Rising air temperatures around the globe are affecting organismal abundance, distribution, and evolution (1, 2). Not surprisingly, biologists are endeavoring to assess and anticipate further impacts of warming. What is usually overlooked in these efforts is the fact that mobile organisms are not prisoners of climate warming: they can use behavioral adjustments (“behavioral thermoregulation”) either to buffer the impact of warming air temperatures or sometimes even to take advantage of them (3–5). However, an evaluation of behavior's roles in modifying organismal responses to climate warming has never been attempted, at least on a large spatial scale. A new study in this issue of PNAS (6) develops a biophysically (heat transfer)-based approach (7–9) that does just that. Kearny et al. (6) quantify whether a diurnal ectotherm's use of behavioral adjustments (e.g., use of shade or burrows) alters the ecological impact of climate warming, and they do so on local, continental, and global scales. For us the key take-home lesson is that behavioral flexibility is critical for organismal survival in a warming world; behavior can buffer the negative consequences of warming, or it can enhance the benefits of warming! The outcome depends on an organism's physiology, availability of shade, and local microclimates, all of which vary with latitude. Many temperate-zone ectotherms live in environments that are considerably cooler than their optimum, and so becoming warmer is now their highest thermoregulatory priority. Warming will be beneficial to them, especially if they can use basking to take advantage of warming temperatures. In contrast, the priority for many tropical and continental-desert ectotherms is staying cool. Climate warming will place them at risk, especially if shade is scant.
Bundling Biophysics with Behavior
Previous studies developed techniques to quantify the impact of behavioral thermoregulation on body temperature (Tb) and performance (10–12) or even on fitness (13) at local sites. The general approach involves comparing some index of the relative performance of a regulator versus that of a nonregulator (12).
Kearney et al. (6) compared the performances of 2 “species” of nonregulators (one always on the surface in full sun, one always in deep shade) with that of a regulator that could shuttle between sun and shade or to retreat below ground, while attempting to maintain a Tb of 33 °C, whenever feasible. [An alternative type of nonregulator, one moving randomly through the environment (12), was not considered, probably because the result is necessarily habitat specific.] If thermoregulation is beneficial during climate warming, then a thermoregulator should perform better on average than a nonregulator. Performance here was indexed as total time spent at near-optimal Tb during the year.
Although the concept here appears simple, its implementation requires detailed data on climate variation across a wide range of scales, all coupled to a biophysical model that allowed organisms to “move” (or not) across landscapes. The first step was to adopt heat-transfer models (integrating morphological and physiological data with Geographic Information System data on climate and terrain) that accurately predict the steady-state temperatures of a generic ectotherm (in sun, shade, or shuttling between sun and shade) at 3 sites in Australia (a coastal tropical, an arid continental, and a coastal temperate site). These models then projected Tb over the year under both contemporary and future climate conditions.
Some Like It Hotter, but Some Can't Take the Heat
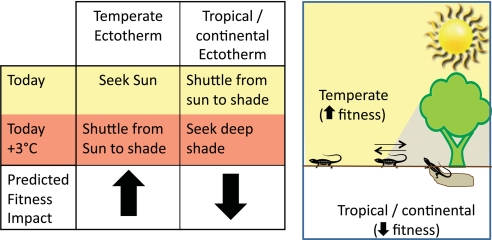
What is striking about the results is that the optimal behavioral strategy varies by site. At a coastal temperate site in today's climate, lizards stationed in shade would be too cool, whereas those in sun would have Tb that are physiologically “just right.” Consequently, the optimal strategy here is to remain in sun as much as possible, at least in today's world. At the tropical and continental sites, any lizard staying in sun would frequently overheat, those remaining in shade would do reasonably well, but those shuttling between sun and shade would spend the most time at their optimal Tb. Clearly, although not surprisingly, the benefits of thermoregulatory behavior are environment specific (see Fig. 1).
Fig. 1.
Schematic showing optimal thermoregulatory behavior and change in fitness of ectotherms in different sites under today's and future (today +3 °C) climate scenarios.
To determine how climate warming influenced these patterns, Kearney et al. (6) reran all calculations after raising air temperature by 3 °C, which represents heating projected by many climate models. [Note: for biophysical reasons, a 3 °C increase in air temperature need not result in a 3 °C increase in Tb.] Dramatic shifts were observed. At the coastal temperate site, lizards should benefit greatly from switching from staying in sun to shuttling between sun and shade. In contrast, at the continental and tropical sites, lizards should suffer from warming, unless they have the option of remaining in deep shade. Overall, warming will benefit temperate species by giving them increased flexibility in their habitat choice, but it will constrain tropical species by restricting their habitat use. These quantitative predictions parallel qualitative ones recently made for insects (14) and lizards (15), even though all studies use very different analytical perspectives.
Kearney et al. (6) next expanded the geographic scale of their calculations, first for all of Australia and then the globe. In Australia, the paramount thermoregulatory priority under contemporary climates is staying cool; and climate warming will exacerbate this concern. With shade at a premium over much of this continent, warming presages bad news.
A similar pattern emerged at the global scale. For much of the land (at least where diurnal ectotherms are abundant), overheating is the central concern, especially in deserts or other areas where shade is rare.
One clear conclusion emerging from this study is that shade will be crucial to ectotherm survival in continental and lowland tropical sites. However, tropical ectotherms may not be safe even where shade is plentiful. In these aseasonal climates, ectotherms are often thermal specialists (14); and those living in shaded forests have few opportunities for behavioral thermoregulation (15). As a result, tropical forest ectotherms may be particularly vulnerable to warming. Given the biodiversity of tropical ectotherms, the specter of climate warming is daunting; as if tropical species didn't already have enough problems (e.g., deforestation)!
Concluding Remarks
Physiological ecologists have long appreciated that behavioral thermoregulation can buffer climate variation. However, one of the most intriguing findings to emerge from this new article (6) addresses the question of whether behavior accentuates or buffers the physiological impact of climate warming? The answer is either, both, or none of the above! At high-latitude sites (other than continental deserts), where warming up is a paramount physiological concern, ectotherms can use shuttling behavior to capitalize on warming. Thus, behavior should enhance their performance and fitness in a warming world. In open and warmer habitats (e.g., deserts and tropical ones), where avoiding heat stress is the prime concern, behavior can buffer the impact of warming, but only if ectotherms have access to shade or a burrow.
Tropical forest ectotherms may be particularly vulnerable to warming.
This model was developed for a generic ectotherm. Obviously, there is ample room for exploring and extending variations on a theme. As Kearney et al. (6) note, optimal regulatory patterns can change in fine-scaled analyses that can include input on wind speed, cloud cover, extent of vegetation cover, surface reflectance, and humidity. Organismal variation (size, shape, color, wet-skinned) can also be important. The buffering impact of acclimation and adaptation are not addressed in that article, but their influence may clearly be important (16). In addition, Kearney et al. focus on adult ectotherms, which can use their mobility to thermoregulate. In contrast, their eggs are immobile, and thus warming-induced selection on eggs may be greater than on adults (17). Of course, if “mother knows best,” mobile moms can potentially use thermal cues to pick the best nest sites, which would represent a cross-generational behavioral buffering (18). Alas, moms sometimes make mistakes (19).
Ultimately, survival will depend on whether ectotherms can use behavior to maintain positive net energy gain (6) and rates of population growth (20, 21) in the face of climate warming. Because the impact of warming will be both site and species specific (15, 22), and biotic interactions directly influence energy gain and fitness (7, 22), much work remains to be done. However, there is no doubt that biophysical approaches that incorporate behavioral options must anchor predictive studies that incorporate biotic interactions.
Decades ago, James Heath (23) dramatically showed that “inanimate objects” (water-filled beer cans) could appear to be thermoregulating, even when they were not. To our knowledge, Heath's beer cans were the first models of nonthermoregulating ectotherms and thus the first “null models” in environmental physiology and perhaps in ecology. The intellectual impact of Heath's article echoes to this day and is evident in the new article by Kearney et al. (6). In effect, Kearney et al. contrast the impact of warming on “Heathian” nonregulators versus a behavioral regulator. They show that regulators generally win, but only when regulation is a physical possibility (e.g., when sufficient shade is available) and seasonal phenology is permissive.
Acknowledgments.
This work was supported by National Science Foundation Grants IOB-0416843 (to R.B.H.) and DEB-0816465 (to J.J.T.).
Footnotes
The authors declare no conflict of interest.
See companion article on page 3835.
References
- 1.Parmesan C. Ecological and evolutionary responses to recent climate change. Annu Rev Ecol Syst. 2006;37:637–669. [Google Scholar]
- 2.Bradshaw WE, Holzapfel CM. Evolutionary response to rapid climate change. Science. 2006;312:1477–1478. doi: 10.1126/science.1127000. [DOI] [PubMed] [Google Scholar]
- 3.Bogert CM. Thermoregulation in reptiles, a factor in evolution. Evolution. 1949;3:195–211. doi: 10.1111/j.1558-5646.1949.tb00021.x. [DOI] [PubMed] [Google Scholar]
- 4.Kingsolver JG, Watt WB. Thermoregulatory strategies of Colias butterflies: Thermal stress and the limits to adaptation in temporally varying environments. Am Nat. 1983;121:32–55. [Google Scholar]
- 5.Huey RB, Hertz PE, Sinervo B. Behavioral drive versus behavioral inertia: A null model approach. Am Nat. 2003;161:357–366. doi: 10.1086/346135. [DOI] [PubMed] [Google Scholar]
- 6.Kearney M, Shine R, Porter WP. The potential for behavioral thermoregulation to buffer “cold-blooded” animals against climate warming. Proc Natl Acad Sci USA. 2009;106:3835–3840. doi: 10.1073/pnas.0808913106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Porter WP, Mitchell JW, Beckman WA, DeWitt CB. Behavioral implications of mechanistic ecology. Thermal and behavioral modeling of desert ectotherms and their microenvironment. Oecologia. 1973;13:1–54. doi: 10.1007/BF00379617. [DOI] [PubMed] [Google Scholar]
- 8.Helmuth B, Kingsolver JG, Carrington E. Biophysics, physiological ecology, and climate change: Does mechanism matter? Annu Rev Physiol. 2005;67:177–201. doi: 10.1146/annurev.physiol.67.040403.105027. [DOI] [PubMed] [Google Scholar]
- 9.Kearney M, Porter WP. Mechanistic niche modeling: Combining physiological and spatial data to predict species' ranges. Ecol Lett. 2009 doi: 10.1111/j.1461-0248.2008.01277.x. 10.1111/j.1461-0248.2008.01277.x. [DOI] [PubMed] [Google Scholar]
- 10.Christian KA, Weavers BW. Thermoregulation of monitor lizards in Australia: An evaluation of methods in thermal biology. Ecol Monogr. 1996;66:139–157. [Google Scholar]
- 11.Huey RB, Peterson CR, Arnold SJ, Porter WP. Hot rocks and not-so-hot rocks: Retreat-site selection by garter snakes and its thermal consequences. Ecology. 1989;70:931–944. [Google Scholar]
- 12.Hertz PE, Huey RB, Stevenson RD. Evaluating temperature regulation by field-active ectotherms: The fallacy of the inappropriate question. Am Nat. 1993;142:796–818. doi: 10.1086/285573. [DOI] [PubMed] [Google Scholar]
- 13.Dunham AE. Population responses to environmental change: Physiologically structured models, operative environments, and population dynamics. In: Kareiva PM, Kingsolver JG, Huey RB, editors. Biotic Interactions and Global Change. Sunderland, MA: Sinauer; 1993. pp. 95–119. [Google Scholar]
- 14.Deutsch CA, et al. Impacts of climate warming on terrestrial ectotherms across latitude. Proc Natl Acad Sci USA. 2008;105:6668–6672. doi: 10.1073/pnas.0709472105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huey RB, et al. Why tropical forest lizards are vulnerable to climate warming. Proc R Soc London Ser B. 2009 doi: 10.1098/rspb.2008.1957. 10.1098/rspb.2008.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kearney M, Porter WP, Williams C, Ritchie SA, Hoffmann AA. Integrating biophysical models and evoutionary theory to predict climate impacts on species' ranges; the dengue mosquito Aedes aegypti in Australia. Funct Ecol. 2009 10.1111/j.1365-2435.2008.01538.x. [Google Scholar]
- 17.Mitchell NJ, Kearney MR, Nelson NJ, Porter WP. Predicting the fate of a living fossil: How will global warming affect sex determination and hatching phenology in tuatara? Proc R Soc London Ser B. 2009;275:2185–2193. doi: 10.1098/rspb.2008.0438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jones JS, Coyne JA, Partridge L. Estimation of the thermal niche of Drosophila melanogaster using a temperature-sensitive mutant. Am Nat. 1987;130:83–90. [Google Scholar]
- 19.Feder ME, Blair N, Figueras H. Oviposition site selection: Unresponsiveness of Drosophila to cues of potential thermal stress. Anim Behav. 1997;53:585–588. [Google Scholar]
- 20.Buckley LB. Linking traits to energetics and population dynamics to predict lizard ranges in changing environments. Am Nat. 2008;171:E1–E8. doi: 10.1086/523949. [DOI] [PubMed] [Google Scholar]
- 21.Dunham AE, Grant BW, Overall KL. Interfaces between biophysical and physiological ecology and the population ecology of terrestrial vertebrate ectotherms. Physiol Zool. 1989;62:335–355. [Google Scholar]
- 22.Buckley LB, Roughgarden J. Effect of species interactions on landscape abundance patterns. J Anim Ecol. 2005;74:1182–1194. [Google Scholar]
- 23.Heath JE. Reptilian thermoregulation: Evaluation of field studies. Science. 1964;145:784–785. doi: 10.1126/science.146.3645.784. [DOI] [PubMed] [Google Scholar]