Abstract
Invasive species' facilitation, or benefiting, of native species is rarely considered in biological invasion literature but could have serious economic consequences should a non-native herbivore facilitate injury by a native pest of high-value crops. Japanese beetle (JB), Popillia japonica, a polyphagous scarab, facilitates feeding by the obligate fruit-feeding native green June beetle (GJB), Cotinis nitida, by biting into intact grape berries that GJB, which has blunt spatulate mandibles, is otherwise unable to exploit. Here, we show JB further facilitates GJB by contaminating fruits with yeasts, and by creating infection courts for yeasts associated with GJB, that elicit volatiles exploited as aggregation kairomones by GJB. Traps baited with combinations of grapes and beetles were used to show that fruits injured by JB alone, or in combination with GJB, become highly attractive to both sexes of GJB. Such grapes emit high amounts of fermentation compounds compared with intact grapes. Beetle feeding on grape mash induced the same volatiles as addition of winemaker's yeast, and similar attraction of GJB in the field. Eight yeast species were isolated and identified from JB collected from grapevine foliage. Establishment and spread of JB throughout fruit-growing regions of the United States is likely to elevate the pest status of GJB and other pests of ripening fruits in vineyards and orchards.
Keywords: Cotinis nitida, facilitation, invasive species, Popillia japonica, tritrophic interaction
Facilitation refers to interactions between species that benefit at least one of the participants and cause harm to neither (1). Invasive species' facilitation of native species is rarely considered in the biological invasion literature that emphasizes economic losses to agriculture and forestry (2, 3) and adverse ecological impacts on biodiversity (4–6). A search of ecological journals from 1993 to 2004 nevertheless found 61 papers reporting evidence of such facilitation across a range of habitats (7). Mechanisms by which invasive species can directly benefit native populations (7) include trophic subsidy, i.e., serving as a food source or providing limiting nutrients to higher trophic levels (8, 9), modification or creation of habitat exploited by native species (10), and pollination of native plants (11, 12). Invasive species may indirectly benefit native ones by ameliorating predation or competition (7), or through plant stress-mediated interactions, e.g., increased colonization of defoliated oaks by wood borers after gypsy moth, Lymantria dispar (L.), outbreaks (13).
Notably, the aforementioned review (7) cited no examples of intraguild facilitation of a native herbivore by an invasive plant-feeding species. There could be serious economic consequences should an invasive herbivore facilitate a native pest's host-finding and injury to high-value crops. This article documents such a relationship between the Japanese beetle (JB; Popillia japonica Newman; Scarabaeidae: Rutelinae) and green June beetle (GJB; Cotinis nitida L.; Scarabaeidae: Cetoniinae) mediated by yeast-induced fermentation volatiles from grapes. We use the term “invasive” herein to mean species that are non-native to the ecosystem under consideration and that cause or are likely to cause economic or environmental harm to human, animal, or plant health (14).
The JB, a polyphagous scarab first found in North America in 1916 and now established throughout most of the eastern United States, continues to expand its range into the Great Plains and south central states. It feeds on ≈300 wild and cultivated plants in 79 families; grapes (Vitis spp.) are among its favored hosts (15). The adults are attracted to diverse plant odors (16), especially blends of feeding-induced volatiles (17, 18), and have inducible gut enzymes able to detoxify myriad secondary compounds (19). Adult JB are mainly leaf-feeders but will exploit sugar-rich foods such as flower petals or ripening fruits when available (20, 21). The GJB, a native to the southeastern and mid-Atlantic United States, is a common pest of ripe or wounded tree and vineyard fruits (21, 22). GJB mandibles are bluntly spatulate, nonopposable, and specialized for feeding on fruit pulp, plant exudates, or similar soft foods (21). JB have sharply pointed, opposable mandibles used to skeletonize leaves and capable of biting through intact skins of ripe fruits (21). In the southeastern United States where viticulture is an emerging industry, proximity of pasture and other grassy larval habitats leads to high numbers of both scarab species in vineyards (ref. 21 and Fig. 1). Besides directly damaging the berries, GJB taint them with odorous secretions (21). Damaged fruits and the beetles themselves may be inadvertently harvested, contaminating the crop.
Fig. 1.
Typical JB and GJB feeding aggregation on early-ripening Reliance grapes in late July 2007.
Insects that feed on overripe, wounded, or decomposing fruits commonly exploit volatiles induced by microbial action on damaged tissues for host-finding (23, 24). Female onion flies, Hylemya antiqua (Meigan), for example, are attracted to bacterially-induced volatiles from decomposing onions (25), and nitidulid sap beetles are attracted to volatiles from fermenting fruits and vegetables (26). In such mutualisms, the microbes are disseminated and able to exploit feeding wounds as infection courts, whereas the herbivores benefit from enhanced host-finding and, in some cases, from microbial detoxification of secondary compounds and breakdown of structural carbohydrates refractory to insect digestive enzymes (23, 24). Volatiles elicited by GJB feeding were shown to elicit aggregations of GJB on peaches (27–29).
Here, we show that the JB facilitates GJB feeding on grapes by biting through the skin providing access to the pulp and by eliciting yeast-mediated fermentation volatiles that GJB exploits in host-finding and aggregation. Implications for the projected impact of JB as it expands its range in fruit-growing regions of the United States are discussed.
Results
Attraction of GJB to JB-Damaged Grapes.
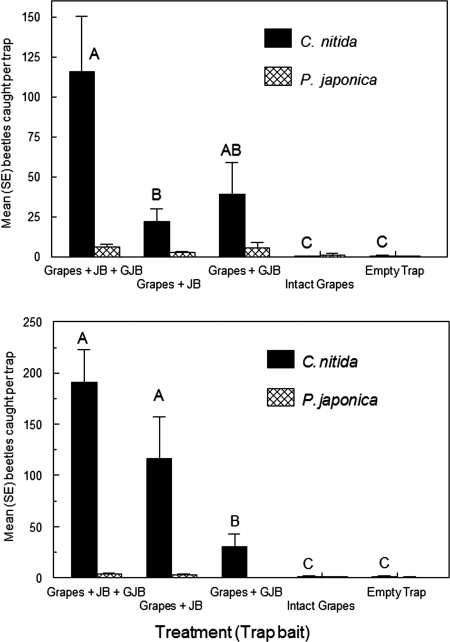
Traps baited with ripe intact grapes without beetles or equivalent clusters with JB alone, GJB alone, or JB and GJB together were exposed in vineyards in July and August. Neither scarab was attracted to empty traps or intact grapes; together, those treatments accounted for <1% of the beetles captured (Fig. 2). GJB fed sparingly in the bait cages, mainly on berries whose skin had ruptured at the pedicel to expose pulp, yet those clusters attracted more GJB than ones without beetles. JB alone damaged many of the grapes and those clusters attracted as many (trial 1) or more (trial 2) GJB as grapes with GJB alone. Clusters with JB and GJB together were extensively fed upon and became highly attractive to GJB (Fig. 2). Both GJB sexes were attracted; the overall sex ratio responding to the 3 most attractive baits was slightly female-skewed (1.26:1). In contrast, few JB were attracted despite a treatment effect [F(4,20) = 12.3, 4.9, in trials 1 and 2, respectively, P < 0.01; Fig. 2].
Fig. 2.
Mean (± SE) numbers of beetles attracted by ripe intact grape clusters or by grapes exposed to feeding by GJB, JB, or both species together. Treatment effect significant for GJB [F(4,20) = 12.3, 58.2 for trial 1 (Upper) and trial 2 (Lower), respectively; means not followed by the same letter differ (Tukey's HSD, P < 0.05)]. Treatment effects also were significant for JB (see the end of the first paragraph of Results).
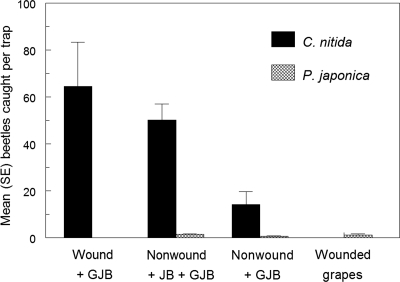
Grapes that were wounded with a hacksaw blade to expose the pulp were not attractive to GJB even after 2–3 days (trial 3; Fig. 3). Intact grapes caged with GJB again sustained little feeding and attracted relatively few GJB, but grapes that were artificially wounded or injured by JB were heavily fed upon by GJB and attracted many additional GJB (Fig. 3). As before, the baits attracted very few JB.
Fig. 3.
GJB response to artificially wounded or nonwounded grapes with or without beetles (Kruskal–Wallis H = 17.5, P < 0.001). Means separation: wounded grapes with GJB = nonwounded grapes with both scarabs > nonwounded grapes with GJB > wounded grapes alone (Mann–Whitney U test, P < 0.05). Note the near-absence of JB response.
JB facilitation of GJB attraction was tested with 2 additional grape cultivars. Reliance, a thin-skinned, early-ripening cultivar with high sugar content, was extensively fed upon by JB and that combination attracted more GJB than did artificially wounded or intact grapes without beetles [means (± SE): 18.0 ± 4.6, 3.4 ± 2.2, and 1.0 ± 0.5, respectively; F(2,8) = 7.4, P < 0.05 for log-transformed data; latter 2 treatments not significantly different (P > 0.05) by Tukey's HSD]. Seyval, a later-ripening cultivar whose berries are still relatively tough and low in sugars, sustained less JB injury but still attracted more GJB than did artificially-wounded or intact grapes without beetles [3.8 ± 1.1, 0.0 ± 0.0, and 0.4 ± 0.4, respectively; Kruskal-Wallis H = 10.6, Mann–Whitney U test, P < 0.05].
Absence of GJB attraction to GJB or JB per se was shown by baiting traps with mixed-species beetle cohorts on late-ripening Chambourcin or Norton grapes whose toughness inhibited feeding, compared with beetle cohorts on early-ripening Foch grapes that were included as a positive control. Mean (± SE) numbers of GJB attracted to those treatments in 24 h were 5 ± 2, 3 ± 1, and 129 ± 9, respectively [F(2,8) = 212, P < 0.001].
Volatiles from Intact Versus Beetle-Damaged Grapes.
Gas chromatography- mass spectroscopic (GC-MS) analyses revealed that JB injury to grapes elicited elevated amounts of 10 fermentation volatiles not emitted by intact grapes (Table 1). All of the compounds induced by JB feeding were elicited in similar amounts by GJB. Notably, many of the compounds were induced at higher levels when both species were present than with either species alone.
Table 1.
Fermentation volatiles collected from Thompson seedless grapes after exposure to P. japonica (JB) and C. nitida (GJB) alone or in combination for 24 h
| Compound | Compound collected (ng/g fresh wt/9 L air) |
P <† | |||
|---|---|---|---|---|---|
| Intact grapes | Grapes + JB* | Grapes + GJB | Grapes + JB + GJB | ||
| Ethyl propionate | 0 ± 0 | 1,144 ± 215* | 616 ± 61 | 2,984 ± 486 | 0.005 |
| Isoamyl alcohol | 0 ± 0 | 799 ± 161* | 1,303 ± 322 | 2,641 ± 899 | 0.05 |
| 2-Methyl-1-butanol | 0 ± 0 | 502 ± 91* | 864 ± 164 | 2,550 ± 460 | 0.005 |
| Ethyl isobutyrate | 0 ± 0 | 57 ± 4* | 15 ± 9 | 121 ± 33 | 0.005 |
| Isobutyl acetate | 0 ± 0 | 560 ± 56* | 241 ± 72 | 1,918 ± 436 | 0.005 |
| Ethyl butonoate | 0 ± 0 | 34 ± 34 | 0 ± 0 | 49 ± 49 | 0.28 |
| Butyl acetate | 0 ± 0 | 47 ± 2b* | 27 ± 16 | 187 ± 49 | 0.01 |
| Hexyl alcohol | 8 ± 8 | 36 ± 14 | 28 ± 17 | 44 ± 15 | 0.31 |
| Isoamyl acetate | 0 ± 0 | 3,601 ± 1053* | 4,335 ± 1,337 | 17,241 ± 8,122 | 0.05 |
| 2-Methylbutyl acetate | 0 ± 0 | 227 ± 54* | 162 ± 48 | 905 ± 227 | 0.005 |
| Hexyl acetate | 0 ± 0 | 76 ± 22* | 44 ± 26 | 214 ± 62 | 0.05 |
| Benzyl alcohol | 0 ± 0 | 60 ± 60 | 0 ± 0 | 54 ± 54 | 0.54 |
| 2-Phenylethyl acetate | 0 ± 0 | 84 ± 22* | 95 ± 16 | 271 ± 65 | 0.01 |
Data represent the mean (±SE) of 4 replications per treatment.
*Amounts emitted by JB-damaged grapes > intact grapes (Mann–Whitney U test, P < 0.05).
†P value for within-row comparison of all 4 treatments (Kruskal–Wallis test).
Role of Yeasts in GJB Attraction to Beetle-Injured Grapes.
Crushed grape mash that had been fed upon overnight by GJB or to which a commercial wine yeast had been added attracted GJB, but fresh or 24-h-old grape mash or yeast slurry alone were not attractive. Means for those treatments were 41.0 ± 12.7, 17.4 ± 3.5, 0, 0, and 0 GJB, respectively (Kruskal–Wallis H = 22.7, P < 0.001), with no difference between mash with beetles or yeast (P = 0.095) that both attracted more GJB than the other baits (Mann–Whitney U tests, P < 0.01). None of the baits were attractive to JB (means: 0.4 ± 0.4, 0.4 ± 0.2, 0, 0, and 0, respectively).
GJB feeding on fresh mash of field-collected Cayuga White grapes elicited increases in 11 volatile compounds that were nondetectable from mash alone (Mann–Whitney U tests, P < 0.05). All of those compounds also were elicited by adding commercial wine yeast. Mean (± SE) amounts (ng/g/9 L) emitted by mash with GJB or with yeast were respectively: 3-hydroxy-2-butanone (289 ± 61, 3,478 ± 1,175), ethyl propionate (371 ± 74, 1,066 ± 16), N-propyl acetate (201 ± 27, 1,930 ± 95), 2-methyl-1-butanol (1,910 ± 693, 41,120 ± 360), isobutyl acetate (94 ± 19, 5,614 ± 127), isoamyl acetate (682 ± 120, 47,447 ± 1173), 2-methylbutyl acetate (71 ± 15, 5,747 ± 32), ethyl hexanoate (159 ± 63, 20,610 ± 1,490), hexyl acetate (608 ± 56, 8,941 ± 782), ethyl octonoate (105 ± 68, 13,455 ± 41), and (Z)-3-hexene-1-ol (681 ± 91, 458 ± 49). Two compounds that were emitted by fresh mash also increased (Kruskal–Wallis, P < 0.05) in the presence of GJB or wine yeast: isoamyl alcohol (476 ± 375, 5,489 ± 1,594, 145,645 ± 45) and hexanol (1,022 ± 87, 4,717 ± 393, 3,334 ± 404), respectively.
Eight yeast species were identified from JB collected from grape foliage: Candida famata (Harrison) (Teleomorph Debaryomyces hansenii), Candida lusitaniae (van Uden et do Carmo-Sousa), Candida guilliermondii (Castellani), Cyptococcus laurentii (Kufferath) Skinner, Rhodotorula minuta (Saito) Harrison, Kloeckera sp., Trichosporon mucoides, and Candida sp.. Six yeasts, C. lusitaniae, Rhodotorula glutinis (Fresinius) Harrison, Rhodotorula mucilaginosa 2 (Jorgensesn) Harrison, Kloekera sp., C. famata, and T. mucoides were isolated from GJB collected from feeding aggregations on grape berries. Four of the species were isolated only from JB, 2 were isolated only from GJB, and 4 were isolated from both scarab species.
Discussion
JB facilitates native GJB by biting into ripe grapes too tough for the latter species to penetrate with their blunt mandibles, creating focal points for GJB feeding (21). JB-associated yeasts contaminate such wounds and elicit fermentation volatiles that both sexes of GJB exploit in host-finding. Early-arriving GJB contaminate such fruits with their own gut flora, inducing additional GJB-attractive odors. Thus, wounding of even a few berries by JB can induce GJB feeding aggregations. The beetles' flight periods overlap, peaking in late July and early August in Kentucky when early-season ripening grape cultivars are harvested and midseason ones are in the later stages of veraison (21). Together the 2 scarab pests can reduce harvestable clusters on nonsprayed vines by 95% or more (30).
Various insects that exploit ripening or decaying fruits use volatiles associated with spoilage microorganisms to locate food sources (23–26, 29, 31, 32). Presence of nonsporulating colonies of powdery mildew (Uncinula necator, syn. Erysiphe necator) on grape berries, for example, was associated with elevated populations of spoilage microbes, increased emission of ethyl acetate, acetic acid, and ethanol, and greater infestation by sap beetles, ants, and yellowjacket wasps (32). Feeding by wasps and birds can break the skin of fruits (32, 33), enabling insects for which intact fruits are too tough or provide insufficient gustatory stimuli to feed. Although skins of intact grapes harbor an indigenous yeast fauna (34), in our trials GJB were not attracted to artificially-wounded berries from unsprayed vines. JB facilitation of GJB therefore involves more than just JB feeding wounds allowing entry of indigenous surface yeasts.
The role of yeasts in eliciting GJB aggregations was first shown when addition of an antifungal antibiotic prevented otherwise attractive baits (peach puree being fed upon by males) from recruiting additional GJB (28). Twelve different yeast species were identified from the gut and feces of GJB that had been feeding on peaches, and GJB transferred yeasts to the food substrate (35). Attraction of GJB to peach slices or puree inoculated with those yeasts alone was inconsistent, however, and less than response to peach substrates being fed upon by GJB (28, 29). It was suggested there may be a GJB-produced aggregation pheromone that is synergized by volatiles from fermenting foods (29). Volatiles were not analyzed in the aforementioned studies.
Our data indicate it is unnecessary to invoke an aggregation pheromone to explain clustering of GJB on fruits. Indeed, grape berries fed upon by JB alone emitted similar odors and recruited as many or more GJB as GJB-damaged grapes. Response to residual sex pheromone cannot account for such aggregations because mated females or males attract no more GJB than do unbaited traps (36). We do not claim GJB cannot feed on grapes in the absence of JB; indeed, GJB was a pest of ripe fruits before JB invaded its geographical range (22). GJB are robust and sometimes able to tear the skin or break a ripe berry from its pedicel with their tarsal claws, and early-ripening grapes may rupture on their own, exposing pulp (21). Our data show that JB injury leads to additive or synergistic increases in GJB feeding and aggregation by the mechanisms outlined above.
Although yeasts have been internally isolated from insects in 8 orders, including at least 3 other scarabs (35, 37), our study demonstrates a yeast fauna associated with JB. How JB acquire yeasts, specifically where they reside on or in JB, and their role in JB nutritional ecology were not addressed in our study. All 5 yeast genera we isolated from JB and GJB occur indigenously on the skin of healthy grapes (34) and other surfaces of vegetation.
None of the yeasts we found associated with beetles collected on grapevines in Kentucky were isolated from feces or guts of field-collected GJB in Arkansas, although 2 that we isolated from JB (D. hansenii and C. guilliermondii), and 2 others from GJB (R. glutinis and R. mucilaginosa) were found in GJB and on peaches in Oklahoma (34). Trichosporon cutaneum Ota, a yeast earlier workers (29, 35) predicted to be central to production of compounds responsible for GJB aggregation, was absent in our samples. The fact that GJB responded to grape mash treated with wine yeast indicates their attraction to fermentation volatiles is not restricted to odors induced by their own endosymbionts. Lures containing blends of such compounds might be useful for monitoring or mass trapping, or fungicides targeting transmission of yeasts might reduce GJB aggregation and injury to fruits.
Day-active scarabs have high energetic requirements requiring calorie-rich foods (20, 21, 38, 39). Compared with GJB, few JB were attracted to beetle-damaged grapes in our assays. Antennal olfactory receptor neurons (ORNs) responsive to food odors have not been characterized for either species, but electrophysiological studies with an African fruit chafer, Pachnoda marginata (Scarabaeidae: Cetoniinae), revealed sets of ORNs selectively tuned to common fruit volatiles and others tuned to odors associated with microbial fermentation (40). JB, in contrast, are attracted to blends of volatiles emitted by JB-damaged leaves (16–18), which may enable them to efficiently locate favored hosts. Differential attraction of GJB and JB to damaged fruits or leaves likely reflects their antennal ORNs being tuned to the type of food resources upon which they mainly depend. JB aggregating on grape foliage nevertheless encounter the sugar-rich berries upon which they will opportunistically feed. The grape/scarab system provides a counterpoint to the induced resistance literature in that fruits injured by either scarab become more attractive to GJB, and leaves fed upon by JB attract additional JB (18). Both beetles exploit feeding-induced volatiles as aggregation kairomones.
Despite ongoing regulatory control efforts, JB is expanding its range in the Great Plains, Great Lakes, and south central states and is an ever-present threat to becoming established in California and the Pacific Northwest (15). Besides damaging fruit and garden crops, adult JB feed on many species of wild indigenous plants including fruits of Vitis, Rubus, and Vaccinium spp (15, 41). JB could impact the fitness of those plants either directly or by facilitating other fruit-feeding arthropods.
Our study illustrates a mechanism by which an invasive insect substantially elevates the economic impact of a native pest through a tri-trophic, yeast-mediated, facilitative interaction. Continued spread of JB likely will aggravate injury from native and invasive pests of ripening fruits. The multicolored Asian lady beetle, Harmonia axyridis (Pallas), for example, which infests ripe grape clusters, resulting in tainted unmarketable wine (42), is unable to break the skin of grapes or apples and exhibits a preference for damaged fruit (31). GJB is abundant in Arkansas, Missouri, and Oklahoma into which JB recently has spread, and Cotinis mutabilis (Gory and Perceron), a cetoniine scarab with habits similar to GJB, is a pest of ripening fruits in the western United States (43). Should JB become established in California it likely would elevate status of C. mutabilis as well. Managing such pests is problematic because most insecticides have a required interval prohibiting their use on fruits in the final few days before harvest. Intraguild facilitation of native herbivores by invasive ones has been overlooked in literature concerning impacts of invasive species (7) but could have serious economic consequences when those herbivores feed on high-value crops.
Materials and Methods
Attraction of GJB to JB-Damaged Grapes.
Field trials were in plantings of grapes and blackberries at the University of Kentucky Horticultural Research Farm, Lexington during July and August, 2007. Traps were designed to allow baiting with grape clusters and beetles while denying incoming beetles' access to the fruit. They consisted of intersecting vanes (31 × 31 cm) of green corrugated plastic (Coroplast) atop a galvanized steel tractor funnel (25.4 cm top diameter) with a standard ventilated metal JB trap container (Ellisco) attached underneath to hold captured beetles. A central cutout (9.5 cm wide, 11.5 cm deep) in the vanes accommodated a slightly smaller cylindrical screened cage containing the baits. Traps were suspended by monofilament fishing line from plant hangers attached to 1.8-m wooden stakes so the baits were 1 m above the ground. Traps were spaced 10 m apart in rows; treatments were replicated 6 times in each trial.
Beetles used to prepare the baits were hand-collected from grape foliage (JB) or grape, blackberry, and peach fruits (GJB) the day before each trial. Sexes were distinguished by differences in their foretibial spurs (21). Only female JB were used to obtain more consistent feeding on the baits (males do not feed while mating). GJB are ≈4 times heavier than JB, and their feeding aggregations consist of both sexes with little mating (21) so unless indicated otherwise, 3 male and 3 female GJB were used for those baits, as opposed to 20 female JB for baits fed upon by that species. Three male and 3 female GJB plus 20 female JB were used for baits fed upon by both species.
Ripe Thompson seedless grapes were used in the first 2 trials. Thirty clusters consisting of 20 intact grape berries attached to a single stem were allocated to 5 treatments: (i) empty bait cage, (ii) cluster of grapes without beetles, (iii) grape cluster with GJB, (iv) grape cluster with JB, and (v) grape cluster with both beetle species. In trial 1, which lasted 96 h (July 11–15, 2007), the traps were baited in the morning as they were hung. For trial 2, which lasted 48 h (July 16–18, 2007), beetles and grapes were preloaded into covered 1-L translucent plastic containers and held at 29 °C with photoperiod of light 15/dark 9 for 24 h before being taken to the field and transferred to bait cages. This process allowed some feeding injury before the start of the trial.
The traps were not 100% efficient in capturing attracted beetles so the trap lines were walked for 30 min about every 2 h from 0900 to 2000 h and any beetles clinging to the outside of bait cages or vanes were knocked into the funnel. Trap captures were frozen and later sorted to compare numbers of GJB and JB that had been attracted.
Another trial tested specificity of attraction of GJB to beetle-injured versus artificially-wounded Thompson seedless grapes. Wounded clusters were prepared by cutting a 7- to 10-mm slit in each berry with a hacksaw blade deep enough to exude juice. Traps, number of beetles and grapes used in baits, and other procedures were as described earlier. The trial ran July 23–25, 2007, comparing beetles' response to 4 treatments: (i) initially-intact grape cluster with GJB, (ii) initially-intact cluster with both beetle species, (iii) artificially-wounded grapes with GJB, and (iv) artificially-wounded grapes alone. The treatments were held overnight as described earlier before being used in the traps.
Generality of GJB attraction to JB-injured fruits was tested with 2 additional grape cultivars, early-ripening Reliance and midseason-ripening Seyval Blanc, collected at the study site. Five replications of 3 treatments for each cultivar were used: (i) intact cluster of 10 berries, (ii) 10-grape cluster with all berries artificially wounded, or (iii) cluster of 10 initially-intact grape berries with 10 female JB. For this trial (July 26–30, 2007) treatments were prepared in the field on the first morning and placed directly into the bait cages.
Volatiles from Intact Versus Beetle-Damaged Grapes.
Clusters of 20 Thompson seedless grapes were weighed, placed in 0.95-L translucent plastic containers, and allocated to 4 treatments: (i) intact cluster without beetles, (ii) cluster with 20 female JB, (iii) cluster with 3 male and 3 female GJB, and (iv) cluster with 20 female JB plus 3 pairs of GJB. The containers were held at 29 °C with photoperiod of light 15/dark 9 for 24 h to allow time for some feeding, then the grapes and beetles were transferred to a push volatiles collection apparatus (see below) the next morning. Four replicates were set up and analyzed over 4 successive days.
Charcoal-filtered air from a commercial cylinder was passed by means of Teflon tubing through 2-L glass bell jars containing the sample to collect headspace volatiles. Air flow rate was regulated at 100 μL·min−1. A glass trap (0.4-cm diameter) packed with 100 mg of Super Q absorbent (Alltech) was connected to the outlet line to collect volatile compounds entrained by the air. After a 3-h collection period the trap was removed and eluted with 400 μL of hexane and cumene (1 μg) was added as an internal standard. Volatile compounds were quantified by 2.0-μl injections into a Hewlett Packard 5890 gas chromatograph equipped with a 60-m × 0.32-mm DB-5 column (J & W Scientific) with a 1-μm film thickness. Operating conditions were: inlet, 220 °C; column 50 °C for 5 min and then programmed at 2 °C min−1 to 220 °C; flame ionization detection, 240°C; He carrier linear flow rate, 30 cm·s−1. Mass spectral analyses were done with a Hewlett Packard GCD 1800B instrument equipped with a 25-m × 0.25-mm DB-5 column with a 0.25-μm film thickness operated under the following conditions: inlet, 250 °C; column 40 °C for 5 min and then programmed at 2 °C min−1 to 200 °C. The scan mass range was from m/z 30 to 450, and the scan time was 0.1 s. Spectra were matched electronically to those in the National Institute of Standards and Technology library, and identifications were confirmed by comparing the retention times of trapped volatiles with authentic compounds.
Role of Yeasts in GJB Attraction to Beetle-Injured Grapes.
The hypothesis that yeasts mediate attraction of GJB to beetle-injured grapes was evaluated by field trapping and comparing grape volatiles elicited by yeasts alone or by beetle feeding. Mash of Cayuga white grapes, an early- to midseason-ripening cultivar, was made by using a mortar and pestle. Samples (100 mL) of mash were used to prepare 6 replicates of 5 treatments: (i) mash with no added yeast, (ii) mash to which a slurry (5 g of yeast/50 mL of water) of Saccharomyces cerevisiae var. bayanus active dry wine yeast (strain Red Star Premier Cuvée; Red Star) had been added (1 mL of slurry/100 mL of mash); (iii) mash plus 3 pairs of GJB, (iv) yeast slurry (20 mL) alone, and (v) fresh grape mash made just before the trial started. Treatments 1–3 were prepared 1 day before the trial and held 24 h in a growth chamber as described earlier to allow fermentation to occur. Treatments were added to open Petri dishes with 5 dental wicks and placed in the traps for 24 h (August 1–2, 2007). Captured beetles were analyzed as described earlier.
Additional 100-ml samples of Cayuga white grape mash were made as above and 3 treatments were prepared to compare yeast or beetle-induced volatiles: (i) fresh mash with no added yeast, (ii) mash with yeast allowed to ferment 24 h, and (iii) mash upon which 3 male and 3 female C. nitida had fed for 24 h. Headspace samples were then analyzed by GC-MS as described earlier.
Beetles were collected at the field site in early August 2007 to survey the yeast fauna associated with each species. GJB collected from feeding aggregations on ripe Reliance grapes were placed directly into sterile plastic centrifuge tubes. Four replicates each containing 2 males and 2 females were prepared. Beetles from different clusters were used for each replicate. Four replicates of 20 JB each were similarly collected from foliage of the same vines. The JB were not taken from fruit clusters to reduce chances they were recently contaminated by microorganisms associated with GJB aggregations. Samples were stored at −80 °C until yeast analysis was conducted.
Whole beetles were placed in sterile whirl packs and 10 mL of 0.1% peptone water was added as a rinse solution. Surface yeasts were isolated and enumerated by plating samples of the rinses in 3 serial 1/10 dilutions with 2 replications per sample onto potato dextrose agar (PDA; Difco). Beetles in the remaining rinse solution were then smashed by hand in the whirl pack for 1 min and those samples were plated as above. The PDA plates were kept at 29 °C under continuous light for 5 days; yeast counts were then taken to estimate the total population. Representative isolates from those enumeration plates were selected for identification based on morphological differences and samples were spread in triplicate on PDA. Those isolates were incubated for 5 days at 29 °C. A total of 25 isolates of yeasts or yeast-like fungi, 12 from JB and 13 from GJB, were selected, plated in triplicate on PDA, and incubated for 24 h at 29 °C. Identity of those isolates was determined with the API 20C AUX yeast identification system (bioMérieux Vitek) (44) and confirmed with identification software (apiweb; bioMérieux Vitek).
Data Analyses.
Data were analyzed with Statistix, version 7.0 (45). Two-way ANOVA was used with Tukey's test in the first 2 field trials in which log (GJB) or square root (JB) transformed data met the assumptions of normality and homogeneity of variances (45). When ANOVA assumptions could not be met in subsequent trials because of all zeros in some treatments, the nonparametric Kruskal–Wallis test was used, followed by Mann–Whitney U tests with Bonferroni correction for preplanned comparisons of selected means (46). All data are reported as original means (± SE).
Acknowledgments.
We thank K. Akers, C. Brady, R. Collins, J. Condra, B. O'Daniel, C. Redmond, D. Slone, and P. Wilson for technical assistance; R. Bessin for statistical guidance; and Talon Winery (Lexington, KY) for providing grapes. This work was partially funded by the New Crop Opportunities Center at the University of Kentucky through a U.S. Department of Agriculture Special Grant and a U.S. Department of Agriculture Sustainable Agriculture Research and Education Graduate Student Grant (to D.L.H.). This is paper 08-08-106 of the Kentucky Agricultural Experiment Station.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Stachowitcz JJ. Mutualism, facilitation, and the structure of ecological communities. BioScience. 2001;51:235–246. [Google Scholar]
- 2.Pimentel D, et al. Economic and environmental threats of alien plant, animal, and microbe invasions. Agric Ecosyst Environ. 2004;84:1–20. [Google Scholar]
- 3.Torchin ME, Mitchell CE. Parasites, pathogens, and invasions by plants and animals. Front Ecol Environ. 2004;2:183–190. [Google Scholar]
- 4.Parker IM, et al. Impact: Toward a framework for understanding the ecological effects of invaders. Biol Invasions. 1999;1:3–19. [Google Scholar]
- 5.Mooney HA, Cleland EE. The evolutionary impact of invasive species. Proc Natl Acad Sci USA. 2001;98:5446–5451. doi: 10.1073/pnas.091093398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Strauss SY, Lau JA, Carroll SP. Evolutionary responses of natives to introduced species: What do introductions tell us about natural communities? Ecol Lett. 2006;9:357–374. doi: 10.1111/j.1461-0248.2005.00874.x. [DOI] [PubMed] [Google Scholar]
- 7.Rodriguez LF. Can invasive species facilitate native species? Evidence of how, when, and why these impacts occur. Biol Invasions. 2006;8:927–939. [Google Scholar]
- 8.Memmott J, Fowler SV, Paynter Q, Sheppar AW, Syrett P. The invertebrate fauna on broom, Cytisus scoparius, in two native and two exotic habitats. Acta Oecologica. 2000;21:213–222. [Google Scholar]
- 9.Richardson DM, Allsopp N, D'Antonio CM, Milton SJ, Rejmanek M. Plant invasions: The role of mutualisms. Biol Rev. 2000;75:65–93. doi: 10.1017/s0006323199005435. [DOI] [PubMed] [Google Scholar]
- 10.Jones CG, Lawton JH, Shachak M. Positive and negative effects of organisms as physical ecosystem engineers. Ecology. 1997;78:1946–1957. [Google Scholar]
- 11.Cox PA. Extincton of Hawaiian avifauna resulted in a change of pollinators for the ieie, Freycinetia arborea. Oikos. 1983;41:195–199. [Google Scholar]
- 12.Horskins K, Turner VB. Resource use and foraging patterns of honeybees, Apis mellifera, and native insects on flowers of Eucalyptus costata. Aust J Ecol. 1999;24:221–227. [Google Scholar]
- 13.Wargo PM. Armillariella mellea and Agrilus bilineatus and mortality of defoliated oak trees. For Sci. 1977;23:485–492. [Google Scholar]
- 14.National Invasive Species Council. [Accessed January 12, 2009];Invasive Species Definition Clarification and Guidance White Paper. 2006 Available at www.invasivespeciesinfo.gov/docs/council/isacdef.pdf.
- 15.Potter DA, Held DW. Biology and management of the Japanese beetle. Annu Rev Entomol. 2002;47:175–205. doi: 10.1146/annurev.ento.47.091201.145153. [DOI] [PubMed] [Google Scholar]
- 16.Loughrin JR, Potter DA, Hamilton-Kemp T. Attraction of Japanese beetles (Coleoptera: Scarabaeidae) to host plant volatiles in field trapping experiments. Environ Entomol. 1998;27:395–400. [Google Scholar]
- 17.Loughrin JR, Potter DA, Hamilton-Kemp T. Feeding-induced volatiles of Malus spp. leaves as aggregation kairomones for the Japanese beetle. J Chem Ecol. 1995;21:1457–1467. doi: 10.1007/BF02035145. [DOI] [PubMed] [Google Scholar]
- 18.Loughrin JH, Potter DA, Hamilton-Kemp TR, Byers ME. Role of feeding-induced plant volatiles in aggregative behavior of the Japanese beetle (Coleoptera: Scarabaeidae) Environ Entomol. 1996;25:1111–1191. [Google Scholar]
- 19.Ahmad S. Mixed-function oxidase activity in a generalist herbivore in relation to its biology, food plants, and feeding history. Ecology. 1983;64:235–243. [Google Scholar]
- 20.Held DW, Potter DA. Floral affinity and benefits of dietary mixing with flowers for a polyphagous scarab, Popillia japonica Newman. Oecologia. 2004;140:312–320. doi: 10.1007/s00442-004-1582-7. [DOI] [PubMed] [Google Scholar]
- 21.Hammons DL, Kurtural SK, Potter DA. Japanese beetles facilitate feeding by green June beetles (Coleoptera: Scarabaeidae) on ripening grapes. Environ Entomol. 2008;37:608–614. doi: 10.1603/0046-225x(2008)37[608:jbffbg]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 22.Chittenden FH, Fink DE. The green June beetle. US Dept Agric Bull. 1922;891:1–52. [Google Scholar]
- 23.Berenbaum MR. Allelochemicals in insect—microbe–plant interactions: Agents provocateurs in the coevolutionary arms race. In: Barbosa P, Letourneau DK, editors. Novel Aspects of Insect–Plant Interactions. New York: Wiley; 1988. pp. 97–123. [Google Scholar]
- 24.Phelan PL, Stinner BR. Microbial mediation of plant-herbivore ecology. In: Rosenthal GA, Berenbaum MR, editors. Herbivores: Their Interactions with Secondary Plant Metabolites: Vol. 2. Evolutionary and Ecological Processes. New York: Academic; 1992. pp. 279–315. [Google Scholar]
- 25.Dindonis LL, Miller JR. Onion fly and little house fly host finding selectively mediated by decomposing onion and microbial volatiles. J Chem Ecol. 1981;7:421–428. doi: 10.1007/BF00995764. [DOI] [PubMed] [Google Scholar]
- 26.Nout MJR, Bartelt RJ. Attraction of a flying nitidulid (Carpophilus humeralis) to volatiles produced by yeasts grown on sweet corn and a corn-based medium. J Chem Ecol. 1998;24:1217–1239. [Google Scholar]
- 27.Domek JM, Johnson DT. Demonstration of semiochemically induced aggregation in the green June beetle, Cotinis nitida (L) (Coleoptera: Scarabaeidae) Environ Entomol. 1988;17:147–149. [Google Scholar]
- 28.Domek JM, Johnson DT. Inhibition of aggregation behavior in the green June beetle, Cotinis nitida (L) (Coleoptera: Scarabaeidae) by fungistatic treatment of food substrate. Environ Entomol. 1990;19:995–1000. [Google Scholar]
- 29.Johnson DT, Vishniac JS. The role of Trichosporon cutaneum in eliciting aggregation behavior in Cotinis nitida (L.) (Coleoptera: Scarabaeidae) Environ Entomol. 1991;20:15–21. [Google Scholar]
- 30.Hammons DL, Kurtural SK, Potter DA. Phenological resistance of grapes to green June beetle damage. In: Coolong T, Snyder J, Smigell C, editors. 2008 Fruit and Vegetable Report PR-572. Lexington, KY: University of Kentucky; 2008. pp. 16–17. [Google Scholar]
- 31.Koch RL, Burkness EC, Wold Burkness SJ, Hutchinson WD. Phytophagous preferences of the multicolored Asian lady beetle (Coleoptera: Coccinellidae) for autumn-ripening fruit. J Econ Entomol. 2004;97:539–544. doi: 10.1093/jee/97.2.539. [DOI] [PubMed] [Google Scholar]
- 32.Gadoury DM, et al. Effects of diffuse colonization of grape berries by Uncinula necator on bunch rots, berry microflora, and juice and wine quality. Phytopathology. 2007;97:1356–1365. doi: 10.1094/PHYTO-97-10-1356. [DOI] [PubMed] [Google Scholar]
- 33.Boudreau GW. Factors related to bird depredations in vineyards. Am J Enol Viticult. 1972;23:50–53. [Google Scholar]
- 34.Rosini G, Federici F, Martini A. Yeast flora of grape berries during ripening. Microb Ecol. 1982;8:83–89. doi: 10.1007/BF02011464. [DOI] [PubMed] [Google Scholar]
- 35.Vishniac HS, Johnson DT. The development of a yeast flora in the adult green June beetle, Cotinis nitida (L) (Coleoptera: Scarabaeidae) Mycologia. 1990;82:470–478. [Google Scholar]
- 36.Domek JM, Johnson DT. Evidence of a sex pheromone in the green June beetle, Cotinis nitida (L) (Coleoptera: Scarabaeidae) J Entomol Sci. 1987;22:264–267. [Google Scholar]
- 37.Vega FE, Dowd PF. The role of yeasts as insect endosymbionts. In: Vega FE, Blackwell M, editors. Insect-Fungal Associations, Ecology, and Evolution. Oxford: Oxford Univ Press; 2005. pp. 211–243. [Google Scholar]
- 38.Chappell MA. Thermoregulation and energetics of the green fig beetles (Cotinis texana) during flight and foraging behavior. Physiol Zool. 1984;57:581–589. [Google Scholar]
- 39.Oertli JJ, Oertli M. Energetics and thermoregulation of Popillia japonica Newman (Coleoptera: Scarabaeidae) during flight and rest. Physiol Zool. 1990;63:921–937. [Google Scholar]
- 40.Stensmyr MC, Larsson MT, Bice S, Hansson B. Detection of fruit- and flower-emitted volatiles by olfactory receptor neurons in the polyphagous fruit chafer, Pachnoda marginata (Coleoptera: Cetoniinae) J Comp Physiol. 2001;187:509–519. doi: 10.1007/s003590100222. [DOI] [PubMed] [Google Scholar]
- 41.Fleming WE. Biology of the Japanese Beetle. Washington, DC: Department of Agriculture; 1972. Technical Bulletin 1449. [Google Scholar]
- 42.Pickering G, et al. Influence of Harmonia axyridis on the sensory properties of white and red wine. Am J Enol Vitic. 2004;55:153–159. [Google Scholar]
- 43.Stone MW. The peach beetle, Cotinis mutabilis (Gory and Percheron) in California (Coleoptera: Scarabaeidae) Pan-Pacific Entomol. 1982;58:159–161. [Google Scholar]
- 44.Smith MB, Dunklee D, Vu H, Woods GL. Comparative performance of the RapID yeast plus system and the API 20C AUX clinical yeast system. J Lin Microbiol. 1999;37:2697–2698. doi: 10.1128/jcm.37.8.2697-2698.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Analytical Software. Statistix Version 8.0: User's Manual. Tallahassee, FL: Analytical Software; 2003. [Google Scholar]
- 46.Sokal RR, Rohlf FJ. Biometry. San Francisco: Freeman; 1969. pp. 380–402. [Google Scholar]