. 2004;350:672–683. doi: 10.1056/NEJMoa031884. [DOI] [PubMed] [Google Scholar]
- 29.Bergers G, et al. Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nature Cell Biol. 2000;2:737–744. doi: 10.1038/35036374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dana MR, Streilein JW. Loss and restoration of immune privilege in eyes with corneal neovascularization. Invest. Ophthalmol. Vis. Sci. 1996;37:2485–2494. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.