Abstract
The generation of high levels of new catalytic activities on natural and artificial protein scaffolds is a major goal of enzyme engineering. Here, we used random mutagenesis and selection in vivo to establish a sugar isomerisation reaction on both a natural (βα)8-barrel enzyme and a catalytically inert chimeric (βα)8-barrel scaffold, which was generated by the recombination of 2 (βα)4-half barrels. The best evolved variants show turnover numbers and substrate affinities that are similar to those of wild-type enzymes catalyzing the same reaction. The determination of the crystal structure of the most proficient variant allowed us to model the substrate sugar in the novel active site and to elucidate the mechanistic basis of the newly established activity. The results demonstrate that natural and inert artificial protein scaffolds can be converted into highly proficient enzymes in the laboratory, and provide insights into the mechanisms of enzyme evolution.
Keywords: chimeric protein, enzyme design, enzyme evolution, half barrel, TIM barrel
Nature has generated enzymes with well-defined active sites in which metabolic reactions are catalyzed with high efficiency and specificity under physiological conditions (1). Recent technical advances have allowed experimental and computational biochemists to alter the activity and stability of these exquisite molecular machines in a predefined manner, either by rational design or directed evolution (2–6). The results of such experiments help to understand the complex interplay between the structure and function of enzymes, and provide insights into the mechanisms by which they have evolved from less sophisticated precursors.
The (βα)8- (or TIM) barrel is the most frequent and most versatile fold among naturally occurring enzymes (7, 8). The canonical TIM-barrel is composed of 8 modular units, each of which consists of a β-strand and a α-helix that are connected by a βα-loop; individual units are linked by αβ-loops. The 8 strands form a central parallel β-sheet, the β-barrel, which is surrounded by the α-helices. In all known (βα)8-barrels, the active site residues are located at the C-terminal ends of the β-strands and in the βα-loops (“catalytic face”). Residues maintaining the stability of the fold are found in the core and on the opposite end of the barrel, including the αβ-loops (“stability face”) (9). As a consequence, residues important for activity can be exchanged without compromising stability. For this reason, (βα)8-barrels provide an ideal scaffold for enzyme design, and are an excellent tool to study the mechanisms of molecular evolution (10–12).
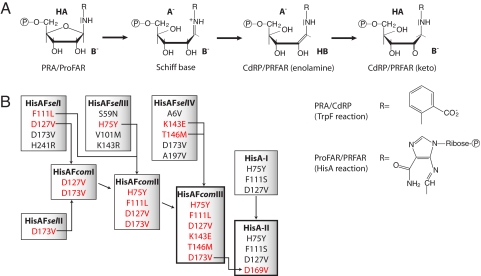
We are studying the evolution and design of (βα)8-barrels involved in aromatic amino acid biosynthesis (9). Along these lines, we earlier used random mutagenesis and selection in vivo to establish the catalytic activity of phosphoribosyl anthranilate (PRA) isomerase (TrpF) on the (βα)8-barrel scaffold of N′-[(5′-phosphoribosyl)formimino]-5-aminoimidazole-4-carboxamide ribonucleotide (ProFAR) isomerase (HisA) from Thermotoga maritima (13, 14). The wild-type TrpF and HisA enzymes catalyze mechanistically related isomerisation reactions of aminoaldoses into the corresponding aminoketoses within tryptophan and histidine biosynthesis (15). However, their substrates, Schiff base intermediates, and products contain a different substituent linked to the nitrogen atom of the corresponding aminosugars (Fig. 1A). Although the sequence identity between TrpF and HisA is only ≈15%, the successful design of detectable PRA isomerisation activity by exchanging only a few HisA residues suggests that the 2 (βα)8-barrel enzymes have evolved from a common ancestor by gene duplication and diversification (9). In further support of this hypothesis, a naturally bifunctional isomerase (PriA) has been identified in several microorganisms that catalyses both the TrpF and the HisA reaction with high efficiency (16, 17).
Fig. 1.
Selection and optimization of HisAF and HisA variants with TrpF activity. (A) Substrates, intermediates, and products of the reactions catalyzed by the TrpF and HisA enzymes. The reactions start with the protonation of the furanose ring oxygen of the aminoaldose (PRA or ProFAR) by a general acid (HA) and subsequent cleavage of the carbon-oxygen bond. Then, the resulting Schiff Base intermediate is deprotonated by a general base (B−) at the C2′ atom of its ribose moiety. The reaction product is the enolamine form of CdRP or PRFAR, which tautomerizes in an enzyme-independent manner to the corresponding aminoketose (15). The subsequent reactions in tryptophan and histidine biosynthesis, which are catalyzed by TrpC and HisF, yield indole glycerol phosphate and imidazole glycerol phosphate plus 5-aminoimidazole-4-carboxamide ribotide, respectively (33, 34). (B) Overview of the experimental workflow. The HisAFselI-IV variants isolated by in vivo selection contain the indicated amino acid exchanges. Bona fide beneficial exchanges of HisAFselI-IV (in red) were combined to generate HisAFcomI-III (for details, see the main text). Substitutions at positions 1–119 are located in the N-terminal half barrel of HisA, and substitutions at positions 120–250 are located in the C-terminal half barrel of HisF (Fig. S1). The earlier isolated HisA-I variant contains the 3 indicated exchanges (13). The additional D169V exchange, which corresponds to the equivalent D173V exchange within HisAFcomIII, was introduced into HisA-I to generate HisA-II. Residues lysine 143 and threonine 146 of HisAF are located within the C-terminal half barrel of HisF, in the long loop between β-strand 5 and α-helix 5 (Fig. S1). This loop has a different conformation in the C-terminal half barrel of HisA (22); therefore, no exchanges equivalent to K143E and T146M in HisAFcomIII could be identified and introduced into HisA-I. The 2 variants with wild-type like TrpF activity, HisAFcomIII and HisA-II, are framed in bold.
Besides gene duplication, the recombination of protein fragments is crucial for the evolution of new enzymes (18, 19). Specifically, the modular construction of (βα)8-barrels suggests that the mixing and matching of (βα)n entities might be a mechanism to generate stable proteins, on which novel catalytic activities can be established (20). Indeed, the striking internal 2-fold symmetry observed for both HisA and imidazole glycerol phosphate synthase (HisF), which catalyzes the subsequent reaction within histidine biosynthesis, suggests that these 2 enzymes are composed of 2 (βα)4-half barrel domains (21, 22). The construction of the chimeric protein HisAF consisting of the 4 N-terminal (βα)1–4 units of HisA and the 4 C-terminal (βα)5–8 units of HisF provides further experimental support for this hypothesis. It forms a stable and monomeric (βα)8-barrel protein with a well-defined tertiary structure and a cooperative unfolding; however, it lacks detectable enzymatic activity (23).
After the successful introduction of TrpF activity into the natural HisA scaffold, we have now used random mutagenesis and selection to establish PRA isomerisation on the artificial HisAF scaffold. The results show that an inert (βα)8-barrel protein generated by the recombination of 2 (βα)4-half barrels can readily be evolved into a highly proficient enzyme. The sequences of the (βα)1–4 units from HisA and HisAF are identical, whereas the sequences of the (βα)5–8 units show a correspondence of ≈25%. Accordingly, we found that within the N-terminal half barrels of HisA and HisAF similar substitutions contribute to the establishment of TrpF activity. However, within the C-terminal half barrels, we identified an additional substitution in HisAF, which was not found in the earlier generated HisA variant. The introduction of the corresponding exchange into this HisA variant resulted in wild-type like TrpF activity, which can be rationalized on the basis of the high-resolution X-ray structure of the protein.
Results and Discussion
Establishing TrpF Activity on the HisAF Scaffold.
We used error-prone PCR to generate a plasmid-encoded hisAF gene library fused to the gene for the maltose-binding protein (MBP), which ensured the solubility of the HisAF protein variants. Those members of this library with TrpF activity were identified by their ability to complement the growth deficiency of an auxotrophic ΔtrpF Escherichia coli strain on minimal medium lacking tryptophan. Retransformation of 7 clones isolated by this approach (hisAFsel I–VII) showed that hisAFselI, hisAFselIII, and hisAFselIV complemented the trpF deficiency most efficiently. These 3 clones, as well as clone hisAFselII, which was interesting because it carries only a single coding mutation, were further investigated. The amino acid substitutions of HisAFselI-IV that result from the acquired nucleotide exchanges are listed in Fig. 1B, and their location in the (βα)8-barrel fold of HisAF is described in supporting information (SI) Fig. S1. It has been shown that the exchanges Y143H and V234M increase the stability and solubility of 2 fused C-terminal half barrels of HisF (24). To allow for soluble expression of the HisAFselI-IV proteins when no longer fused to MBP, the corresponding exchanges Y140H and V231M were introduced by site-directed mutagenesis. Steady-state enzyme kinetic measurements with the purified recombinant proteins showed that the stabilized HisAFselI-IV proteins catalyzed the TrpF reaction in vitro with only modest efficiency (Table 1).
Table 1.
Steady-state PRA isomerisation activities and ligand affinities of HisAF and HisA variants in comparison with TrpF wild-type enzymes
| Protein | kcat, min−1 | KmPRA, μM | kcat/KmPRA, M−1 s−1 | (A)KdrCdRP (B)KiProFAR, μM |
|---|---|---|---|---|
| Selected HisAF variants | ||||
| HisAFselI | 0.57 | 84‡ | 113 | — |
| HisAFselII | 0.017 | 53 | 5.4 | — |
| HisAFselIII | 0.019 | 205‡ | 1.5 | — |
| HisAFselIV | 0.081 | 67 | 20.2 | — |
| Combined HisAF variants | ||||
| HisAFcomI | 1.9 ± 0.1 | 19 ± 7 | (1.9 ± 0.7) × 103 | — |
| HisAFcomII | 3 ± 1 | 14 ± 3§ | (3 ± 1) × 103 | 8.8B |
| HisAFcomIII | 4.0 ± 0.4 | 2.9 ± 0.5 | (2.4 ± 0.5) × 104 | 5.6A/3.1B |
| HisA variants | ||||
| HisA-I* | 7 ± 2 | 35 ± 4 | (3.3 ± 0.7) × 103 | — |
| HisA-II | 67 ± 4 | 16 ± 2 | (7.2 ± 0.8) × 104 | 4.5A/0.10B |
| HisA-II-D8N | 0.020 ± 0.004 | 57 ± 24 | 8 ± 4 | — |
| Wild-type enzymes | ||||
| HisAF, HisA | <0.005 | — | — | — |
| ecTrpF† | 2070 | 12 | 2.9 × 106 | 6.8A |
| tmTrpF† | 222 | 0.28 | 1.3 × 107 | — |
Reaction conditions for all experiments were 50 mM Hepes, pH 7.5/4 mM MgCl2/4 mM EDTA/2 mM DTT/25 °C. All selected and combined HisAF variants contain the Y140H exchange to improve solubility. The variants HisAFselI, II, and IV additionally contain the V231M exchange (24). The shown values for the combined HisAF and HisA variants are the mean and SD, as deduced from at least 3 PRA saturation curves of different protein preparations. The values for the selected HisAF variants were deduced from single PRA saturation curves. The estimated error is less than factor 2.
*The HisA-I variant containing the exchanges H75Y, F111S, and D127V originally could not be saturated with PRA (13), probably due to the presence of residual orthophosphate, which is a competitive inhibitor of substrate binding. After extensive dialysis against phosphate-free buffer, we were able to record complete saturation curves and determine values for the Michaelis constant and the turnover number of HisA-I.
‡KmPRA values were determined in presence of >0.8 mM orthophosphate.
§KmPRA value was determined in presence of >0.1 mM orthophosphate.
Generation of a TrpF-Active HisAF Variant with Wild-Type Substrate Affinity.
To generate more proficient HisAF variants, bona fide beneficial amino acid exchanges found in the different HisAFselI-IV variants were combined by site-directed mutagenesis to generate the variants HisAFcomI-III (Fig. 1B). The choice of residues to be combined was guided by the earlier generated TrpF-active HisA-I variant, which contained the exchanges H75Y, F111S, and D127V. The analysis of HisA-I showed that the single D127V exchange leads to weak TrpF activity, which is increased by the additional exchanges H75Y and F111S (13).
The HisAFselI variant contains the exchange D127V, and the single exchange D173V present in HisAFselII is obviously sufficient to establish weak TrpF activity on the HisAF scaffold. Therefore, in a first step, the D127V and D173V exchanges were combined to generate HisAFcomI. HisAFselIII contains the H75Y exchange and HisAFselI contains the F111L exchange, which were both introduced into HisAFcomI to generate HisAFcomII. HisAFselIV has a higher turnover number than HisAFselII (Table 1). It was plausible to assume that this difference is caused by the K143E and T146M substitutions of HisAFselIV, because these exchanges are located in the putative active site region between β-strand 5 and α-helix 5 (Fig. S1). Therefore, we added K143E and T146M to HisAFcomII, generating HisAFcomIII.
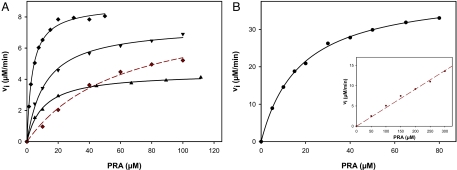
PRA saturation curves showed that the 3 combination variants HisAFcomI-III have high TrpF activities (Fig. 2A); the deduced catalytic efficiencies are listed in Table 1. The turnover number of the best design HisAFcomIII (kcat = 4 min−1) is ≈55- and 520-fold lower than the turnover numbers of the wild-type PRA isomerases from T. maritima (tmTrpF; kcat = 222 min−1) and E. coli (ecTrpF; kcat = 2,070 min−1), respectively. However, the Michaelis constant of HisAFcomIII (KmPRA = 2.9 μM) is ≈4-fold lower than the Michaelis constant of ecTrpF (KmPRA = 12 μM), suggesting that the designed active site embedded into the artificial scaffold has a substrate affinity at least as high as the naturally evolved ecTrpF active site. This finding was corroborated by fluorescence titration studies with the product analogue reduced 1-(2-carboxyphenylamino)-1-deoxyribulose-5-phosphate (rCdRP), which yielded similar dissociation constants for HisAFcomIII and ecTrpF (Table 1) (25). Attempts to further increase the TrpF activity by performing another round of random mutagenesis and selection did not lead to the identification of variants with turnover numbers or Michaelis constants surpassing those of HisAFcomIII.
Fig. 2.
Representative PRA saturation curves of TrpF-active HisAF and HisA variants. (A) HisAFcomI (▲), HisAFcomII (▼), HisAFcomIII (♦), and HisAFcomIII in the presence of 50 μM ProFAR (♦). (B) HisA-II (·); inset: HisA-II in presence of 5 μM ProFAR (·). Reaction conditions for all experiments were 50 mM Hepes, pH 7.5/4 mM EDTA/4 mM MgCl2/2 mM DTT/25 °C/PRA at the indicated concentration, and 2.5 μM HisAF variant or 0.5 μM HisA-II. The solid lines show the result of a hyperbolic fit (linear fit in case of ·) of the data points, which yielded values for Vmax and KmPRA (Vmax/KmPRA in case of ·).
Generation of a TrpF-Active HisA Variant with a Wild-Type Turnover Number.
The earlier generated TrpF-active HisA-I variant contains the exchanges H75Y, F111S, and D127V (Fig. 1B). However, HisA-I does not carry the D169V exchange, which would be equivalent to the D173V substitution present in all HisAFcom variants (Fig. 1B). We reasoned that the addition of the D169V substitution might further improve the TrpF activity of HisA-I and, therefore, introduced it by site-directed mutagenesis, generating HisA-II (Fig. 1B). Indeed, the analysis of a PRA saturation curve of HisA-II (Fig. 2B) revealed a boost in catalytic efficiency, compared with HisA-I (Table 1). Whereas the Michaelis constant of HisA-II (KmPRA = 16 μM) is similar to the one of ecTrpF (KmPRA = 12 μM), its turnover number (kcat = 67 min−1) amounts to a respectable 30% of the turnover number of tmTrpF (kcat = 222 min−1). Such a level of enzymatic proficiency has not been achieved with any of the currently available design methods.
We tested whether HisAFcomIII and HisA-II, in addition to their high TrpF activity, also show residual HisA activity (Fig. 1B). However, neither of the 2 variants converted ProFAR into N′-[(5′-phosphoribulosyl) formimino]-5-aminoimidazole-4-carboxamide-ribonucleotide (PRFAR) at a detectable rate. This result was not unexpected, because aspartate 127, which is replaced by valine in both HisAFcomIII and HisA-II, is essential for the activity of wild-type HisA (15). Nevertheless, we investigated whether ProFAR can still bind to HisAFcomIII and HisA-II by measuring its effect on the TrpF activity of both variants. When PRA saturation curves of HisAFcomIII and HisA-II were recorded in the presence of 50 and 5 μM ProFAR, respectively (Figs. 2 A and B), the KmPRA values increased from 2.9 to 60.5 μM for HisAFcomIII, and from 16 to ≈800 μM for HisA-II. These results show that ProFAR efficiently competes with PRA for binding to the active sites of HisAFcomIII and HisA-II. Also, the deduced competitive inhibition constants Ki of 0.10 and 3.1 μM indicate that HisA-II binds ProFAR with much higher affinity than PRA, whereas HisAFcomIII has a comparable affinity for ProFAR as for PRA (Table 1). Nevertheless, attempts to introduce HisA activity into the HisAFcomIII scaffold by random mutagenesis and selection were not successful, for reasons unknown so far.
X-Ray Structure Analysis and Ligand Docking.
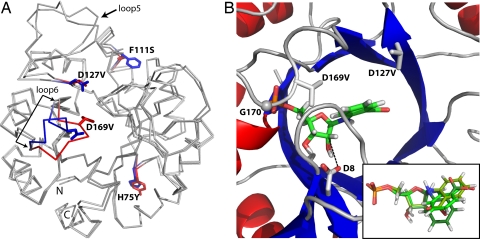
To elucidate the structural basis of the high TrpF activities of HisAFcomIII and HisA-II, we tried to crystallize both proteins. Our attempts were successful in the case of HisA-II, and we could solve its X-ray structure at a resolution of 1.85 Å. The comparison of the HisA-II structure with that of wild-type HisA (PDB ID 1qo2) reveals a different conformation of loop β6α6. This loop harbours the exchange D169V (Fig. 3A), which is crucial for the high TrpF-activity of HisA-II (Fig. 1B and Table 1). The other 3 substitutions do not significantly change the protein backbone.
Fig. 3.
Crystal structure of HisA-II and modeling of PRA into its active site. (A) Comparison of the structures of HisA-II and wild-type HisA (PDB ID 1qo2). The 4 side-chain exchanges are indicated (blue sticks, wild-type HisA residues; red sticks, new residues present in HisA-II). The largest backbone difference is observed in loop β6α6, which carries the D169V exchange. Some variation can also be seen in the long loop β5α5; however, its conformation differs in the 2 molecules of the asymmetric units of both the HisA-II and the HisA structures illustrating its general flexibility. (B) Close-up of the active site of HisA-II with the best docking solution for PRA. A comparison of the top 3 PRA poses is shown in the inset [Glide scores XP-docking: −7.22 (green), −6.85 (pea), and −6.71 (forest)]. In HisA-II, glycine 170 (G170) (shown as sphere) forms a hydrogen bond with an oxygen atom of the phosphate group, which is enabled through a conformational change of loop β6α6 caused by the exchange D169V. The removal of negatively charged carboxylate groups caused by the exchanges D169V and D127V (new side chain shown as sticks) favour the accommodation of the negatively charged anthranilate moiety of PRA. The proximity of the wild-type residue aspartate 8 (D8) (shown as sticks) to the ribose moiety of PRA (distance between the closest carboxylate oxygen and the C2′ atom, 3.45 Å; indicated by broken line) suggests that it acts as the catalytic base of the TrpF reaction (Fig. 1A).
Attempts to cocrystallize the stable product analogue rCdRP with HisA-II or to soak it into the preformed crystals failed. Therefore, we modeled the substrate PRA into the active site of HisA-II to identify residues involved in its binding and turnover to CdRP (Fig. 3B). The 3 best poses of PRA shown in the inset of Fig. 3B are identical with respect to the phosphate moiety and the furanose ring, but differ in the orientation of the anthranilate part. Although the HisA-II protein contains 2 phosphate binding sites, the model indicates that the phosphate moiety of PRA is bound exclusively to the C-terminal one, where it interacts with the backbone atoms of 3 glycines at positions 170, 196, 223, and an arginine at position 224. Remarkably, the alternative conformation of loop β6α6 allows for the formation of a hydrogen bond between an oxygen atom of the substrate phosphate group and the backbone nitrogen of glycine 170 (bond length, 3.06 Å). Also, we note that the crucial exchanges D127V and D169V both lead to the removal of a negative charge, which might facilitate binding of the negatively charged PRA due to relief of electrostatic repulsion (14).
The PRA isomerization reaction catalyzed by TrpF requires a general acid to protonate the furanose ring oxygen, and a general base to deprotonate the Schiff base intermediate at the C2′ atom of its ribose moiety (Fig. 1A) (15). The proximity of the side chain of aspartate 8, which is located at the C-terminal end of β-strand 1, to the ribose moiety of PRA (Fig. 3B) suggested that it acts as the general base. In accordance with this hypothesis, the D8N exchange introduced into HisA-II resulted in an ≈10,000-fold decrease of its TrpF activity (Table 1). Because the sequences of the N-terminal halves of HisA and HisAF are identical, it is very likely that aspartate 8 is also active as catalytic base in the TrpF reaction of HisAFcomIII. Also, the same aspartate 8 in HisA, as well as the corresponding cysteine 7 in tmTrpF, are the catalytic bases in the wild-type isomerisation reactions (15). In tmTrpF, aspartate 126 at the C-terminal end of β-strand 6 most likely acts as the general acid. The corresponding residue in HisA-II is threonine 164, which is highly unlikely to donate the proton of its hydroxyl group to the furanose ring, and also is located too far away to fulfil this function (distance of hydroxyl group to ring oxygen, 5.15 Å). Also, aspartate 127, which is the general acid in the wild-type HisA reaction, has been replaced by the catalytically inert valine in HisA-II, and no other residue is located close enough in the model to protonate the ring oxygen. It has been suggested that the carboxylate of the anthranilate moiety of PRA could take over this task (26). Indeed, there is plenty of space in the HisA-II binding pocket for the modeled anthranilate moiety, and it appears feasible that in one or more of its various plausible conformations the carboxylate group could protonate the furanose ring oxygen.
Implications for Protein Design and Evolution.
The most efficient catalysts HisAFcomIII and HisA-II exhibit substrate affinities and turnover numbers that come close to those of TrpF wild-type enzymes, although the proteins share a sequence identity of only ≈15%. Remarkably, as neither wild-type HisAF nor HisA show detectable TrpF activity (kcat < 0.005 min−1; Table 1), the amino acid exchanges present in HisAFcomIII (kcat = 4 min−1) and HisA-II (kcat = 67 min−1) must have increased the turnover number for PRA by at least 3–4 orders of magnitude. Relatively few residue substitutions (6 in the case of HisAFcomIII, 4 in the case of HisA-II) were sufficient to achieve this high level of activity, which demonstrates the power of combining random mutagenesis with selection in vivo (27), and underlines the catalytic versatility of the ubiquitous (βα)8-barrel fold (8). Admittedly, the newly evolved catalysts are taking advantage of already existing features of the design scaffold (e.g., a phosphate-binding pocket). However, this is how we imagine divergent evolution to occur in nature: to recruit parts of existing functionalities and, thus, get a head start in an evolutionary race.
The successful establishment of TrpF activity on the artificial scaffold HisAF has further implications for the model of (βα)8-barrel evolution from half barrels (9). The mixing and matching of half barrels and related scaffolds has allowed us to generate new (βα)8-barrel proteins, although without enzymatic activity (23, 24, 28). The properties of the HisAFsel and HisAFcom variants now show that newly combined half barrels originating from existing enzymes can achieve significant catalytic efficiency by the acquisition of only few mutations. These findings underscore that the combinatorial assembly of protein modules is highly relevant for the evolution of protein folds and functions (18, 19).
Materials and Methods
Random Mutagenesis and Selection in Vivo.
When expressed in E. coli, the HisAF protein is mainly produced in insoluble form and found in inclusion bodies (23). In contrast, HisAF fused to the MBP is soluble, which allowed us to select for mutants with TrpF activity by in vivo complementation. To this end, a plasmid-encoded gene library was generated, which consisted of randomised hisAF variants being fused to the 3′-end of the MBP-encoding gene malE. The sequencing of the hisAF inserts of 10 clones showed that the used PCR protocol resulted in the introduction of 2–7 mutations per gene. From the total number of 38 mutations, 17 (45%) were AT to GC exchanges and 21 (55%) were GC to AT exchanges; the transversion/transition ratio was 5/3. The pTNA-malE-(hisAF)randomized library, which contained ≈1.1 × 106 independent clones, was used to transform cells of an auxotrophic ΔtrpF E. coli strain. This strain lacks the trpF gene on its chromosome and is, therefore, unable to grow on medium without tryptophan (29). The transformants were incubated either on selective agar plates or in selective liquid medium (Fig. S2).
Protein Production.
Genes encoding HisAF and HisA variants with TrpF activity were subcloned into pET plasmids and expressed in E. coli T7-Express cells (New England Biolabs) containing the pRARE plasmid isolated from Rosetta(DE3)pLysS (Novagen). The recombinant proteins were purified from the soluble cell extract by metal chelate affinity chromatography by using a C-terminally added hexa-histidine tag, followed by Blue Sepharose 6 FF affinity chromatography in the case of the HisAF variants. To exclude that the high TrpF activity of HisA-II was caused by contamination with ecTrpF, the protein was also expressed in E. coli strain W3110 trpEA2, which lacks on its chromosome the entire trp operon (30), and purified by Ni2+ chelate affinity chromatography as described above. The kcat and KmPRA values of HisA-II expressed in E. coli T7-Express and W3110 trpEA2 were identical within experimental error.
Functional Characterization.
The TrpF activities of the purified HisAF and HisA variants were followed at 25 °C by a fluorimetric assay and analyzed as described (14, 25). The binding of the product analogue rCdRP to HisAFcomIII and HisA-II was followed by fluorescence energy transfer and analyzed as described (15, 31).
X-Ray Structure Determination and Ligand Docking.
The X-ray structure of HisA-II was solved by molecular replacement, using the published HisA structure (22) as the template. Data collection and refinement statistics are shown in Table S1. The TrpF substrate PRA and its product CdRP were docked into the active sites of the HisA-II and HisA (PDB ID 1qo2) structures by using the software Glide 4.5 (Schrödinger) (32).
Further details of the methods used are described in SI Methods.
Supplementary Material
Acknowledgments.
We thank Marcus Hartmann and Kornelius Zeth for help with the crystallographic data analysis, and Stephen J. Benkovic, Frank M. Raushel, Franz X. Schmid, Felix List, and Rainer Merkl for discussion and comments on the manuscript. This work was supported by the Deutsche Forschungsgemeinschaft Grant STE 891/4-3.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The atomic coordinates and structure factors have been deposited in the Protein Data Bank, www.rcsb.org (PDB ID code 2w79).
This article contains supporting information online at www.pnas.org/cgi/content/full/0810342106/DCSupplemental.
References
- 1.Walsh C. Enabling the chemistry of life. Nature. 2001;409:226–231. doi: 10.1038/35051697. [DOI] [PubMed] [Google Scholar]
- 2.Aharoni A, Griffiths AD, Tawfik DS. High-throughput screens and selections of enzyme-encoding genes. Curr Opin Chem Biol. 2005;9:210–216. doi: 10.1016/j.cbpa.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 3.Bloom JD, et al. Evolving strategies for enzyme engineering. Curr Opin Struct Biol. 2005;15:447–452. doi: 10.1016/j.sbi.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 4.Jäckel C, Kast P, Hilvert D. Protein design by directed evolution. Annu Rev Biophys. 2008;37:153–173. doi: 10.1146/annurev.biophys.37.032807.125832. [DOI] [PubMed] [Google Scholar]
- 5.Toscano MD, Woycechowsky KJ, Hilvert D. Minimalist active-site redesign: Teaching old enzymes new tricks. Angew Chem Int Ed Engl. 2007;46:3212–3236. doi: 10.1002/anie.200604205. [DOI] [PubMed] [Google Scholar]
- 6.Zanghellini A, et al. New algorithms and an in silico benchmark for computational enzyme design. Protein Sci. 2006;15:2785–2794. doi: 10.1110/ps.062353106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gerlt JA, Raushel FM. Evolution of function in (β/α)8-barrel enzymes. Curr Opin Chem Biol. 2003;7:252–264. doi: 10.1016/s1367-5931(03)00019-x. [DOI] [PubMed] [Google Scholar]
- 8.Wierenga RK. The TIM-barrel fold: A versatile framework for efficient enzymes. FEBS Lett. 2001;492:193–198. doi: 10.1016/s0014-5793(01)02236-0. [DOI] [PubMed] [Google Scholar]
- 9.Sterner R, Höcker B. Catalytic versatility, stability, and evolution of the (βα)8-barrel enzyme fold. Chem Rev. 2005;105:4038–4055. doi: 10.1021/cr030191z. [DOI] [PubMed] [Google Scholar]
- 10.Schmidt DM, et al. Evolutionary potential of (β/α)8-barrels: Functional promiscuity produced by single substitutions in the enolase superfamily. Biochemistry. 2003;42:8387–8393. doi: 10.1021/bi034769a. [DOI] [PubMed] [Google Scholar]
- 11.Jiang L, et al. De novo computational design of retro-aldol enzymes. Science. 2008;319:1387–1391. doi: 10.1126/science.1152692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Röthlisberger D, et al. Kemp elimination catalysts by computational enzyme design. Nature. 2008;453:190–195. doi: 10.1038/nature06879. [DOI] [PubMed] [Google Scholar]
- 13.Jürgens C, et al. Directed evolution of a (βα)8-barrel enzyme to catalyze related reactions in two different metabolic pathways. Proc Natl Acad Sci USA. 2000;97:9925–9930. doi: 10.1073/pnas.160255397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leopoldseder S, Claren J, Jürgens C, Sterner R. Interconverting the catalytic activities of (βα)8-barrel enzymes from different metabolic pathways: Sequence requirements and molecular analysis. J Mol Biol. 2004;337:871–879. doi: 10.1016/j.jmb.2004.01.062. [DOI] [PubMed] [Google Scholar]
- 15.Henn-Sax M, et al. Two (βα)8-barrel enzymes of histidine and tryptophan biosynthesis have similar reaction mechanisms and common strategies for protecting their labile substrates. Biochemistry. 2002;41:12032–12042. doi: 10.1021/bi026092h. [DOI] [PubMed] [Google Scholar]
- 16.Barona-Gomez F, Hodgson DA. Occurrence of a putative ancient-like isomerase involved in histidine and tryptophan biosynthesis. EMBO Rep. 2003;4:296–300. doi: 10.1038/sj.embor.embor771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuper J, Dönges C, Wilmanns M. Two-fold repeated (βα)4 half-barrels may provide a molecular tool for dual substrate specificity. EMBO Rep. 2005;6:134–139. doi: 10.1038/sj.embor.7400330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Riechmann L, Winter G. Early protein evolution: Building domains from ligand-binding polypeptide segments. J Mol Biol. 2006;363:460–468. doi: 10.1016/j.jmb.2006.08.031. [DOI] [PubMed] [Google Scholar]
- 19.Yadid I, Tawfik DS. Reconstruction of functional β-propeller lectins via homo-oligomeric assembly of shorter fragments. J Mol Biol. 2007;365:10–17. doi: 10.1016/j.jmb.2006.09.055. [DOI] [PubMed] [Google Scholar]
- 20.Gerlt JA, Babbitt PC. Barrels in pieces? Nat Struct Biol. 2001;8:5–7. doi: 10.1038/83048. [DOI] [PubMed] [Google Scholar]
- 21.Höcker B, Beismann-Driemeyer S, Hettwer S, Lustig A, Sterner R. Dissection of a (βα)8-barrel enzyme into two folded halves. Nat Struct Biol. 2001;8:32–36. doi: 10.1038/83021. [DOI] [PubMed] [Google Scholar]
- 22.Lang D, Thoma R, Henn-Sax M, Sterner R, Wilmanns M. Structural evidence for evolution of the β/α barrel scaffold by gene duplication and fusion. Science. 2000;289:1546–1550. doi: 10.1126/science.289.5484.1546. [DOI] [PubMed] [Google Scholar]
- 23.Höcker B, Claren J, Sterner R. Mimicking enzyme evolution by generating new (βα)8-barrels from (βα)4-half-barrels. Proc Natl Acad Sci USA. 2004;101:16448–16453. doi: 10.1073/pnas.0405832101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seitz T, Bocola M, Claren J, Sterner R. Stabilisation of a (βα)8-barrel protein designed from identical half barrels. J Mol Biol. 2007;372:114–129. doi: 10.1016/j.jmb.2007.06.036. [DOI] [PubMed] [Google Scholar]
- 25.Hommel U, Eberhard M, Kirschner K. Phosphoribosyl anthranilate isomerase catalyzes a reversible amadori reaction. Biochemistry. 1995;34:5429–5439. doi: 10.1021/bi00016a014. [DOI] [PubMed] [Google Scholar]
- 26.Wright H, et al. The structure/function relationship of a dual-substrate (βα)8-isomerase. Biochem Biophys Res Commun. 2008;365:16–21. doi: 10.1016/j.bbrc.2007.10.101. [DOI] [PubMed] [Google Scholar]
- 27.Park HS, et al. Design and evolution of new catalytic activity with an existing protein scaffold. Science. 2006;311:535–538. doi: 10.1126/science.1118953. [DOI] [PubMed] [Google Scholar]
- 28.Bharat TAM, Eisenbeis S, Zeth K, Höcker B. A βα-barrel built by the combination of fragments from different folds. Proc Natl Acad Sci USA. 2008;105:9942–9947. doi: 10.1073/pnas.0802202105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sterner R, et al. (βα)8-barrel proteins of tryptophan biosynthesis in the hyperthermophile Thermotoga maritima. EMBO J. 1995;14:4395–4402. doi: 10.1002/j.1460-2075.1995.tb00118.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schneider WP, Nichols BP, Yanofsky C. Procedure for production of hybrid genes and proteins and its use in assessing significance of amino acid differences in homologous tryptophan synthetase α polypeptides. Proc Natl Acad Sci USA. 1981;78:2169–2173. doi: 10.1073/pnas.78.4.2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bisswanger H, Kirschner K, Cohn W, Hager V, Hansson E. N-(5-Phosphoribosyl)anthranilate isomerase-indoleglycerol-phosphate synthase. 1. A substrate analogue binds to two different binding sites on the bifunctional enzyme from Escherichia coli. Biochemistry. 1979;18:5946–5953. doi: 10.1021/bi00593a029. [DOI] [PubMed] [Google Scholar]
- 32.Friesner RA, et al. Glide: A new approach for rapid, accurate docking and scoring. 1. Method and assessment of docking accuracy. J Med Chem. 2004;47:1739–1749. doi: 10.1021/jm0306430. [DOI] [PubMed] [Google Scholar]
- 33.Beismann-Driemeyer S, Sterner R. Imidazole glycerol phosphate synthase from Thermotoga maritima. Quaternary structure, steady-state kinetics, and reaction mechanism of the bienzyme complex. J Biol Chem. 2001;276:20387–20396. doi: 10.1074/jbc.M102012200. [DOI] [PubMed] [Google Scholar]
- 34.Hennig M, Darimont BD, Jansonius JN, Kirschner K. The catalytic mechanism of indole-3-glycerol phosphate synthase: Crystal structures of complexes of the enzyme from Sulfolobus solfataricus with substrate analogue, substrate, and product. J Mol Biol. 2002;319:757–766. doi: 10.1016/S0022-2836(02)00378-9. [DOI] [PubMed] [Google Scholar]
- 35.Sterner R, et al. Phosphoribosyl anthranilate isomerase from Thermotoga maritima is an extremely stable and active homodimer. Prot Sci. 1996;5:2000–2008. doi: 10.1002/pro.5560051006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.