Abstract
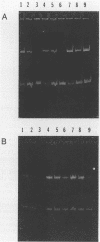
The emergence of epidemic multiple-drug-resistant (MDR) strains of Mycobacterium tuberculosis in conjunction with an increase in the number of reported cases of tuberculosis (TB) represents a major public health problem. In light of a recent outbreak of MDR M. tuberculosis at our center, we began the development of a polymerase chain reaction (PCR) assay for the rapid diagnosis of pulmonary TB using two sets of primers, one based on the IS6110 repeated sequence of M. tuberculosis and the other based on the protein antigen b (PAB). Reaction conditions were first optimized as to the appropriate extraction protocol and the concentrations of primer pairs, nucleotides, and MgCl2. Following a preliminary evaluation of the assay with clinical specimens, extraction and amplification procedures were further modified. PAB and IS6110 primers detected between 2 and 23 and 0.023 and 0.23 CFU of M. tuberculosis, respectively, in pooled, M. tuberculosis-negative sputa by our optimized PCR assay. After routine processing for mycobacteria, 734 specimens were subsequently amplified. DNA for amplification was obtained by boiling and beating the sediments with Tween 20. For each reaction, DNA (10 microliters) was added to an amplification mixture containing 12 pmol of IS6110 primers, 20 pmol of PAB primers, 2 mM MgCl2, 200 microM nucleotides, and 2.5 U of Taq polymerase and the mixture was then amplified for 40 cycles. The sensitivity and specificity of our PCR assay were 87.2 and 97.7%, respectively. We were unable to interpret the results for seven specimens (1%). In our experience, PCR proved to be a useful rapid diagnostic test for TB in a clinical setting and a valuable epidemiological tool for determining exposure groups in the hospital setting. Our findings also underscore the need for the systematic optimization of PCR assay conditions.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brisson-Noël A., Gicquel B., Lecossier D., Lévy-Frébault V., Nassif X., Hance A. J. Rapid diagnosis of tuberculosis by amplification of mycobacterial DNA in clinical samples. Lancet. 1989 Nov 4;2(8671):1069–1071. doi: 10.1016/s0140-6736(89)91082-9. [DOI] [PubMed] [Google Scholar]
- Buck G. E., O'Hara L. C., Summersgill J. T. Rapid, simple method for treating clinical specimens containing Mycobacterium tuberculosis to remove DNA for polymerase chain reaction. J Clin Microbiol. 1992 May;30(5):1331–1334. doi: 10.1128/jcm.30.5.1331-1334.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böddinghaus B., Rogall T., Flohr T., Blöcker H., Böttger E. C. Detection and identification of mycobacteria by amplification of rRNA. J Clin Microbiol. 1990 Aug;28(8):1751–1759. doi: 10.1128/jcm.28.8.1751-1759.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousins D. V., Wilton S. D., Francis B. R., Gow B. L. Use of polymerase chain reaction for rapid diagnosis of tuberculosis. J Clin Microbiol. 1992 Jan;30(1):255–258. doi: 10.1128/jcm.30.1.255-258.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Wit D., Steyn L., Shoemaker S., Sogin M. Direct detection of Mycobacterium tuberculosis in clinical specimens by DNA amplification. J Clin Microbiol. 1990 Nov;28(11):2437–2441. doi: 10.1128/jcm.28.11.2437-2441.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Portillo P., Murillo L. A., Patarroyo M. E. Amplification of a species-specific DNA fragment of Mycobacterium tuberculosis and its possible use in diagnosis. J Clin Microbiol. 1991 Oct;29(10):2163–2168. doi: 10.1128/jcm.29.10.2163-2168.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenach K. D., Cave M. D., Bates J. H., Crawford J. T. Polymerase chain reaction amplification of a repetitive DNA sequence specific for Mycobacterium tuberculosis. J Infect Dis. 1990 May;161(5):977–981. doi: 10.1093/infdis/161.5.977. [DOI] [PubMed] [Google Scholar]
- Eisenach K. D., Sifford M. D., Cave M. D., Bates J. H., Crawford J. T. Detection of Mycobacterium tuberculosis in sputum samples using a polymerase chain reaction. Am Rev Respir Dis. 1991 Nov;144(5):1160–1163. doi: 10.1164/ajrccm/144.5.1160. [DOI] [PubMed] [Google Scholar]
- Hermans P. W., Schuitema A. R., Van Soolingen D., Verstynen C. P., Bik E. M., Thole J. E., Kolk A. H., van Embden J. D. Specific detection of Mycobacterium tuberculosis complex strains by polymerase chain reaction. J Clin Microbiol. 1990 Jun;28(6):1204–1213. doi: 10.1128/jcm.28.6.1204-1213.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermans P. W., van Soolingen D., Dale J. W., Schuitema A. R., McAdam R. A., Catty D., van Embden J. D. Insertion element IS986 from Mycobacterium tuberculosis: a useful tool for diagnosis and epidemiology of tuberculosis. J Clin Microbiol. 1990 Sep;28(9):2051–2058. doi: 10.1128/jcm.28.9.2051-2058.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pao C. C., Yen T. S., You J. B., Maa J. S., Fiss E. H., Chang C. H. Detection and identification of Mycobacterium tuberculosis by DNA amplification. J Clin Microbiol. 1990 Sep;28(9):1877–1880. doi: 10.1128/jcm.28.9.1877-1880.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierre C., Lecossier D., Boussougant Y., Bocart D., Joly V., Yeni P., Hance A. J. Use of a reamplification protocol improves sensitivity of detection of Mycobacterium tuberculosis in clinical samples by amplification of DNA. J Clin Microbiol. 1991 Apr;29(4):712–717. doi: 10.1128/jcm.29.4.712-717.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sjöbring U., Mecklenburg M., Andersen A. B., Miörner H. Polymerase chain reaction for detection of Mycobacterium tuberculosis. J Clin Microbiol. 1990 Oct;28(10):2200–2204. doi: 10.1128/jcm.28.10.2200-2204.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soini H., Skurnik M., Liippo K., Tala E., Viljanen M. K. Detection and identification of mycobacteria by amplification of a segment of the gene coding for the 32-kilodalton protein. J Clin Microbiol. 1992 Aug;30(8):2025–2028. doi: 10.1128/jcm.30.8.2025-2028.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victor T., du Toit R., van Helden P. D. Purification of sputum samples through sucrose improves detection of Mycobacterium tuberculosis by polymerase chain reaction. J Clin Microbiol. 1992 Jun;30(6):1514–1517. doi: 10.1128/jcm.30.6.1514-1517.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]