Abstract
Activated EGF receptor (EGFR) plays an oncogenic role in several human malignancies. Although the intracellular effects of EGFR are well studied, its ability to induce and modulate tumor angiogenesis is less understood. We found previously that oncogenic EGFR can be shed from cancer cells as cargo of membrane microvesicles (MVs), which can interact with surfaces of other cells. Here we report that MVs produced by human cancer cells harboring activated EGFR (A431, A549, DLD-1) can be taken up by cultured endothelial cells, in which they elicit EGFR-dependent responses, including activation of MAPK and Akt pathways. These responses can be blocked by annexin V and its homodimer, Diannexin, both of which cloak phosphatidylserine residues on the surfaces of MVs. Interestingly, the intercellular EGFR transfer is also accompanied by the onset of VEGF expression in endothelial cells and by autocrine activation of its key signaling receptor (VEGF receptor-2). In A431 human tumor xenografts in mice, angiogenic endothelial cells stain positively for human EGFR and phospho-EGFR, while treatment with Diannexin leads to a reduction of tumor growth rate and microvascular density. Thus, we propose that oncogene-containing tumor cell-derived MVs could act as a unique form of angiogenesis-modulating stimuli and are capable of switching endothelial cells to act in an autocrine mode.
Keywords: tumor angiogenesis, antiangiogenesis, exosomes, annexin V, oncogenes
Processes of cellular activation and transformation frequently lead to shedding of microvesicles (MVs) from the plasma membrane (1). Depending on their nature, size and origin MVs are referred to as ectosomes, exosomes, or microparticles, but their generation and biological roles are still poorly understood (2, 3). In various instances, MVs have been implicated in secretory processes, immunomodulation, inflammation, coagulation, and intercellular communication (4). These functions are usually attributed to the transfer of the MV cargo between adjacent or remote cells, and often involve interaction between phosphatidylserine (PS) residues exposed on the MV surface and the cellular plasma membrane, a process that can be blocked by annexin V (1, 5). MV-mediated intercellular exchange could include lipids, soluble proteins (6), or nucleic acids (7), but also functional transmembrane proteins (8), chemokine receptors (9), transferrin receptors (3), tissue factor (8, 10), and receptor tyrosine kinases (1, 11), such as HER-2 (12) and EGF receptor (EGFR) (1).
The latter property is especially thought-provoking in the context of cancer, in which EGFR-like kinases play transforming and oncogenic roles (13). Notably, the expression of the activated EGFR in glioma cells was found to result in markedly enhanced cellular vesiculation, capture, and intercellular transfer of this oncoprotein to adjacent tumor cells via MVs (1). This form of EGFR acquisition precipitated a series of molecular events in the MV recipient cells, including activation of the EGFR downstream pathways (MAPK and Akt), changes in growth and gene expression, as well as production of angiogenic mediators, especially VEGF (1). Collectively, these observations provide a glimpse into the role of MVs as extracellular carriers of active oncoproteins, and are also consistent with the known ability of the oncogenic EGFR to contribute to the angiogenic phenotype of the affected cancer cells (14). Indeed, the relative successes of targeting EGFR in human cancer may be, at least in part, attributed to the antiangiogenic properties of EGFR kinase inhibitors (EKI) and the respective neutralizing antibodies (14, 15).
Another source of encouragement to explore EGFR as a potential target in cancer and angiogenesis is related to the observation that, although this receptor is largely absent from normal endothelial cells, it is often selectively overexpressed by the tumor-associated vasculature (16–18). Thus, EGFR-directed agents could possess both indirect and direct antiangiogenic activity. Endothelial expression of EGFR is poorly understood, and it could be related to intrinsic abnormalities these cells sustain within the tumor context (19), but also to the paracrine stimulation by cancer cells expressing EGFR ligands and other mediators (16), tumor hypoxia (20), and various unspecified stress responses (18). It is also unclear whether EGFR-activated pathways are similar in endothelial cells to those operative in cancer cells and what their specific consequences for tumor angiogenesis might be.
Here we report a mechanism leading to endothelial cell expression of EGFR, namely by MV-mediated transfer of this receptor from EGFR-transformed cancer cells. Incorporation of EGFR-containing MVs by endothelial cells leads to activation of MAPK and Akt pathways and triggers endogenous expression of VEGF, followed by the activation of VEGF receptor-2 (VEGFR-2). Blockade of this MV exchange with annexin V derivatives aborts these effects in vitro, whereas in vivo these agents (e.g., Diannexin) exert an antitumor and antiangiogenic effect. Thus, oncogene-containing MVs may represent a distinct type of angiogenesis-modulating mechanism, and a therapeutic target in cancer.
Results
Microvesicular Transfer of the Oncogenic EGFR to Endothelial Cells.
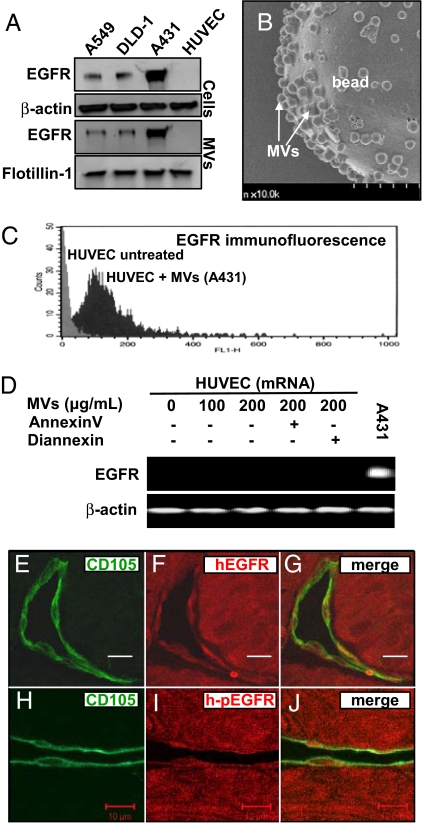
Aggressive properties of the A431 human squamous cell carcinoma cell line are largely attributed to the oncogenic effects of the WT EGFR, which these cells over-express in remarkable quantities (14). Interestingly, A431 cells (also A459 and DLD-1 cells) not only produce a standard membrane-bound form of EGFR, but also release this receptor into the conditioned medium (Fig. 1A). This latter pool of EGFR can be recovered from the soluble material by ultracentrifugation of the culture supernatants, with a pelleted fraction that is also positive for flotillin-1, a marker of membrane lipid rafts and their derived MVs (1, 8). Indeed, such intact, A431-derived MVs can be directly visualized by adsorption of this material onto poly-L-lysine particles/beads followed by scanning electron microscopy (Fig. 1B). In contrast, human umbilical vein endothelial cells are devoid of EGFR, whether cell-associated or in MVs (Fig. 1A).
Fig. 1.
Microvesicular transfer of the oncogenic EGFR to endothelial cells. (A) EGFR associated with cells and MVs. A549, DLD-1, and A431 cancer cells express high levels of EGFR and shed this oncogenic receptor into their conditioned medium as cargo of MVs. In contrast, neither HUVECs nor their derived MVs contain detectable EGFR. Protein loading was normalized to β-actin (cell lysates) or to flotillin-1 (MVs), a marker of membrane lipid rafts from which MVs originate (1, 8). (B) Scanning electron micrograph of A431-derived MVs adsorbed on poly-L-lysine beads. (Scale bar, 1 μm.) (C) Uptake of EGFR by HUVEC cells upon exposure to EGFR-containing MVs derived from cancer cells (A431). FACS analysis reveals a prominent shift in EGFR immunofluorescence of HUVECs after 24 h incubation with MVs followed by extensive washing. (D) Exposure to tumor cell-derived MVs does not induce endogenous EGFR gene expression in endothelial cells. HUVECs were treated with EGFR-containing MVs for 24 h and then processed for EGFR mRNA detection by RT-PCR. (E–G) Transfer of tumor-derived EGFR to endothelial cells in vivo. Co-localization of the human EGFR immunofluorescence (red) with staining for the mouse endothelial marker CD105 (green) in A431 xenografts (SCID mice). (H–J) Co-staining for phosphorylated human EGFR (h-pEGFR; red) and A431 tumor-associated mouse endothelium (CD105; green) (Scale bar, 10 μm.) For more information see Figs. S6–S8 and Movies S1–S3.
MVs can merge with membranes of heterotypic cells via a PS-dependent mechanism (1, 8). We reasoned that similar events are possible between EGFR-positive tumor cells and endothelial cells, whereby this oncogenic receptor could be incorporated into the membranes of the latter. Indeed, incubation with MV preparations obtained from 3 different EGFR-expressing cancer cell lines (A431, A549, and DLD1) resulted in the appearance of the specific EGFR immunofluorescence on the surface of human umbilical vein endothelial cells (HUVECs) and human microvascular endothelial cells [FACS; Fig. 1C and Figs. S1 and S2]. This uptake of EGFR was dose-dependent and inhibitable by pretreatment of MVs with annexin V (ref. 1 and data not shown). Importantly, the incubation of endothelial cells with tumor-derived MVs and the acquisition of EGFR protein expression did not result from the activation of the endogenous EGFR gene, as documented by the consistent absence of a detectable EGFR transcript in all HUVEC preparations analyzed (Fig. 1D), even though MVs did contain traces of EGFR mRNA (Fig. S2C). Moreover, antibodies against human EGFR and phosphorylated human EGFR readily decorated tumor blood vessels in A431 tumor xenografts, as indicated by the co-localization of these respective fluorescent signals with markers of mouse endothelial cells (CD105/endoglin). As expected, anti-EGFR antibodies that react with both human and mouse receptor decorated normal keratinocytes, but not cutaneous endothelial cells (Fig. 1 E–J). These results suggest that cancer cells shed MVs containing the EGFR oncoprotein, which can be transferred to endothelial cells in vitro and in vivo.
EGFR Signaling in Endothelial Cells Exposed to Tumor-Derived MVs.
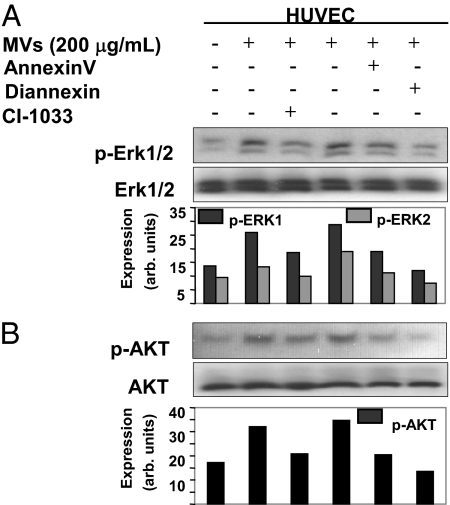
Oncogenic EGFR activates a number of cellular signaling pathways, notably MAPK and Akt (13). To determine whether the aforementioned micro-vesicular transfer of this receptor is of any functional consequence to the recipient endothelial cells, we examined their status of MAPK and Akt phosphorylation (Fig. 2). Interestingly, addition of EGFR-containing MVs to serum-starved HUVECs resulted in activation of both pathways, in a manner that could be inhibited by pretreatment of this material (i.e., MVs) with annexin V and its recently described homodimer designated as Diannexin. The latter differs from annexin V in that it has a higher affinity to PS on cellular surfaces, longer half-life, and longer action in vivo (21) (Fig. 2 A and B). Phosphorylation of MAPK and Akt was also prevented when MVs were exposed to the irreversible pan-Erb kinase inhibitor, CI-1033, before their addition to endothelial cells, and similar effects were also observed with EGFR-neutralizing antibody (cetuximab; Fig. S3). Thus, the activation of these respective signaling pathways by tumor cell-derived MVs was dependent on the accessibility of their surface PS residues, and the intact EGFR kinase activity present in the MV cargo.
Fig. 2.
Signaling events triggered in endothelial cells upon the uptake of MV-derived EGFR. (A) Phosphorylation of MAPK (Erk1/2) in endothelial cells that have taken up EGFR upon incubation with MVs isolated from A431 conditioned medium. These effects were obliterated by preincubation of MVs with PS blocking agents: annexin V and Diannexin (both at 2 μg/mL) or with irreversible EGFR inhibitor CI-1033 (50 μM) before addition to HUVEC. The phospho-Erk1 (p-Erk1) signal was somewhat more robust than p-Erk2, and both were normalized to the corresponding Erk1/2 bands for the purpose of densitometric quantification, which is expressed in arbitrary units. (B) Impact of the microvesicular EGFR transfer on Akt phosphorylation in endothelial cells (HUVECs, all conditions as in A).
EGFR-Dependent Onset of the Autocrine VEGF Production in Endothelial Cells.
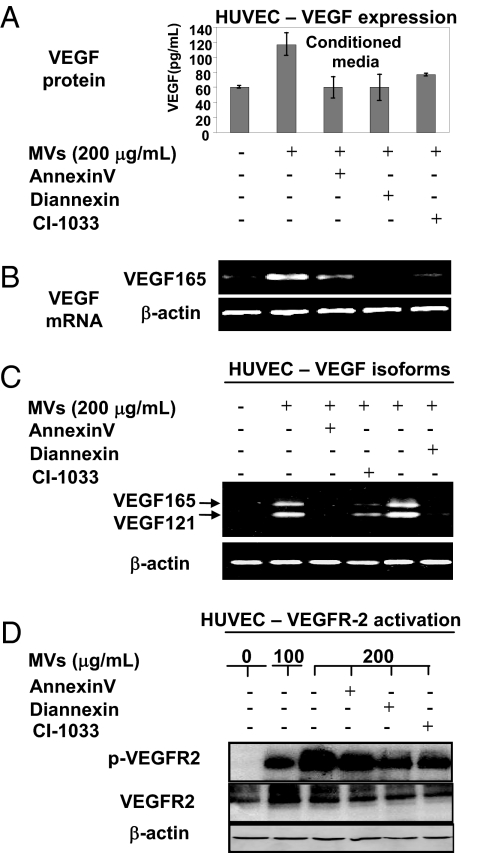
One of the important mechanisms by which oncogenic EGFR contributes to tumor angiogenesis is through up-regulation of VEGF in tumor cells (14). Interestingly, VEGF was recently found to be expressed by endothelial cells, where it plays an important homeostatic role (22). We reasoned that the latter property could be modulated by the uptake of MV-associated EGFR. Indeed, we observed that HUVEC cells exposed to EGFR-containing MVs (from A431 cells) secreted elevated amounts of VEGF to their conditioned media and turned on the expression of VEGF mRNA. The RT-PCR analysis of this mRNA using VEGF isoform specific primers revealed that the MV-stimulated endothelial cells produced mainly VEGF121 and VEGF165 (Fig. 3 A–C). These effects were blocked by preincubation of the MV preparations with annexin V, Diannexin, or CI-1033, a result suggesting the requirement for PS-mediated MV merger with the target cells and the intact EGFR activity. Moreover, whereas, in un-stimulated and serum-starved HUVECs, the endogenous VEGFR-2 remained un-phosphorylated, microvesicular transfer of EGFR from cancer cells provoked its robust phosphorylation. Once again, these effects were obliterated when MVs were treated with annexin V, Diannexin, or CI-1033 before their addition to endothelial cell cultures. The effects of MVs were also blocked by VEGFR-2 kinase inhibitor (SU5416), but not by addition of VEGF-neutralizing antibody (Avastin/bevacizumab), suggesting that this material does not contain VEGF and acts through an autocrine mechanism (22) (Figs. S4 and S5). Intact MVs also stimulated growth and viability of HUVECs (data not shown). These results suggest that tumor-derived EGFR may trigger the expression of VEGF and the autocrine stimulation of VEGFR-2 in endothelial cells exposed to cancer cell-related MVs.
Fig. 3.
Microvesicular uptake of EGFR triggers VEGF expression and autocrine signaling in endothelial cells. (A) Secretion of VEGF into conditioned medium of serum-starved HUVECs upon their incubation with EGFR-containing MVs. This effect was markedly diminished by preincubation of MVs with agents blocking PS residues, annexin V and Diannexin (both at 2 μg/mL), and with the EGFR/panErb inhibitor CI-1033 (50 μM). The baseline level of VEGF represents the limit of detectability and should be considered as negative. (B) The onset of VEGF165 mRNA expression (RT-PCR) in HUVEC cells that have taken up MV-associated EGFR. Again, annexin V, Diannexin, and CI-1033 pre-treatments diminish the effects of MVs. (C) Expression of the main VEGF isoforms (VEGF165 and VEGF 121) in HUVECs is triggered by the transfer of tumor cell-derived MVs in a manner dependent on PS-mediated uptake and intact activity of the MV-associated EGFR. An independent set of primers was used to amplify VEGF121 and VEGF165 in HUVECs after exposure to either intact A431-derived MVs or MVs pretreated with annexin V, Diannexin, or CI-1033 (conditions as in A and B). (D) Autocrine activation of VEGFR-2 upon the uptake of EGFR-containing MVs by cultured endothelial cells (HUVECs). Serum starved HUVECs were treated with MVs as in A–C and assayed for VEGFR-2 phosphorylation (p-VEGFR-2). Interference with MV-related PS (annexin V and Diannexin) and EGFR kinase activity (i.e., CI-1033) diminishes the level of pVEGFR-2 in parallel with reduction of VEGF produced by HUVEC cells (compare A vs. C). The respective signals were quantified by densitometry, normalized to the total VEGFR-2, and expressed in relative units (Fig. S5).
In Vivo Inhibition of EGFR-Driven Tumor Growth and Angiogenesis by Blocking Membrane PS.
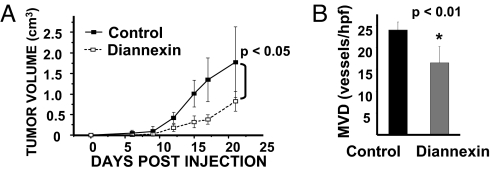
Our data suggest that tumor cell-derived EGFR can be transferred to endothelial cells in vitro and in vivo, leading to activation of the autocrine VEGF/VEGFR-2 pathway in a manner dependent on PS-mediated exchange of MVs. To examine the functional significance of these events, we generated A431 xenografts in SCID mice and subjected these animals to daily i.p. injections of Diannexin to impair the intratumoral MV exchange (Fig. 4). As predicted, tumors exposed to this treatment grew more slowly than their control counterparts, and attained lower microvascular densities (MVDs), as determined by staining for CD105, a marker of endothelial cells (Fig. 4). Collectively, these results suggest that cancer cell-derived MVs may play an important role during tumor angiogenesis, notably as carriers of oncoproteins capable of inducing VEGF expression, and thereby switching tumor endothelial cells from a predominantly paracrine to a more autocrine mode of response to this angiogenic growth factor.
Fig. 4.
Inhibition of tumor growth and angiogenesis by interference with membrane PS. (A) Delay of A431 tumor growth in SCID mice treated with daily doses of Diannexin (1 mg/kg i.p.). Average tumor volume ± SD; n = 5; P < 0.05. (B) Reduced MVD in A431 tumors exposed to Diannexin. Tumor sections were stained with anti-CD105 antibody to visualize endothelial cells and vascular counts were performed in “hot spots” at ×200 magnification.
Discussion
Our results demonstrate that cancer cells harboring oncogenic EGFR emit this receptor in an intact form as cargo of membrane MVs. This material is probably derived from membrane lipid rafts, because the MV fraction of cancer cell-conditioned medium was uniformly positive for flotillin-1, which is often associated with these domains (1, 8). This interpretation is also consistent with our recent results, which suggest that lipid raft-related material containing oncogenic mutant of EGFR (EGFRvIII) may be shed from glioma cells within flotillin-1-positive MVs. It is of note that intercellular trafficking of this receptor led to transformation-like changes in adjacent tumor cells, a finding that may potentially apply also to other membrane-associated oncogenic proteins (1, 23).
Microvesicles (24) and exosomes (25) readily interact with endothelial cells. An intriguing observation made during the present study relates to the impact on these cells exerted by EGFR-containing, tumor cell-derived MVs (1). Thus, incubation of primary cultures of 2 different types of endothelial cells with MV preparations led to the uptake of this material and retention of the intact and functional EGFR. The latter is documented by the activation of the signaling events, such as phosphorylation of MAPK and Akt, both of which could be prevented by blocking of the MV-associated surface PS with annexin V or Diannexin, or EGFR kinase activity (CI-1033). These findings suggest that the uptake by endothelial cells of tumor-derived MVs, at least those containing EGFR, occurs by a mechanism involving exposed PS residues. This is operationally similar to the recently described uptake of tissue factor-containing MVs derived from activated macrophages by platelets participating in coagulation processes (8). It is noteworthy that other forms of micro-vesiculation may clearly be operative in transformed, activated, and secretory cells and lead to a release of material from membrane (ectosomes) or endosomal subcellular compartments (exosomes) (2, 3, 11, 26–28). In many of these instances, there is strong evidence for the secondary attachment and uptake mechanisms that involve various specific receptors, either for PS (e.g., Tim4, BAI1, or KIM1) (29–31) or for other MV-associated surface molecules (e.g., P-selectin glycoprotein ligand-1) (32). The involvement of these mechanisms in formation and transfer of EGFR-containing tumor MVs is presently unknown, and in our hands, cloaking of PS is sufficient to prevent their uptake by endothelial cells.
Our study suggests that one source of EGFR expression by tumor-associated endothelial cells is through the transfer of MVs from adjacent cancer cells harboring this oncogenic receptor. Although the expression and role of EGFR in endothelial cells has been a subject of some controversy (18), such expression has clearly been documented in the context of tumor angiogenesis (16, 17, 33), especially in tumors expressing EGFR ligands (16), and is viewed as one of the reasons for the antiangiogenic and anticancer activity of EKIs (16). A corollary to this point is that the clinical activity of the latter agents can be dependent upon cancer-specific mutations, e.g., in the subset of patients with non-small-cell lung carcinoma whose disease is highly responsive to gefitinib and similar agents, but only if they harbor a mutant EGFR (34). Therefore, it would be of considerable interest to examine whether patients harboring EGFR mutations that sensitize (or desensitize) this receptor to EKIs also express the corresponding (WT or mutant) tumor cell-derived receptor also on the surface of tumor-associated endothelial cells. The latter could be expected, if the clinical effects of EKIs are indeed dependent on their direct impact on the tumor vasculature. Indeed, we have previously observed that mutant forms of EGFR (e.g., EGFRvIII) can be transferred between cells via MVs (1), a result confirmed and extended in a recent study (23).
The mode of pro-angiogenic action of endothelial EGFR, whether MV-related or endogenous, is poorly understood and rather implicit (16). In this regard, our study offers a mechanism whereby MV-derived EGFR re-wires endothelial cells to express, and respond to, VEGF in an autocrine manner. VEGF was originally considered to act as a strictly paracrine mediator of vascular development and growth, including in cancer, principally because of difficulties with the detection of the respective protein and mRNA in the endothelial compartment in vivo (22, 35). However, in some instances, VEGF protein was, in fact, detected in association with tumor blood vessels (35), and VEGF synthesis could be induced in cultured endothelial cells by hypoxia (36) or malignant transformation (37). Importantly, in a recent study, Lee et al. demonstrated that very small quantities of intracellular VEGF are expressed by normal endothelial cells in vivo, activate VEGFR-2, and are required for vascular homeostasis. Withdrawal of this influence leads to destabilization of the micro-vasculature, ischemia, and thrombosis (22). Our study suggests that, in the context of cancer, a transfer of oncogenic EGFR (see Fig. 1 E–J, Figs. S6–S8, and Movies S1–S3) may trigger and amplify the expression and function of endothelial VEGF, perhaps contributing to the distinct nature of tumor blood vessels and their responsiveness to therapy.
In agreement with this notion, we observed that administration of an agent blocking PS (Diannexin), and thereby MV transfer, exerts a noticeable anti-cancer and anti-angiogenic effect in vivo. Diannexin is a homodimeric form of annexin V, which is being developed as an antithrombotic agent for cardiovascular indications (21). We suggest that, as in the case of cultured cells, the antitumor effect of this agent in vivo may, at least in part, involve interference with MV exchange between tumor and endothelial cells. It should be kept in mind that exposed PS is not only a feature of MVs, but is also present on the surface of apoptotic cells and activated/angiogenic endothelium (30, 38). However, targeting endothelial PS to achieve antiangiogenic effects in cancer, e.g., by using monoclonal antibodies (bavituximab), involves agents that often act in a manner that is dependent on β2-glycoprotein, are mediated by cytotoxic cells, and may require co-administration of chemotherapy (38), none of which was involved in our experiments. Instead, in our hands, Diannexin treatment by itself inhibited growth and angiogenesis of A431 tumors. These tumors are known to heavily depend on EGFR and VEGF for their expansion (39), and in this context human oncogenic EGFR was detected on tumor-associated (mouse) endothelial cells.
Collectively, our data suggest that tumor-derived MVs harboring oncogenic EGFR could interact with endothelial cells and act as a distinct type of a more complex angiogenic regulator. Indeed, we observed that incorporation of MVs leads to re-programming of endothelial cells and to their stimulation with the autocrine VEGF. There is no reason to think that these effects are limited to EGFR, because many other receptors and oncogenes could potentially undergo similar intercellular exchange and elicit responses in the recipient cells (1), including in the tumor-associated endothelium. We postulate that these events could contribute to the distinct nature of the latter cells and, potentially, serve as targets for anticancer therapies.
Materials and Methods
Reagents and Cells.
Antibodies recognizing EGFR (sheep polyclonal and mouse monoclonal), polyclonal antibodies against MAPK (Erk1/2), Akt, and flotillin-1were purchased from Cell Signaling Technologies. Other reagents included monoclonal antibodies against human EGFR (NeoMarker), β-actin antibody (Sigma), HRP-conjugated secondary antibodies (Cell Signaling Technologies), Alexa Fluor secondary antibodies (Molecular Probes), cell culture reagents (Invitrogen), HUVEC medium (Clontech), and annexin V (BD-PharMingen). As gifts, we received CI-1033 from C. Marsolais and L. Levesque (Pfizer, Montreal) and Diannexin from Alavita. A431, A549, and HUVEC cells were purchased from the American Type Tissue Collection. DLD-1 cells were a kind gift from S. Shirasawa (Tokyo) (40). Human microvascular endothelial cells/adult dermal human microvascular endothelial cells (HMVECad) were purchased from Cascade Biologics/Invitrogen/Cell Culture. The cells were cultured in their appropriate MV-depleted media as previously described (1).
Isolation and Analysis of MVs.
Conditioned medium was collected from cells at approximately 80% confluence and subjected to 2 consecutive centrifugations at 300 × g for 5 min, and then at 12,000 × g for 20 min to eliminate cells and debris. MV fraction was obtained after centrifugation for 2 h at 100,000 × g and washed twice with a large volume of PBS. The amount of microvesicles proteins recovered was measured using the Bradford assay (Bio-Rad) and assayed as described in SI Methods.
Flow Cytometry.
To detect EGFR on the surface of viable HUVECs, the cells were treated with MVs for 24 h, harvested using 2 mM EDTA, and processed as described previously (1). Following the staining with monoclonal antibody against EGFR and Alexa Fluor 488 goat anti-mouse secondary antibody (Molecular Probes), the cells were fixed with 1% paraformaldehyde, resuspended in PBS solution, and analyzed using a FACScalibur flow cytometer (BD Biosciences).
Scanning Electron Microscopy.
Images were collected after adsorption of the MV preparations to poly-L-lysine beads formed during the glass slide coating process. The slides were fixed with 2.5% glutaraldehyde in 0.1 M PBS solution, washed 3 times with 0.1 M PBS solution and then with 0.1 M cacodylate buffer, followed by staining with 1% osmium tetraoxide (OsO4). The slides were then dehydrated, dried, fixed on a stud, covered with gold, and microphotographed using the JEOL 840A instrument.
VEGF ELISA.
HUVEC cells were treated with MVs for 24 h as indicated, washed extensively, and fed with fresh medium. After 24 h, conditioned medium was collected and assayed for VEGF content using Quantikine Human VEGF Immunoassay (R&D Systems) according to the manufacturer's protocol. The readings were collected from multiple (n = 3–4) independent samples for each experimental condition, normalized to the cell number, and read at several dilutions against a standard curve.
Detection of mRNA.
Treatment of HUVEC cells with MV preparations was followed by extensive washing and extraction of RNA using Trizol reagent (Invitrogen). RT-PCR analysis was performed using a single-step method (Qiagen) whereby VEGF isoforms were detected using the primer sets 5′ATGAACTTTCTGCTGTCTTG3′ and 5′TCACCGCCTCGGCTTGTCACAT3′, which hybridize to regions flanking exons 1 and 8 of the VEGF transcript, respectively. The amplified species of 688, 656, 584, 524, and 452 bp correspond to VEGF isoforms 206, 189, 165, 145, and 121, respectively (41). For specific amplification of other species, we used the following primer sets: VEGF165, GCAAGACAAGAAAATCCCTGTGGG and TTCTGTCGATGGTGATGGTGTGG; and β-actin, TTCCTGGGCATGGAGTCCTGTGG and CGCCTAGAAGCATTTGCGGTGG for sense and antisense, respectively (42). Reactions were carried out in 50 μL in which the initial Taq activation at 95 °C for 30 min was followed by 40 cycles of denaturation at 95 °C for 30 seconds, primer annealing at 60 °C for 1 min, and extension at 72 °C for 30 seconds. The products were resolved on 1% agarose and photographed. The 473-bp EGFR transcript was detected in a similar manner and using the following set of primers: 5′-TCT CAG CAA CAT GTC GATGG-3′; antisense, 5′-TCG CAC TTC TTA CAC TTG CC-3′, as recently described (43).
Tumor Analysis.
All in vivo experiments were performed in 6- to 8-week-old SCID female mice (Charles River), as previously described (1, 40), upon institutional approval and in accordance with the guidelines of the Canadian Council of Animal Care. Briefly, single cell suspension of A431 cells was injected s.c. (2 × 106 cells/mouse; 5 mice per group) and daily i.p. injections of the vehicle or Diannexin (1 mg/kg) commenced the day after. In some experiments, the cells were premixed with 0.1 mg/mL of Diannexin at the time of injection, and the results of both of these treatment protocols were comparable. Tumors were measured 2 to 3 times per week and their volumes were calculated according to the formula a2× b × 0.54, where a and b are the shorter and longer diameters, respectively. Upon reaching the experimental end-point, tumor tissues were harvested, embedded in paraffin, sectioned (5 μm), and used for immunofluorescent staining for human EGFR and CD105 (endoglin) with the respective secondary antibodies tagged with Alexa Fluor 594 (EGFR) or Alexa Fluor 488 (CD105). The images were collected using an LSM confocal microscope against controls in which primary antibodies were omitted. Goat anti-mouse CD105 (R & D Systems) and chicken anti-goat Alexa Fluor 488 (Molecular Probes) were used to visualize tumor-associated endothelial cells and quantify MVD at ×200 magnification. MVD was averaged from counts in vascular hot spots located in 3 independent fields per section of each tumor.
Data Analysis.
All experiments were reproduced at least twice with similar results. The quantitative data were presented as the average value of replicates within the representative experiment ± SD. Statistical significance was evaluated using a computerized 2-tailed Student t test. Differences were considered significant at P < 0.05.
Supplementary Material
Acknowledgments.
We thank our McGill colleagues for their assistance: J. Laliberte in confocal microscopy, L. Mongeon in electron microscopy, and M. Dahho in statistics, as well as our families for their support. This project was supported by a grant from the Canadian Cancer Society Research Institute (J.R.). J.R. is the Jack Cole Chair in Pediatric Oncology. Infrastructure contribution was received from Fonds de la recherche en santé du Quebec.
Footnotes
Conflict of interest statement: A.C.A. is employed by Alavita, which develops therapeutic applications of Diannexin, mainly in cardiovascular medicine.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0804543106/DCSupplemental.
References
- 1.Al-Nedawi K, et al. Intercellular transfer of the oncogenic receptor EGFRvIII by microvesicles derived from tumour cells. Nat Cell Biol. 2008;10:619–624. doi: 10.1038/ncb1725. [DOI] [PubMed] [Google Scholar]
- 2.Pilzer D, Gasser O, Moskovich O, Schifferli JA, Fishelson Z. Emission of membrane vesicles: roles in complement resistance, immunity and cancer. Springer Semin Immunopathol. 2005;27:375–387. doi: 10.1007/s00281-005-0004-1. [DOI] [PubMed] [Google Scholar]
- 3.Johnstone RM. Exosomes biological significance: a concise review. Blood Cells Mol Dis. 2006;36:315–321. doi: 10.1016/j.bcmd.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 4.Janowska-Wieczorek A, et al. Microvesicles derived from activated platelets induce metastasis and angiogenesis in lung cancer. Int J Cancer. 2005;113:752–760. doi: 10.1002/ijc.20657. %20; [DOI] [PubMed] [Google Scholar]
- 5.Mandal SK, Iakhiaev A, Pendurthi UR, Rao LV. Acute cholesterol depletion impairs functional expression of tissue factor in fibroblasts: modulation of tissue factor activity by membrane cholesterol. Blood. 2005;105:153–160. doi: 10.1182/blood-2004-03-0990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iero M, et al. Tumour-released exosomes and their implications in cancer immunity. Cell Death Differ. 2008;15:80–88. doi: 10.1038/sj.cdd.4402237. [DOI] [PubMed] [Google Scholar]
- 7.Valadi H, et al. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 8.del Conde , Shrimpton CN, Thiagarajan P, Lopez JA. Tissue-factor-bearing microvesicles arise from lipid rafts and fuse with activated platelets to initiate coagulation. Blood. 2005;106:1604–1611. doi: 10.1182/blood-2004-03-1095. [DOI] [PubMed] [Google Scholar]
- 9.Mack M, et al. Transfer of the chemokine receptor CCR5 between cells by membrane-derived microparticles: a mechanism for cellular human immunodeficiency virus 1 infection. Nat Med. 2000;6:769–775. doi: 10.1038/77498. [DOI] [PubMed] [Google Scholar]
- 10.Yu JL, Rak JW. Shedding of tissue factor (TF)-containing microparticles rather than alternatively spliced TF is the main source of TF activity released from human cancer cells. J Thromb Haemost. 2004;2:2065–2067. doi: 10.1111/j.1538-7836.2004.00972.x. [DOI] [PubMed] [Google Scholar]
- 11.Sanderson MP, et al. Generation of novel, secreted epidermal growth factor receptor (EGFR/ErbB1) isoforms via metalloprotease-dependent ectodomain shedding and exosome secretion. J Cell Biochem. 2008;103:1783–1797. doi: 10.1002/jcb.21569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koga K, et al. Purification, characterization and biological significance of tumor-derived exosomes. Anticancer Res. 2005;25:3703–3707. [PubMed] [Google Scholar]
- 13.Citri A, Yarden Y. EGF-ERBB signalling: towards the systems level. Nat. Rev. Mol Cell Biol. 2006;7:505–516. doi: 10.1038/nrm1962. [DOI] [PubMed] [Google Scholar]
- 14.Viloria-Petit AM, et al. Neutralizing antibodies against EGF and ErbB-2/neu receptor tyrosine kinases down-regulate VEGF production by tumor cells in vitro and in vivo: angiogenic implications for signal transduction therapy of solid tumors. Am J Pathol. 1997;151:1523–1530. [PMC free article] [PubMed] [Google Scholar]
- 15.Baselga J, Arteaga CL. Critical update and emerging trends in epidermal growth factor receptor targeting in cancer. J Clin Oncol. 2005;23:2445–2459. doi: 10.1200/JCO.2005.11.890. [DOI] [PubMed] [Google Scholar]
- 16.Baker CH, et al. Blockade of epidermal growth factor receptor signaling on tumor cells and tumor-associated endothelial cells for therapy of human carcinomas. Am J Pathol. 2002;161:929–938. doi: 10.1016/S0002-9440(10)64253-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuwai T, et al. Phosphorylated epidermal growth factor receptor on tumor-associated endothelial cells is a primary target for therapy with tyrosine kinase inhibitors. Neoplasia. 2008;10:489–500. doi: 10.1593/neo.08200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Luca A, et al. The role of the EGFR signaling in tumor microenvironment. J Cell Physiol. 2008;214:559–567. doi: 10.1002/jcp.21260. [DOI] [PubMed] [Google Scholar]
- 19.Hida K, et al. Tumor-associated endothelial cells with cytogenetic abnormalities. Cancer Res. 2004;64:8249–8255. doi: 10.1158/0008-5472.CAN-04-1567. [DOI] [PubMed] [Google Scholar]
- 20.Franovic A, et al. Translational up-regulation of the EGFR by tumor hypoxia provides a nonmutational explanation for its overexpression in human cancer. Proc Natl Acad Sci USA. 2007;104:13092–13097. doi: 10.1073/pnas.0702387104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuypers FA, Larkin SK, Emeis JJ, Allison AC. Interaction of an annexin V homodimer (Diannexin) with phosphatidylserine on cell surfaces and consequent antithrombotic activity. Thromb Haemost. 2007;97:478–486. [PubMed] [Google Scholar]
- 22.Lee S, et al. Autocrine VEGF signaling is required for vascular homeostasis. Cell. 2007;130:691–703. doi: 10.1016/j.cell.2007.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Skog J, et al. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol. 2008;10:1470–1476. doi: 10.1038/ncb1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Millimaggi D, et al. Tumor vesicle-associated CD147 modulates the angiogenic capability of endothelial cells. Neoplasia. 2007;9:349–357. doi: 10.1593/neo.07133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Al-Nedawi K, Szemraj J, Cierniewski CS. Mast cell-derived exosomes activate endothelial cells to secrete plasminogen activator inhibitor type 1. Arterioscler Thromb Vasc Biol. 2005;25:1744–1749. doi: 10.1161/01.ATV.0000172007.86541.76. [DOI] [PubMed] [Google Scholar]
- 26.Williams RL, Urbe S. The emerging shape of the ESCRT machinery. Nat Rev Mol Cell Biol. 2007;8:355–368. doi: 10.1038/nrm2162. [DOI] [PubMed] [Google Scholar]
- 27.Trajkovic K, et al. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science. 2008;319:1244–1247. doi: 10.1126/science.1153124. [DOI] [PubMed] [Google Scholar]
- 28.Gesierich S, Berezovskiy I, Ryschich E, Zoller M. Systemic induction of the angiogenesis switch by the tetraspanin D6.1A/CO-029. Cancer Res. 2006;66:7083–7094. doi: 10.1158/0008-5472.CAN-06-0391. [DOI] [PubMed] [Google Scholar]
- 29.Miyanishi M, et al. Identification of Tim4 as a phosphatidylserine receptor. Nature. 2007;450:435–439. doi: 10.1038/nature06307. [DOI] [PubMed] [Google Scholar]
- 30.Zhou Z. New phosphatidylserine receptors: clearance of apoptotic cells and more. Dev Cell. 2007;13:759–760. doi: 10.1016/j.devcel.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 31.Ichimura T, et al. Kidney injury molecule-1 is a phosphatidylserine receptor that confers a phagocytic phenotype on epithelial cells. J Clin Invest. 2008;118:1657–1668. doi: 10.1172/JCI34487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Polgar J, Matuskova J, Wagner DD. The P-selectin, tissue factor, coagulation triad. J Thromb Haemost. 2005;3:1590–1596. doi: 10.1111/j.1538-7836.2005.01373.x. [DOI] [PubMed] [Google Scholar]
- 33.Amin DN, Hida K, Bielenberg DR, Klagsbrun M. Tumor endothelial cells express epidermal growth factor receptor (EGFR) but not ErbB3 and are responsive to EGF and to EGFR kinase inhibitors. Cancer Res. 2006;66:2173–2180. doi: 10.1158/0008-5472.CAN-05-3387. [DOI] [PubMed] [Google Scholar]
- 34.Lynch TJ, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–2139. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 35.Brown LF, et al. Vascular stroma formation in carcinoma in situ, invasive carcinoma, and metastatic carcinoma of the breast. Clin Cancer Res. 1999;5:1041–1056. [PubMed] [Google Scholar]
- 36.Namiki A, et al. Hypoxia induces vascular endothelial growth factor in cultured human endothelial cells. J Biol Chem. 1995;270:31189–31195. doi: 10.1074/jbc.270.52.31189. [DOI] [PubMed] [Google Scholar]
- 37.Arbiser JL, et al. Oncogenic H-ras stimulates tumor angiogenesis by two distinct pathways. Proc Natl Acad Sci USA. 1997;94:861–866. doi: 10.1073/pnas.94.3.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huang X, Bennett M, Thorpe PE. A monoclonal antibody that binds anionic phospholipids on tumor blood vessels enhances the antitumor effect of docetaxel on human breast tumors in mice. Cancer Res. 2005;65:4408–4416. doi: 10.1158/0008-5472.CAN-05-0031. [DOI] [PubMed] [Google Scholar]
- 39.Viloria-Petit A, et al. Acquired resistance to the antitumor effect of epidermal growth factor receptor-blocking antibodies in vivo: a role for altered tumor angiogenesis. Cancer Res. 2001;61:5090–5101. [PubMed] [Google Scholar]
- 40.Yu JL, et al. Oncogenic events regulate tissue factor expression in colorectal cancer cells: implications for tumor progression and angiogenesis. Blood. 2005;105:1734–1741. doi: 10.1182/blood-2004-05-2042. [DOI] [PubMed] [Google Scholar]
- 41.Abdel-Rahman MH, Craig EL, Davidorf FH, Eng C. Expression of vascular endothelial growth factor in uveal melanoma is independent of 6p21-region copy number. Clin Cancer Res. 2005;11:73–78. [PubMed] [Google Scholar]
- 42.von Marschall Z, et al. Dual mechanism of vascular endothelial growth factor upregulation by hypoxia in human hepatocellular carcinoma. Gut. 2001;48:87–96. doi: 10.1136/gut.48.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Papouchado B, et al. Epidermal growth factor receptor and activated epidermal growth factor receptor expression in gastrointestinal carcinoids and pancreatic endocrine carcinomas. Mod Pathol. 2005;18:1329–1335. doi: 10.1038/modpathol.3800427. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.