Abstract
DNA damage response (DDR) acts as a tumorigenesis barrier, and any defects in the DDR machinery may lead to cancer. SOX4 expression is elevated in many types of tumors; however, its role in DDR is still largely unknown. Here, we show that SOX4, a new DNA damage sensor, is required for the activation of p53 tumor suppressor in response to DNA damage. Notably, SOX4 interacts with and stabilizes p53 protein by blocking Mdm2-mediated p53 ubiquitination and degradation. Furthermore, SOX4 enhances p53 acetylation by interacting with p300/CBP and facilitating p300/CBP/p53 complex formation. In concert with these results, SOX4 promotes cell cycle arrest and apoptosis, and it inhibits tumorigenesis in a p53-dependent manner. Therefore, these findings highlight SOX4 as a potential key factor in regulating DDR-associated cancer.
Keywords: Mdm2, ubiquitination, tumorigenesis
DNA damage response (DDR), a highly conserved response to genotoxic stresses, is the guardian of genomic integrity (1, 2). It has been shown that DDR serves as a barrier to constrain tumor progression in its early stages by inducing cell cycle arrest, DNA repair, or apoptosis (3). A number of components are involved in cellular DDR machinery, in which ATM-Chk2-p53 and ATR-Chk1-p53 cascade are the key signaling pathways involved (2). A central component of DDR, p53, is one of the most important tumor suppressor proteins (4–8). The major consequence of p53 activation upon DNA damage is the induction of specific target genes, such as p21WAF, Bax, and Puma, to initiate cell cycle arrest, apoptosis, and DNA repair (4). Cells lacking functional p53 exhibit a partial deficiency in DNA damage repair, resulting in uncontrolled cell proliferation and malignancy. Indeed, p53 gene is either lost or mutated in more than half of all human cancers (9). Around p53 there is a highly regulated network consisting of numerous proteins that interact with p53 and regulate its activity by protein stabilization, posttranscriptional modifications, protein–protein interaction, and protein subcellular localization (10), among which stabilization of p53 is presumed to play a major role in its activation. Under normal conditions, amount and activity of p53 are maintained at low levels by Mdm2, a ubiquitin E3 ligase, which binds to the N terminus of p53 and targets its C-terminal lysine residues for ubiquitination and degradation (11, 12). However, in response to DNA damage, p53 protein is rapidly stabilized and activated mostly through multiple posttranslational modifications, such as phosphorylation and acetylation of specific residues in the N-terminal and C-terminal domains. DNA damage-induced p53 phosphorylation, which is mediated by ATM kinase (13, 14), contributes to p53 stability (15). Acetylation of p53 C-terminal lysine residues in p53 stabilizes the protein by preventing Mdm2-mediated ubiquitination of the same residues (16, 17). In addition, the activity of p53 is also modulated by its recruitment of transcriptional coactivators or corepressors.
SOX4 is a member of the SOX (SRY-related HMG-box) transcription factor family, which is characterized as a highly conserved HMG-box, DNA-binding domain (18). It has been shown that SOX4 plays important roles in many developmental processes, such as embryonic cardiac development, thymocyte development, and nervous system development (19–21). In addition, SOX4 also is involved in many cellular processes. For example, SOX4 expression is induced by progestin, leading to increased SOX-mediated transcriptional activity in breast cancer cells (22), and enhances β-catenin/TCF activity (23). Recently, increasing evidence has shown that SOX4 is highly up-regulated in a number of tumors, including breast cancer (22), lung cancer (24), colon cancer (25), meduloblastoma (26), salivary gland cancer (27), and hepatocellular carcinoma (28). Furthermore, higher SOX4 expression correlates with better survival in bladder tumor patients (29), and it promotes prostaglandina-induced apoptosis in hepatocellular carcinoma (30), suggesting that SOX4 has a potential tumor-suppressive function. However, the precise mechanism by which SOX4 is involved in tumorigenesis remains largely unknown.
Here, we find that SOX4 is induced in response to DNA damage. Notably, the induction of SOX4 upon DNA damage contributes to p53-related functions, such as cell cycle arrest, apoptosis, and tumorigenesis. We further show that SOX4 physically interacts with p53 and enhances its transcriptional activity by stabilizing p53 protein via enhancing p53 acetylation and thus inhibiting Mdm2-mediated p53 ubiquitination. Therefore, SOX4 is a novel mediator for p53 activation in response to DNA damage.
Results
SOX4 Is Required for the Activation of p53 in Response to DNA Damage.
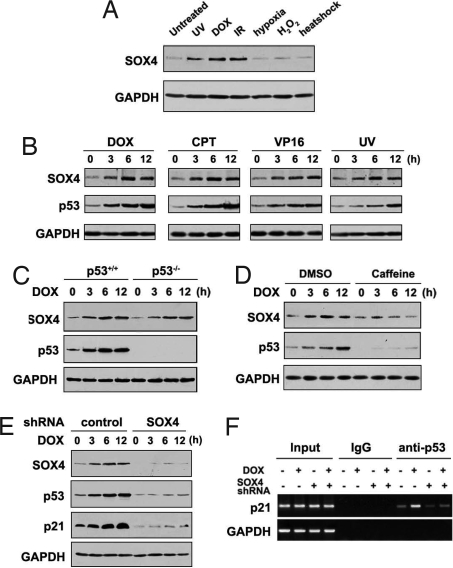
To investigate the involvement of SOX4 in genotoxic stresses, we treated human lung non-small cell carcinoma H460 cells (harboring wild-type p53) with different agents. Induction of SOX4 was observed upon treatments by DNA damage agents doxorubicin (DOX), UV irradiation, and ionizing radiation (IR), but not hypoxia, H2O2, and heat shock (Fig. 1A). Then, the kinetics of SOX4 were examined following the treatment of 4 different DNA damage agents. Interestingly, DNA damage-induced increase of SOX4 protein parallelled with the induction of p53 protein (Fig. 1B). The induction of SOX4 upon DNA damage occurred on a posttranscriptional level, because SOX4 protein levels but not mRNA levels were increased following DOX treatment [supporting information (SI) Fig. S1]. To test whether SOX4 induction relies on p53 activation, we examined SOX4 levels upon DOX treatment in colon cancer HCT116 p53+/+ and HCT116 p53−/− cells. As shown in Fig. 1C, a similar increase in SOX4 protein was observed in both cells. SOX4 induction after DNA damage was inhibited by caffeine (Fig. 1D), a specific inhibitor of ATM/ATR, which are the key kinases involved in DDRs. These results indicate that SOX4 induction upon DNA damage is p53-independent and ATM/ATR-dependent.
Fig. 1.
SOX4 is required for p53 activation in response to DNA damage. (A) H460 cells were treated with UV irradiation (50 J/m2), DOX (1 μg/mL, 6 h), IR (5 Gy), hypoxia (1 h), H2O2 (0.4 mM, 24 h), or heat shock (43 °C, 1 h). Cells were harvested, and then SOX4 and GAPDH protein levels were determined by immunoblotting. (B) H460 cells were treated with 1 μg/mL DOX, 2 μM camptothecin (CPT), 30 μM etoposide (VP16), and 50 J/m2 UV irradiation, and they were harvested at the indicated times. Cell lysates then were analyzed by immunoblotting for SOX4 and p53. (C) Lysates from HCT116 p53+/+ and HCT116 p53−/− cells treated with DOX for the indicated times were analyzed by immunoblotting. (D) H460 cells pretreated with 10 mM caffeine for 2 h were treated with DOX for the indicated times, and the cell lysates were analyzed by immunoblotting. (E) HCT116 p53+/+ control or SOX4 shRNA-stable cells were treated with DOX for the indicated times, and cell lysates were analyzed by immunoblotting. (F) ChIP assay was performed using HCT116 p53+/+ control or SOX4 shRNA-stable cells treated with or without DOX for 6 h. Primers specific for p21 or GAPDH promoter were used to amplify the DNAs associated with p53 in vivo.
To test whether SOX4 contributes to the induction of p53 in response to DNA damage, we knocked down SOX4 in HCT116 cells (wild-type p53) by shRNA, and we treated the cells with DOX. DOX-induced increases of p53 and its target p21 proteins were dramatically decreased in the SOX4 shRNA cells compared with control cells (Fig. 1E). To avoid off-target effects or cloning effects of shRNA, we performed the same experiments by using an additional clone of SOX4 shRNA-stable cell line or specific SOX4 siRNA, and similar results were obtained (Fig. S2 A and B). Similar results also were obtained when these cells were treated with other DNA damage agents (Fig. S2C). Consistently, SOX4 knockdown in HCT116 cells led to the decrease of p21 and Bax promoter activity following DOX treatment (Fig. S2D). The effect of SOX4 knockdown on p53 activity was further confirmed by chromatin immunoprecipitation assay (Fig. 1F). Taken together, these data demonstrated that SOX4 is required for p53 activation in response to DNA damage.
SOX4 Regulates p53 Protein Stability and Activity.
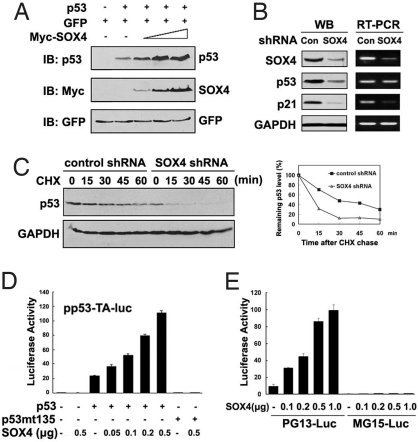
To extend the above observations, we tested whether the forced expression of SOX4 leads to the accumulation of p53 protein. As shown in Fig. 2A, the protein amount of p53 was dramatically elevated when SOX4 was cotransfected in HCT116 p53−/− cells. SOX4 knockdown resulted in a decrease in p53 protein level, whereas the mRNA level of p53 was not affected (Fig. 2B and Fig. S3). Therefore, the regulation of p53 by SOX4 is unlikely at the transcriptional level. Instead, SOX4 regulates p53 posttranslationally, because the half-life of p53 protein was notably shortened when the SOX4 expression was knocked down (Fig. 2C). Overexpression of SOX4 did not further potentiate the accumulation of p53 protein in the presence of specific proteasome inhibitor MG132 (Fig. S4), suggesting that SOX4 is involved in the regulation of p53 stability.
Fig. 2.
SOX4 regulates p53 protein stability and activity. (A) HCT116 p53−/− cells were transfected with equal amounts of 0.2 μg of p53, 0.1 μg of GFP plasmids, and increasing amounts of Myc-SOX4 plasmids (0.5, 1, and 2 μg). Cell lysates were subjected to immunoblotting. Levels of GFP are shown as equal transfection efficiencies. (B) Lysates from HCT116 p53+/+ control or SOX4 shRNA cells were subjected to immunoblotting (Left), and total RNA from the same cells was extracted and analyzed by RT-PCR (Right). (C) Lysates from HCT116 p53+/+ control or SOX4 shRNA cells treated with 20 μM cycloheximide (CHX) for the indicated times were subjected to immunoblotting (Left). Relative p53 levels were quantified by densitometry (Right). (D) H1299 (p53 null) cells were cotransfected with 0.5 μg of p53-responsive reporter pp53-TA-Luc, 50 ng of p53 or p53 transcriptional-inactive mutant p53mt135 expression vectors, and increasing amounts of SOX4 expression vectors. Luciferase activity was measured at 24 h after the transfection. (E) H460 cells were cotransfected with PG13-Luc or MG15-Luc reporters and increasing amounts of SOX4 expression plasmids as indicated, and luciferase activity was measured as in D. (D and E) Results are means ± SEM of 3 independent experiments.
Consistent with the above observation, SOX4 expression increased p53-mediated transcription measured with a p53 response reporter, but it had no effect on the transcriptional activity of the p53mt135 protein in H1299 cells (Fig. 2D). Similar results were obtained with natural p53-responsive luciferase reporters (Fig. S5A). SOX4 overexpression also strongly increased endogenous p53 activity from the PG13-Luc vector, but it had no effect on the expression from MG15-Luc, which cannot be regulated by p53 (Fig. 2E). In contrast, SOX4 knockdown led to reduced PG13-Luc activity in HCT116 p53+/+ cells (Fig. S5B). Unlike SOX4, SOX2, a member of SOX family, failed to increase the activity of p53 (Fig. S5C). Taken together, these results indicate that SOX4 regulates p53 protein stability and increases its transcriptional activity.
SOX4 Interacts with p53.
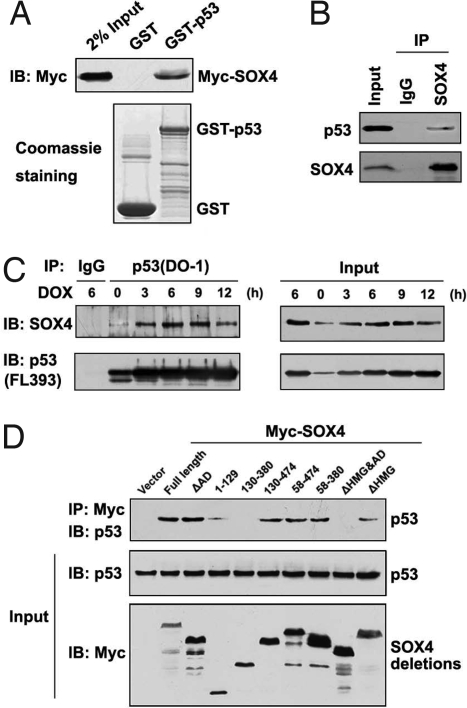
We next investigated the potential mechanism by which SOX4 activates p53. Immunofluorescence staining and confocal laser scanning microscopy showed that endogenous p53 and SOX4 proteins colocalize in the nucleus of H460 cells (Fig. S6A). This raised the possibility that SOX4 and p53 interact directly or are in the same complex. To explore these possibilities, we performed GST pull-down assay with recombinant GST-fusion proteins. Specific interactions of GST-p53 with transfected Myc-tagged SOX4 were observed (Fig. 3A), indicating that SOX4 physically interacts with p53. This interaction was further confirmed by coimmunoprecipitation experiments with either anti-Myc or anti-HA antibody when Myc-SOX4 and HA-p53 were coexpressed in 293 cells (Fig. S6B). Importantly, we verified the association of SOX4 and p53 endogenously expressed in HCT116 p53+/+ cells by immunoprecipitation with anti-SOX4 antibody (Fig. 3B). Notably, when SOX4 and p53 are induced in H460 cells by DOX treatment, the interaction between SOX4 and p53 was detected more robustly (Fig. 3C). Thus, SOX4 interacts with p53 in vitro and in vivo, and the interaction is augmented in response to DNA damage.
Fig. 3.
SOX4 interacts with p53. (A) GST pull-down assays were performed with the indicated GST-fused proteins and the cell lysates from HEK293 cells transfected with Myc-SOX4 plasmid. IB indicates immunoblotting. (B) Cell lysates were immunoprecipitated (IP) with anti-SOX4 antibody, and normal IgG was used as a negative control. (C) Cell lysates from H460 cells treated with DOX for the indicated times were immunoprecipitated with IgG or anti-p53 (DO-1) antibody, and immunoprecipitants (Left) or the whole-cell lysates (Right) were analyzed by immunoblotting. (D) HEK293 cells were cotransfected with p53 expression plasmids and Myc-SOX4 or its truncations vectors. Cell lysates were immunoprecipitated by using Myc antibody, and the bound proteins were analyzed by immunoblotting with anti-p53 polyclonal antibody.
The Interaction Between SOX4 and p53 Is Essential for the Regulatory Effect of SOX4 on p53 Stability.
Next, we tested whether the interaction between SOX4 and p53 is essential for the SOX4 regulatory effect on p53 stability. GST pull-down assay revealed that DNA-binding domain (DBD; 102–292 amino acids) and regulatory domain (RD; 359–393 amino acids) of p53 were responsible for its interaction with SOX4 (Fig. S6C). To identify which portion of SOX4 protein is required for its interaction with p53, we constructed 8 different SOX4 mutant expression vectors. Among these 8 mutants of SOX4, only 2 of them, the mutant 130-380 and ΔHMGΔAD (ΔHΔA), could not immunoprecipitate p53 protein (Fig. 3D). Interestingly, neither of these 2 mutants has the HMG box or the C-terminal transactivation domain (AD) of SOX4 protein, and other mutants having 1 of the 2 domains are still capable of interacting with p53 (see schematic representation in Fig. S6D). The same conclusions were obtained with GST pull-down experiments (Fig. S6D).
To further define the interaction between SOX4 and p53, we examined the binding preference of each of these domains by performing GST pull-down assays with GST-p53 fusion proteins. As shown in Fig. S6E, the SOX4ΔAD mutant lost its ability to interact with the DBD of p53, but it still bound to the RD of p53. In contrast, the SOX4ΔHMG mutant interacted with the DBD of p53, but it failed to bind to the RD of p53. These data indicate that the SOX4 AD domain is required for the interaction of SOX4 with the DBD of p53, and SOX4 HMG-box domain is necessary for its interaction with the RD of p53.
To test whether the interaction between SOX4 and p53 is essential for the SOX4 regulatory effect on p53, we transfected the full-length SOX4 and the SOX4ΔHΔA mutant and found that full-length SOX4 but not the truncation, which fails to bind p53, could prolong the half-life of p53 and increase p53 level and transcriptional activity (Fig. S7), indicating the interaction between SOX4 and p53 is essential for SOX4 to stabilize p53.
SOX4 Blocks Mdm2-Mediated p53 Ubiquitination.
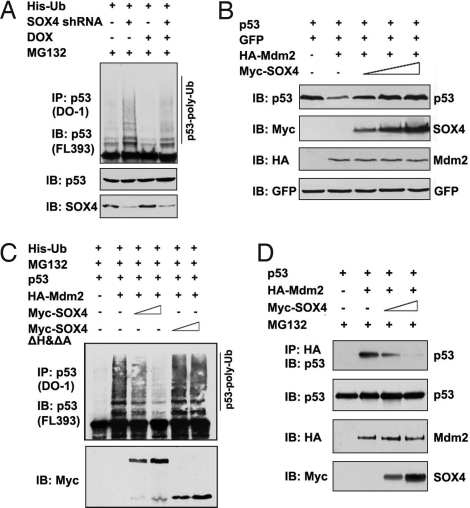
Because p53 is degraded through the ubiquitin-proteasome pathway, we investigated whether SOX4 regulates the ubiquitination of p53. SOX4 overexpression and DOX treatment resulted in a decrease of p53 ubiquitination (Fig. S8). Consistently, when SOX4 expression was knocked down, the amount of ubiquitinated p53 was dramatically increased, and SOX4 knockdown also effectively eliminated the inhibitory effect of DOX on p53 ubiquitination (Fig. 4A), indicating that SOX4 inhibits p53 ubiquitination. As it is well-known that the ubiquitin ligase Mdm2 is the major player in regulating the ubiquitination and degradation of p53 (31, 32), we then investigated whether SOX4 stabilizes p53 through blocking Mdm2-mediated p53 degradation. As expected, p53 protein level was reduced when Mdm2 was cotransfected in HCT116 p53−/− cells. However, overexpression of full-length SOX4 but not SOX4ΔHΔA mutant significantly eliminated the destabilization effect of Mdm2 on p53 protein (Fig. 4B and Fig. S9A), implying that the interaction between SOX4 and p53 is critical for the antagonistic effect of SOX4 on Mdm2-mediated p53 degradation. Consistently, the Mdm2-mediated increase of p53 ubiquitination was inhibited by overexpression of SOX4 but not the SOX4ΔHΔA mutant (Fig. 4C). Therefore, these data demonstrated that SOX4 blocks Mdm2-mediated p53 ubiquitination and degradation.
Fig. 4.
SOX4 blocks Mdm2-mediated p53 ubiquitination. (A) HCT116 p53+/+ control or SOX4 shRNA cells were transfected with 4 μg of ubiquitin-expressing plasmids. At 36 h after the transfection, cells were treated with or without DOX for 6 h, followed by the treatment of 20 μM MG132 for 6 h. Cell lysates were immunoprecipitated (IP) with anti-p53 (DO-1) antibody and analyzed by immunoblotting (IB) with anti-p53 (FL-393) antibody. (B) HCT116 p53−/− cells were transfected with p53, GFP, HA-Mdm2, and Myc-SOX4-expressing vectors as indicated. Cell lysates were analyzed by immunoblotting. (C) HCT116 p53−/− cells were cotransfected with p53, HA-Mdm2, Myc-SOX4, or Myc-SOX4ΔHΔA together with ubiquitin expression vectors in different combinations as indicated. Cells were treated with 20 μM MG132 for 6 h before harvest. (D) HCT116 p53−/− cells were cotransfected with p53, HA-Mdm2, and Myc-SOX4 plasmids as indicated. Cells lysates were immunoprecipitated with anti-HA antibody and analyzed by immunoblotting using anti-p53 (FL-393) antibody.
The interaction between p53 and Mdm2 is pivotal for Mdm2 to control p53 stability. We then investigated whether SOX4 stabilizes p53 by disrupting the p53–Mdm2 interaction. Indeed, when SOX4 was overexpressed, the coprecipitation of p53 with Mdm2 was dramatically decreased (Fig. 4D), suggesting that SOX4 disrupts the interaction between p53 and Mdm2. However, we failed to detect an interaction between SOX4 and Mdm2 (Fig. S10). Therefore, SOX4 interferes with the binding of Mdm2 to p53 through its interaction with p53.
Because the nuclear exportation of p53 is dependent on its Mdm2-mediated ubiquitination (33), we then tested by immunofluorescence microscopy whether SOX4 blocks the Mdm2-mediated p53 nuclear export. As previously reported, p53 protein was entirely located in the nucleus when expressed alone, but it translocated into the cytoplasm when coexpressed with Mdm2 (34). As expected, p53 redistributed to the nucleus when SOX4 was coexpressed with Mdm2 and p53; however, the SOX4ΔHΔA mutant failed to do so (Fig. S9B). Therefore, SOX4 inhibits Mdm2-mediated p53 nuclear export.
SOX4 Enhances p53 Acetylation.
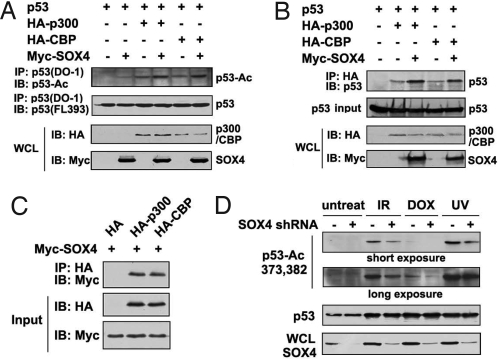
To gain insight into the mechanism by which SOX4 blocks p53–Mdm2 interaction, we studied the effect of SOX4 on p53 posttranscriptional modifications. It is believed that the acetylation of p53, which is mainly mediated by acetyltransferase p300 and CBP, plays a vital role in p53 stabilization; particularly, that the acetylation of p53 destabilizes the interaction between Mdm2 and p53 (17). We then tested whether SOX4 achieved its regulatory effect on p53 stability by enhancing the acetylation of p53. We first found that p300/CBP-mediated acetylation of p53 at Lys-373 and Lys-382 was increased in the presence of SOX4 (Fig. 5A). Further, we found that expression of SOX4 increased the interaction of p53 with p300 or CBP, indicating that SOX4 may strengthen the formation of the p53–p300 or p53–CBP complexes (Fig. 5B). Interestingly, SOX4 was found to interact with either p300 or CBP (Fig. 5C). In addition, SOX4 silencing led to reduced levels of p53 acetylation following IR, DOX, or UV treatment (Fig. 5D). These data suggest that SOX4 may act as a cofactor of p53 by recruiting acetyltransferases and mediating its acetylation. Therefore, SOX4 stabilizes p53, at least partially, by enhancing p53 acetylation.
Fig. 5.
SOX4 enhances p53 acetylation. (A) HCT116 p53−/− cells were transfected with p53, Myc-SOX4, and HA-p300 or HA-CBP plasmids as indicated. Cell lysates adjusted to equal p53 amount were immunoprecipitated (IP) with anti-p53 (DO-1) antibody and immunoblotted (IB) with anti-p53-Ac373, 382, and anti-p53 (FL393) antibodies. Whole-cell lysates also were analyzed by immunoblotting. (B) HCT116 p53−/− cells were transfected with 1 μg of p53, 2 μg of Myc-SOX4, and 1 μg of HA-p300 or HA-CBP expression vector as indicated. Cell lysates adjusted to equal p53 amount were immunoprecipitated with anti-HA antibody and immunoblotted with anti-p53 (FL-393) antibody. Whole-cell lysates also were analyzed by immunoblotting. (C) Cell lysates from HCT116 cells transfected with HA-p300 or HA-CBP and Myc-SOX4 expression plasmids were immunoprecipitated with anti-HA antibody and immunoblotted with anti-Myc antibody. (D) HCT116 p53+/+ SOX4 control or shRNA cells were treated with IR, DOX, or UV. Cells were harvested, and the normalized lysates for equal p53 amounts were analyzed by immunoblotting for p53 acetylation at lysines 373 and 382. SOX4 protein levels of the whole-cell lysates also are shown.
SOX4 Regulates Cell Cycle Arrest, Apoptosis, and Tumor Growth in a p53-Dependent Manner.
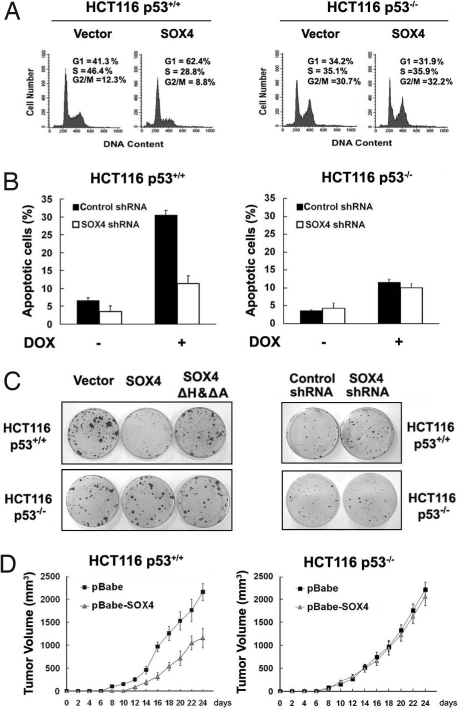
We next examined whether the effect of SOX4 on p53 activation has any consequence for p53-dependent biological functions. Overexpression of SOX4 in HCT116 p53+/+ cells resulted in an increased proportion of cells in G1 phase (Fig. 6A Left), whereas no change in cell cycle was observed when SOX4 was overexpressed in p53−/− cells (Fig. 6A Right), suggesting that SOX4 promotes p53-mediated cell cycle arrest in G1 phase. p53 plays important roles in apoptosis, and many proteins regulate apoptosis via p53 (35, 36), so the effect of SOX4 on apoptosis was examined. Knockdown of SOX4 led to a decrease of DOX-induced apoptosis in HCT116 p53+/+ but not p53−/− cells (Fig. 6B), indicating that SOX4 promotes p53-mediated apoptosis.
Fig. 6.
SOX4 regulates cell cycle arrest, apoptosis, and tumorigenesis in a p53-dependent manner. (A) HCT116 p53+/+ or HCT116 p53−/− cells were cotransfected with GFP and Myc-SOX4 or its empty vector. At 48 h after transfection, cells were fixed, stained with propidium iodide (PI), and analyzed for DNA content by flow cytometry. (B) HCT116 p53+/+ or HCT116 p53−/− stably shRNA-expressing cells were treated with DOX for 24 h and then analyzed by flow cytometry for apoptosis using Annexin-V/PI. Results are the mean ± SEM of 3 independent experiments. (C) (Left) The indicated cells were transfected with pBabe-SOX4 or its deletion mutant expression vectors. Puromycin-resistant colonies were stained 14 days later. (Right) The same cells as in Left were transfected with the indicated vectors. Puromycin-resistant colonies were stained 2 weeks later. (D) A total of 5 × 106 HCT116 p53+/+/pBabe or pBabe-SOX4 cells (Left) or HCT116 p53−/−/pBabe or pBabe-SOX4 cells (Right) were injected into the flank region of female nude mice. Tumor volume was measured at the indicated days.
The tumor suppressor p53 plays significant roles in the regulation of tumor progression (5, 37). To explore the effect of SOX4 on the tumor-suppressive function of p53, we performed colony-formation assays in HCT116 p53+/+ and HCT116 p53−/− cells transfected with expression vectors of SOX4 or SOX4ΔHΔA mutant. Overexpression of SOX4 in HCT116 p53+/+ cells inhibited cell proliferation and resulted in sparse colonies, whereas overexpression of the SOX4ΔHΔA mutant had no effect on the formation of colonies compared with the control cells (Fig. 6C Left). Conversely, knockdown of SOX4 protein in HCT116 p53+/+ cells resulted in an increase in colony formation, whereas SOX4 silencing in HCT116 p53−/− cells had no effect on colony formation (Fig. 6C Right). Similarly, soft agar assays showed that expression of SOX4 decreased the colony numbers in HCT116 p53+/+ but not p53−/− cells on soft agar (Fig. S11). Next, a tumorigenicity assay was performed with immunodeficient strains of mice. As shown in Fig. 6D, SOX4 expression in HCT116 p53+/+ but not p53−/− cells significantly suppressed tumor progression in nude mice. Taken together, these findings demonstrate that SOX4 promotes cell cycle arrest and apoptosis and inhibits tumor growth in a p53-dependent manner.
Discussion
We have identified SOX4 as a new DNA damage-induced protein that is crucial for p53 activation in response to genotoxic stress. Under normal conditions, p53 is tightly controlled by Mdm2, which acts as an E3 ligase to target p53 for ubiquitination and degradation (11, 12). In response to DNA damage, p53 is rapidly accumulated, in part through the activation of ATM/ATR kinases, which directly or indirectly trigger the posttranslational modification cascade of p53, such as phosphorylation and acetylation (10). The stabilization of p53 upon DNA damage is largely dependent on these modifications. Several proteins have been identified to regulate p53 activity by affecting its stabilization, whereas each has a somewhat different detailed mechanism of action (31). In this study, we report that the increased p53 stabilization is achieved via abrogated p53–Mdm2 interaction by SOX4. This disruption is unlikely due to their competitive binding to p53, because SOX4 and Mdm2 bind separate regions of p53. It seems that SOX4 does not act as a cofactor of Mdm2, like p14ARF (38), Rb (39), and YY1 (40); nor does SOX4 bind to Mdm2. Instead, we observed that SOX4 interacts with p300/CBP transacetylases and facilitates the formation of stable p53/p300 or p53/CBP complexes and consequently promotes p53 acetylation in residues Lys-373 and Lys-382. It is believed that the acetylation of p53 leads to less ubiquitination, because the 2 modifications compete for the same lysine residues and increased p53 activity by enhancing the DNA-binding ability of p53 (41). Moreover, it is recently reported that acetylation of p53 destabilizes the p53–Mdm2 complex formation (17). Thus, the augmentation of acetylation by SOX4 may be accountable for SOX4-mediated p53 stabilization and activation.
We demonstrate that SOX4 is induced in response to DNA damage in a p53-independent manner. The significance of the SOX4 induction in this event lies in the fact that loss of SOX4 impairs p53 activation and consequent tumor-suppressive functions, such as cell cycle arrest and apoptosis. Based on the observation that SOX4 interacts with p53 in physiological conditions, it is possible that SOX4 is required for maintaining a basal level of p53 expression. However, when DNA damage occurs, SOX4 protein is rapidly induced to form a more stable complex with p53 to promote its stabilization. It is noteworthy that the induction of SOX4 upon DNA damage is dependent on ATM kinase, which is a key kinase involved in DDR. The ATM/ATR-regulated DDR machinery serves as an anticancer barrier in early tumorigenesis by inducing cell cycle arrest or cell death (2). It is believed that DDR is constitutively activated in many human cancer cell lines (1). Thus, the aberrant overexpression of SOX4 in many tumors, presumably induced by the constitutively activated DDR, may provide a stress signal in triggering p53 activation, which provides a safeguard against oncogenic proliferation. Mutations in DDR components are strongly associated with cancers (42). Indeed, SOX4 gene mutations have been reported in human lung cancers (43), and we found that these mutations of SOX4 in human lung cancer abolished the normal function of SOX4 in regulating p53 activity and, more importantly, some of these SOX4 mutants may even function as dominant-negative mutants by blocking p53 activity instead. These findings thus raise the possibility that SOX4 acts as a component of DDR in sustaining genomic integrity by enhancing p53 function.
The significance of tumor-suppressive function of SOX4 in tumorigenesis has been emphasized by clinical research revealing that higher SOX4 expression correlates with better survival in bladder tumor and medulloblastoma patients (29, 44). Nevertheless, SOX4 was also reported to be an oncogene for human prostate cancer cells (45), and SOX4 knockdown promotes apoptosis in adenoid cystic carcinoma cells (46). Thus, whether SOX4 is a tumor suppressor or an oncogene is presumably context-dependent, which is worth further investigation.
In conclusion, we established SOX4 as an important p53 regulator that is up-regulated by DNA damage and exerts its inhibitory role on cell cycle progression and tumorigenesis through p53. Furthermore, SOX4 interacts with p53 to enhance its transcriptional activity by inhibiting Mdm2-mediated p53 ubiquitination and degradation. Taken together, our findings shed light on the molecular mechanistic insight into the role of SOX4 in tumorigenesis, and drugs targeting SOX4 may be a potential anticancer therapeutic approach for future studies.
Materials and Methods
Plasmid Constructions.
Myc-SOX4 full-length and truncated mutant expression plasmids were constructed as described previously (47). HA-p53 and HA-Mdm2 plasmids were generated by PCR amplification and cloned into pXJ40-HA vector. pBabe-SOX4 and pBabe-SOX4ΔHMGΔAD plasmids were generated by PCR and cloned into pBabe-retro-puro vectors. GST-p53 mutant constructs were kindly provided by R. Schuele (Frauenklinik der Albert-Ludwigs-University, Freiburg, Germany). PG13-Luc and MG13-Luc were kindly provided by B. Vogelstein (Johns Hopkins University, Baltimore). p21-Luc and Bax-Luc reporters were from A. Fusco (Università degli Studi di Napoli, Naples, Italy). SOX2 expression plasmid was from C. Basilico (New York University School of Medicine, New York). HA-p300 and HA-CBP plasmids were provided by D. Spengler (Max Planck Institute of Psychiatry, Germany). pBabe-retro-puro retrovirus vector was provided by Y.-S. Cong (Beijing Normal University, Beijing, China). pCMV-p53, pCMV-p53mt135, and pp53-TA-Luc reporters were purchased from Clontech.
Cell Culture, Transfection, and Luciferase Assays.
HEK293 cells were cultured in DMEM containing 10% newborn calf serum at 37 °C in a humidified atmosphere of 5% CO2. HCT116 cells were maintained in McCoy 5A containing 10% FBS. H1299 and H460 cells were cultured in RPMI 1640 containing 10% FBS. Cells were transfected by using Lipofectamine 2000 (Invitrogen) following the manufacturer's instructions. Luciferase assays were performed as described previously (48, 49).
Immunoprecipitation, Immunoblotting, and Antibodies.
Immunoprecipitation and immunoblotting were performed as described previously (48). To detect p53 acetylation, cells were lysed in lysis buffer [20 mM Tris·HCl (pH 8.0), 150 mM NaCl, 0.5% Nonidet P-40, 5 mM MgCl2, 2 mM EDTA, 1 mM Na3VO4, 5 mM trichostatin A, and protease inhibitor mixture]. The p53 monoclonal antibody (DO-1), p53 polyclonal antibody (FL-393), HA, Myc epitope, and p21 antibodies were purchased from Santa Cruz Biotechnology. Anti-acetyl-p53 (Lys-373, Lys-382) antibody was purchased from Upstate Biotechnology. SOX4 monoclonal antibody, GAPDH, and GFP polyclonal antibodies were prepared in our lab.
GST Pull-Down Assay.
GST and GST fusion proteins were expressed and purified according to the manufacturer's instructions (Amersham Pharmacia). Myc-SOX4 proteins, obtained from the whole-cell lysates of HEK293 cells, which were transfected with pXJ40-Myc-SOX4 and/or its mutant plasmids, were incubated with GST and GST–p53 fusion protein bound to Sepharose beards in 1 mL of binding buffer [20 mM Tris·HCl, (pH 8.0), 150 mM NaCl, 1 mM EDTA, 10% glycerol, and 0.1% Nonidet P-40] at 4 °C for 4 h. Beads then were washed and eluted in 20 μL of 2× SDS/PAGE sample buffer and detected by immunoblotting.
Protocols for ChIP analysis, flow cytometry, fluorescence microscopy, RNA interference, and stable cell line construction can be found in SI Materials and Methods.
Supplementary Material
Acknowledgments.
We thank Drs. Bert Vogelstein, Roland Schule, Dietmar Spengler, Alfredo Fusco, Claudio Basilico, and Yu-Sheng Cong for generously providing materials. This work was supported by grants from the National Natural Science Foundation of China (30525021, 30672357, 30770471, 30500583, 30600246, and 30621063), the National High Technology Research and Development Program of China (Grant 2006AA02Z340), and the Major State Basic Research Development Program of China (Grants 2006CB500700, 2006CB910802).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0810147106/DCSupplemental.
References
- 1.Bartek J, Bartkova J, Lukas J. DNA damage signalling guards against activated oncogenes and tumour progression. Oncogene. 2007;26:7773–7779. doi: 10.1038/sj.onc.1210881. [DOI] [PubMed] [Google Scholar]
- 2.Harper JW, Elledge SJ. The DNA damage response: Ten years after. Mol Cell. 2007;28:739–745. doi: 10.1016/j.molcel.2007.11.015. [DOI] [PubMed] [Google Scholar]
- 3.Bartkova J, et al. DNA damage response as a candidate anti-cancer barrier in early human tumorigenesis. Nature. 2005;434:864–870. doi: 10.1038/nature03482. [DOI] [PubMed] [Google Scholar]
- 4.Vogelstein B, Lane D, Levine AJ. Surfing the p53 network. Nature. 2000;408:307–310. doi: 10.1038/35042675. [DOI] [PubMed] [Google Scholar]
- 5.Kastan MB. Wild-type p53: Tumors can't stand it. Cell. 2007;128:837–840. doi: 10.1016/j.cell.2007.02.022. [DOI] [PubMed] [Google Scholar]
- 6.Lu X. P53: A heavily dictated dictator of life and death. Curr Opin Genet Dev. 2005;15:27–33. doi: 10.1016/j.gde.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 7.Vousden KH, Prives C. P53 and prognosis: New insights and further complexity. Cell. 2005;120:7–10. doi: 10.1016/j.cell.2004.12.027. [DOI] [PubMed] [Google Scholar]
- 8.Budanov AV, Karin M. P53 target genes sestrin1 and sestrin2 connect genotoxic stress and mtor signaling. Cell. 2008;134:451–460. doi: 10.1016/j.cell.2008.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levine AJ. P53, the cellular gatekeeper for growth and division. Cell. 1997;88:323–331. doi: 10.1016/s0092-8674(00)81871-1. [DOI] [PubMed] [Google Scholar]
- 10.Horn HF, Vousden KH. Coping with stress: Multiple ways to activate p53. Oncogene. 2007;26:1306–1316. doi: 10.1038/sj.onc.1210263. [DOI] [PubMed] [Google Scholar]
- 11.Haupt Y, Maya R, Kazaz A, Oren M. Mdm2 promotes the rapid degradation of p53. Nature. 1997;387:296–299. doi: 10.1038/387296a0. [DOI] [PubMed] [Google Scholar]
- 12.Kubbutat MH, Jones SN, Vousden KH. Regulation of p53 stability by mdm2. Nature. 1997;387:299–303. doi: 10.1038/387299a0. [DOI] [PubMed] [Google Scholar]
- 13.Banin S, et al. Enhanced phosphorylation of p53 by atm in response to DNA damage. Science. 1998;281:1674–1677. doi: 10.1126/science.281.5383.1674. [DOI] [PubMed] [Google Scholar]
- 14.Canman CE, et al. Activation of the atm kinase by ionizing radiation and phosphorylation of p53. Science. 1998;281:1677–1679. doi: 10.1126/science.281.5383.1677. [DOI] [PubMed] [Google Scholar]
- 15.Shieh SY, Ikeda M, Taya Y, Prives C. DNA damage-induced phosphorylation of p53 alleviates inhibition by mdm2. Cell. 1997;91:325–334. doi: 10.1016/s0092-8674(00)80416-x. [DOI] [PubMed] [Google Scholar]
- 16.Li M, Luo J, Brooks CL, Gu W. Acetylation of p53 inhibits its ubiquitination by mdm2. J Biol Chem. 2002;277:50607–50611. doi: 10.1074/jbc.C200578200. [DOI] [PubMed] [Google Scholar]
- 17.Tang Y, et al. Acetylation is indispensable for p53 activation. Cell. 2008;133:612–626. doi: 10.1016/j.cell.2008.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van de Wetering M, Oosterwegel M, van Norren K, Clevers H. Sox-4, an sry-like hmg box protein, is a transcriptional activator in lymphocytes. EMBO J. 1993;12:3847–3854. doi: 10.1002/j.1460-2075.1993.tb06063.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schilham MW, Moerer P, Cumano A, Clevers HC. Sox-4 facilitates thymocyte differentiation. Eur J Immunol. 1997;27:1292–1295. doi: 10.1002/eji.1830270534. [DOI] [PubMed] [Google Scholar]
- 20.Schilham MW, et al. Defects in cardiac outflow tract formation and pro-b-lymphocyte expansion in mice lacking sox-4. Nature. 1996;380:711–714. doi: 10.1038/380711a0. [DOI] [PubMed] [Google Scholar]
- 21.Cheung M, Abu-Elmagd M, Clevers H, Scotting PJ. Roles of sox4 in central nervous system development. Brain Res Mol Brain Res. 2000;79:180–191. doi: 10.1016/s0169-328x(00)00109-1. [DOI] [PubMed] [Google Scholar]
- 22.Graham JD, Hunt SM, Tran N, Clarke CL. Regulation of the expression and activity by progestins of a member of the sox gene family of transcriptional modulators. J Mol Endocrinol. 1999;22:295–304. doi: 10.1677/jme.0.0220295. [DOI] [PubMed] [Google Scholar]
- 23.Sinner D, et al. Sox17 and sox4 differentially regulate beta-catenin/t-cell factor activity and proliferation of colon carcinoma cells. Mol Cell Biol. 2007;27:7802–7815. doi: 10.1128/MCB.02179-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Friedman RS, et al. Molecular and immunological evaluation of the transcription factor sox-4 as a lung tumor vaccine antigen. J Immunol. 2004;172:3319–3327. doi: 10.4049/jimmunol.172.5.3319. [DOI] [PubMed] [Google Scholar]
- 25.Reichling T, et al. Transcriptional profiles of intestinal tumors in apc(min) mice are unique from those of embryonic intestine and identify novel gene targets dysregulated in human colorectal tumors. Cancer Res. 2005;65:166–176. [PubMed] [Google Scholar]
- 26.Lee CJ, et al. Differential expression of sox4 and sox11 in medulloblastoma. J Neurooncol. 2002;57:201–214. doi: 10.1023/a:1015773818302. [DOI] [PubMed] [Google Scholar]
- 27.Frierson HF, Jr., et al. Large scale molecular analysis identifies genes with altered expression in salivary adenoid cystic carcinoma. Am J Pathol. 2002;161:1315–1323. doi: 10.1016/S0002-9440(10)64408-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ahn SG, et al. Identification of cdnas for sox-4, an hmg-box protein, and a novel human homolog of yeast splicing factor ssf-1 differentially regulated during apoptosis induced by prostaglandin a2/delta12-pgj2 in hep3b cells. Biochem Biophys Res Commun. 1999;260:216–221. doi: 10.1006/bbrc.1999.0856. [DOI] [PubMed] [Google Scholar]
- 29.Aaboe M, et al. Sox4 expression in bladder carcinoma: Clinical aspects and in vitro functional characterization. Cancer Res. 2006;66:3434–3442. doi: 10.1158/0008-5472.CAN-05-3456. [DOI] [PubMed] [Google Scholar]
- 30.Ahn SG, et al. Sox-4 is a positive regulator of hep3b and hepg2 cells' apoptosis induced by prostaglandin (pg)a(2) and delta(12)-pgj(2) Exp Mol Med. 2002;34:243–249. doi: 10.1038/emm.2002.34. [DOI] [PubMed] [Google Scholar]
- 31.Lavin MF, Gueven N. The complexity of p53 stabilization and activation. Cell Death Differ. 2006;13:941–950. doi: 10.1038/sj.cdd.4401925. [DOI] [PubMed] [Google Scholar]
- 32.Oren M. Regulation of the p53 tumor suppressor protein. J Biol Chem. 1999;274:36031–36034. doi: 10.1074/jbc.274.51.36031. [DOI] [PubMed] [Google Scholar]
- 33.Li M, et al. Mono- versus polyubiquitination: Differential control of p53 fate by mdm2. Science. 2003;302:1972–1975. doi: 10.1126/science.1091362. [DOI] [PubMed] [Google Scholar]
- 34.Maya R, et al. Atm-dependent phosphorylation of mdm2 on serine 395: Role in p53 activation by DNA damage. Genes Dev. 2001;15:1067–1077. doi: 10.1101/gad.886901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Piu F, Aronheim A, Katz S, Karin M. Ap-1 repressor protein jdp-2: Inhibition of uv-mediated apoptosis through p53 down-regulation. Mol Cell Biol. 2001;21:3012–3024. doi: 10.1128/MCB.21.9.3012-3024.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wei X, Xu H, Kufe D. Human muc1 oncoprotein regulates p53-responsive gene transcription in the genotoxic stress response. Cancer Cell. 2005;7:167–178. doi: 10.1016/j.ccr.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 37.Aylon Y, Oren M. Living with p53, dying of p53. Cell. 2007;130:597–600. doi: 10.1016/j.cell.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 38.Pomerantz J, et al. The ink4a tumor suppressor gene product, p19arf, interacts with mdm2 and neutralizes mdm2's inhibition of p53. Cell. 1998;92:713–723. doi: 10.1016/s0092-8674(00)81400-2. [DOI] [PubMed] [Google Scholar]
- 39.Hsieh JK, et al. Rb regulates the stability and the apoptotic function of p53 via mdm2. Mol Cell. 1999;3:181–193. doi: 10.1016/s1097-2765(00)80309-3. [DOI] [PubMed] [Google Scholar]
- 40.Sui G, et al. Yin yang 1 is a negative regulator of p53. Cell. 2004;117:859–872. doi: 10.1016/j.cell.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 41.Brooks CL, Gu W. Ubiquitination, phosphorylation and acetylation: The molecular basis for p53 regulation. Curr Opin Cell Biol. 2003;15:164–171. doi: 10.1016/s0955-0674(03)00003-6. [DOI] [PubMed] [Google Scholar]
- 42.Kastan MB, Bartek J. Cell-cycle checkpoints and cancer. Nature. 2004;432:316–323. doi: 10.1038/nature03097. [DOI] [PubMed] [Google Scholar]
- 43.Chen QL, et al. Analysis of sox4 gene mutation in non-small cell lung cancer tissues. Zhonghua Yi Xue Yi Chuan Xue Za Zhi. 2007;24:505–509. [PubMed] [Google Scholar]
- 44.de Bont JM, et al. Differential expression and prognostic significance of sox genes in pediatric medulloblastoma and ependymoma identified by microarray analysis. Neuro Oncol. 2008;10:648–660. doi: 10.1215/15228517-2008-032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu P, et al. Sex-determining region y box 4 is a transforming oncogene in human prostate cancer cells. Cancer Res. 2006;66:4011–4019. doi: 10.1158/0008-5472.CAN-05-3055. [DOI] [PubMed] [Google Scholar]
- 46.Pramoonjago P, Baras AS, Moskaluk CA. Knockdown of sox4 expression by rnai induces apoptosis in acc3 cells. Oncogene. 2006;25:5626–5639. doi: 10.1038/sj.onc.1209566. [DOI] [PubMed] [Google Scholar]
- 47.Pan X, et al. Ubc9 interacts with sox4 and represses its transcriptional activity. Biochem Biophys Res Commun. 2006;344:727–734. doi: 10.1016/j.bbrc.2006.03.194. [DOI] [PubMed] [Google Scholar]
- 48.Zhang PJ, et al. Cue domain containing 2 regulates degradation of progesterone receptor by ubiquitin-proteasome. EMBO J. 2007;26:1831–1842. doi: 10.1038/sj.emboj.7601602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Man JH, et al. Pias3 induction of prb sumoylation represses prb transactivation by destabilizing its retention in the nucleus. Nucleic Acids Res. 2006;34:5552–5566. doi: 10.1093/nar/gkl691. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.