Abstract
Hepcidin is a hormone secreted in response to iron loading and inflammation. Hepcidin binds to the iron exporter ferroportin, inducing its degradation and thus preventing iron entry into plasma. We determined that hepcidin binding to ferroportin leads to the binding and activation of the protein Janus Kinase2 (Jak2), which is required for phosphorylation of ferroportin. Ferroportin is a dimer and both monomers must be capable of binding hepcidin for Jak2 to bind to ferroportin. Once Jak2 is bound to the ferroportin dimer, both ferroportin monomers must be functionally competent to activate Jak2 and for ferroportin to be phosphorylated. These results show that cooperativity between the ferroportin monomers is required for hepcidin-mediated Jak2 activation and ferroportin down-regulation. These results provide a molecular explanation for the dominant inheritance of hepcidin resistant iron overload disease.
Keywords: iron, dimer, phosphorylation
Iron homeostasis in vertebrates is regulated by the binding of hepcidin to the cell surface iron transporter ferroportin (Fpn) inducing its internalization and degradation (1). Removal of Fpn from the cell surface leads to cellular iron retention and an increase in cellular ferritin, which stores the retained iron. Hepcidin is synthesized mainly by hepatocytes, but macrophages can also synthesize and secrete hepcidin (2). Hepcidin synthesis by the liver is increased by inflammation and iron stores and is decreased by hypoxia and anemia (2). Hepcidin, a member of the defensin family, has antimicrobial activity (3). The antimicrobial activity of hepcidin is due both to a direct effect on bacteria and to hepcidin's effect on iron homeostasis. High levels of hepcidin down-regulate cellular iron export into plasma leading to hypoferremia, which deprives bacteria of a required nutrient. Chronic inflammation and persistent hypoferremia leads to iron-restricted erythropoiesis. Conversely, low levels of hepcidin permit increased iron acquisition.
The recessive hemochromatosis disorders are diseases in which single gene defects lead to the absence of hepcidin or inadequate hepcidin secretion in response to iron stores (4). These disorders result in excessive iron acquisition, which may lead to organ damage. Mutations in Fpn also result in an inability to respond to adequate levels of hepcidin, leading to a dominant form of hemochromatosis (Fpn-linked hemochromatosis or Fpn-disease). Hepcidin binding to Fpn results in the phosphorylation of Fpn, a step necessary for Fpn internalization by clathrin-coated pits (5). Here we demonstrate that the kinase responsible for the phosphorylation of Fpn is Janus kinase 2 (Jak2). Binding of Jak2 to Fpn is highly cooperative and requires that hepcidin bind to both monomers of the Fpn dimer and that both monomers be capable of activating Jak2.
Results
Jak2 Is Required for Hepcidin-Mediated Fpn Internalization.
Hepcidin-mediated internalization of Fpn requires the phosphorylation of either of 2 adjacent tyrosines (Y302,Y303) on a cytosolic loop of cell surface Fpn (5). Analysis of the amino acid sequence surrounding these tyrosines suggests that they represent a potential Jak2 phosphorylation site. To determine if Jak2 is required for hepcidin-mediated Fpn phosphorylation, we used a human fibrosarcoma cell line (γ2A) that does not express Jak2 mRNA or protein (ref. 6 and Fig. 1). Transfection of mutant cells (γ2A) or the WT parent (2C4) with a plasmid expressing Fpn with a carboxyl terminal GFP (Fpn-GFP) under the control of the CMV promoter resulted in the expression of cell surface Fpn-GFP. Incubation of WT cells with hepcidin resulted in Fpn-GFP internalization and degradation, as shown by both fluorescence and Western analysis. In contrast, Fpn-GFP was not internalized in γ2A cells.
Fig. 1.
Jak2 is required for hepcidin-mediated Fpn internalization. WT (2C4) and Jak2 deficient cells (γ2A) were transfected with a plasmid containing a CMV-regulated Fpn-GFP. Eighteen hours posttransfection, cells were incubated in the presence or absence of 1.0 μg/ml hepcidin for 4 or 24 h. Fpn-GFP localization was determined by epifluorescence microscopy. Cells were lysed and protein samples were analyzed by Western blot using rabbit anti-Fpn, mouse anti-tubulin, or rabbit anti-Jak2 followed by a peroxidase-conjugated goat anti-rabbit IgG or goat anti-mouse IgG.
We confirmed that Jak2 is the kinase responsible for hepcidin-mediated Fpn internalization although the use of siRNA. HEK293TFpn-GFP is a stable human cell line expressing Fpn-GFP under the control of the ecdysone inducible promoter (1). Incubation of these cells with the ecdysone analogue ponasterone results in the expression of cell surface Fpn-GFP. Cells were transfected with nonspecific or human Jak2 specific siRNA oligonucleotide pools, and 48 h later ponasterone was added to induce expression of Fpn-GFP. Silencing of Jak2 was effective as shown by Western blot analysis and did not affect the cell surface localization of Fpn-GFP (Fig. 2A). Addition of hepcidin led to the internalization and degradation of Fpn-GFP in cells transfected with nonspecific oligonucleotide pools but not in cells transfected with Jak2 specific siRNA. Hepcidin-induced internalization of Fpn-GFP was restored by transfecting cells with a plasmid containing CMV-mouse Jak2, which is resistant to human Jak2 siRNA (Fig. 2B).
Fig. 2.
Silencing of Jak2 in human cells prevents hepcidin-mediated Fpn internalization. (A) HEK293TFpn-GFP cells were transfected with either nonspecific siRNA (N.S.) or human Jak2 specific siRNA oligonucleotide pools using OligofectAMINE. Forty-eight hours later cells were induced to express Fpn-GFP, and after 18 h cells were incubated in the presence or absence of hepcidin. Fpn-GFP localization was examined by epifluorescence microscopy. Silencing was assessed by Western blot with tubulin as a loading control. (B) HEK293TFpn-GFP cells were silenced as in (A). Forty-eight hours later, cells were transfected with pCMV-mouse Jak2 and induced to express Fpn-GFP. After 18 h cells were incubated in the presence or absence of hepcidin and Fpn-GFP localization was examined by epifluorescence microscopy 1 hr later. The data were quantified by determining the percent of cells with internalized Fpn-GFP in 2 data sets of >100 cells each, and error bars indicate standard deviations.
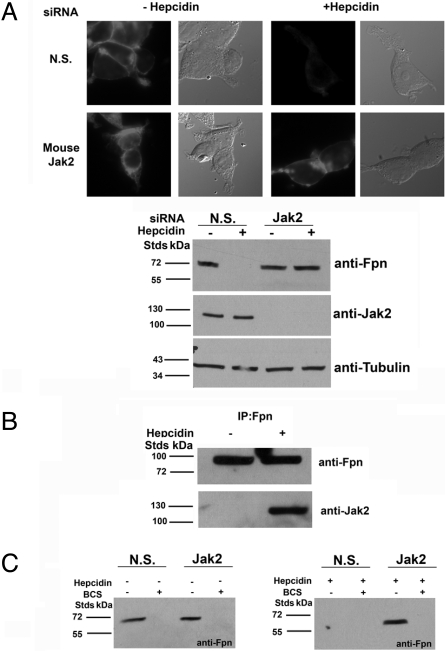
Macrophages recycle iron from aged or damaged red blood cells and play a critical role in iron homeostasis. Cultured murine bone marrow macrophages express low levels of Fpn but expression is increased by addition of iron (7, 8). Iron-treated macrophages were transfected with nonspecific siRNA or siRNA specific to mouse Jak2. Addition of hepcidin resulted in the internalization and degradation of Fpn in cells transfected with nonspecific siRNA but not in macrophages transfected with Jak2 specific siRNA (Fig. 3A). If Jak2 is required for hepcidin-induced Fpn-mediated internalization, we would expect that it must bind to Fpn. To examine that possibility, we immunoprecipitated Fpn in the absence or presence of hepcidin and probed the immunoprecipitate for Jak2. No Jak2 bound to Fpn in the absence of hepcidin could be detected but Jak2 bound to Fpn in the presence of hepcidin was detected. (Fig. 3B).
Fig. 3.
Silencing Jak2 in macrophages specifically inhibits hepcidin-mediated Fpn internalization. (A) Mouse bone marrow macrophages were transfected with either nonspecific (N.S.) or mouse Jak2 specific siRNA pools using OligofectAMINE. Forty-eight hours later, cells were incubated with FAC (10 μM Fe) to induce the expression of Fpn. After a further 24 h the cells were incubated in the presence or absence of hepcidin and Fpn localization was examined by immunofluorescence microscopy. Silencing efficiency was assessed by Western blot. (B) Mouse bone marrow macrophages were incubated with FAC (10 μM Fe) to induce the expression of Fpn and after 24 h cells were incubated in the presence or absence of 1 μg/ml hepcidin for 30 min. Cells were placed at 0 °C, solubilized, and samples immunoprecipitated with rabbit anti-Fpn antibodies. Immunoprecipitated samples were analyzed by Western blots probing for Jak2 or Fpn. (C) Cells as in (A) were incubated in the presence or absence of BCS (250 μM) for 18 h and levels were analyzed by Western blot.
Fpn expressed by macrophages and cultured glial cells is internalized and degraded in the absence of the multicopper oxidase ceruloplasmin (9). The ferroxidase activity of ceruloplasmin, by converting Fe(II) to Fe(III), provides the driving force for Fpn to export iron. In the absence of ceruloplasmin, Fpn cannot export iron and iron bound to Fpn signals the rapid internalization and degradation of Fpn in a hepcidin-independent process, which does not require phosphorylation of Fpn (8). To determine if Jak2 silencing affects hepcidin-independent internalization of Fpn, bone marrow macrophages were incubated with the copper chelator bathocuproinedisulfonic acid (BCS), which reduces intracellular copper concentrations and leads to synthesis of an inactive GPI-linked apoceruloplasmin. Macrophages silenced for Jak2 and incubated with BCS still showed loss of Fpn in the absence of hepcidin (Fig. 3C). Together these results show that Jak2 is necessary for hepcidin-mediated internalization of Fpn but not for hepcidin-independent internalization of Fpn.
Hepcidin-Induced Binding of Jak2 to Fpn Is Necessary but Not Sufficient for Fpn Internalization.
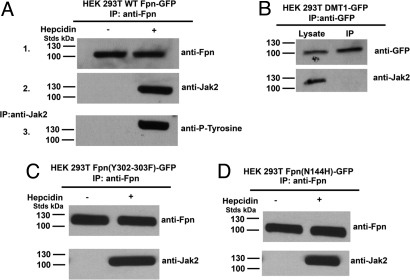
Studies on the mechanism of Jak2-induced internalization of growth factor and cytokine receptors have shown that binding of ligands induces the binding of Jak2 to the cytosolic tails of receptors (see refs. 10, 11 for review). Fpn is rapidly phosphorylated and internalized following hepcidin addition and binding of Jak2 to Fpn is expected to be transient. To ensure that we could examine cell surface events, we blocked endocytosis of Fpn-hepcidin using a dominant negative dynamin construct (K44A) to capture the binding of Jak2 to Fpn (5). Dynamin is a GTPase required for clathrin-mediated endocytosis (12). HEK293T cells were cotransfected with plasmids containing DynaminK44A and WT Fpn-GFP followed by immunoprecipitation of Fpn (Fig. 4A 1). Jak2 did not coprecipitate with Fpn in the absence of hepcidin but did coprecipitate upon addition of hepcidin (Fig. 4A 2). Fpn bound Jak2 was tyrosine phosphorylated, indicating that Jak2 is activated (Fig. 4A 3). We confirmed that the tyrosine phosphorylated molecule was Fpn by using a phosphotyrosine-specific Fpn antibody (Y302/Y303). The interaction of Jak2 with Fpn-GFP was specific, as Jak2 did not coimmunoprecipitate with overexpressed DMT1-GFP (Fig. 4B). These results show that the binding of Jak2 to Fpn is hepcidin dependent.
Fig. 4.
Hepcidin binding to Fpn leads to the binding and activation of Jak2. (A) HEK293T cells were cotransfected with plasmids containing Fpn-GFP and DynaminK44A and incubated in the presence or absence of 1.0 μg/ml hepcidin for 30 min. Cells were placed at 0 °C and solubilized. Samples were immunoprecipitated with rabbit anti-Fpn antibodies as described in Methods. Immunoprecipitated samples were analyzed by Western blots probed using rabbit anti-Fpn (1) or rabbit anti-Jak2 (2) followed by a peroxidase-conjugated goat anti-rabbit IgG. Samples from the Fpn immunoprecipitation were immunoprecipitated for a second time with rabbit anti-Jak2 antibodies. Immunoprecipitated samples were analyzed by Western blot using mouse anti-phosphotyrosine followed by a peroxidase-conjugated goat anti-mouse IgG (3). (B) HEK293T cells were transfected with plasmids containing DMT1-EGFP-N1 and DynaminK44A. Cells were solubilized and immunoprecipitated with rabbit anti-GFP antibodies. Immunoprecipitated samples were analyzed by Western blot using rabbit anti-Jak2 or rabbit anti-GFP followed by a peroxidase-conjugated goat anti-rabbit IgG. (C) HEK293T cells were cotransfected with plasmids containing Fpn(Y302–303F)-GFP and Dynamin K44A. Cells were incubated in the presence or absence of 1 μg/ml hepcidin for 30 min, solubilized and immunoprecipitated with rabbit anti-Fpn antibodies. Immunoprecipitated samples were analyzed by Western blot using rabbit anti-Jak2 or rabbit anti-Fpn followed by a peroxidase-conjugated goat anti-rabbit IgG. (D) HEK293T cells were cotransfected with plasmids containing Fpn(N144H)-GFP and Dynamin K44A. Cells were incubated in the presence or absence of 1 μg/ml hepcidin for 30 min, solubilized, and immunoprecipitated with rabbit anti-Fpn antibodies. Immunoprecipitated samples were analyzed by Western blots using rabbit anti-Jak2 or rabbit anti-Fpn followed by a peroxidase-conjugated goat anti-rabbit IgG.
We next determined whether binding of Jak2 to Fpn occurs if Fpn cannot be phosphorylated. Cells were transfected with a construct of Fpn in which tyrosines 302 and 303 were mutated to phenylalanines. Fpn(Y302–303F)-GFP binds hepcidin but is not phosphorylated or internalized (5). Jak2 did not coimmunoprecipitate with Fpn(Y302–303F)-GFP in the absence of hepcidin but did coprecipitate with Fpn(Y302–303F)-GFP in the presence of hepcidin (Fig. 4C). We previously characterized a human Fpn mutant (N144H) responsible for hepcidin resistant iron-overload disease that bound hepcidin but was not phosphorylated or internalized (13). Immunoprecipitation of Fpn(N144H)-GFP from transfected cells showed Jak2 bound to Fpn in the presence but not absence of hepcidin (Fig. 4D). Further, Jak2 bound to Fpn (N144H) and Fpn (Y302–303F) but was not phosphorylated (see below). These results indicate that Jak2 activation subsequent to binding requires conformational changes in Fpn. In particular, the Y302–303F mutations may have effects beyond the loss of a kinase target. These data demonstrate that hepcidin binding is required for Jak2 binding to Fpn but binding of Jak2 does not require phosphorylation of target tyrosines.
Binding of Jak2 to Fpn Requires Two Functional Hepcidin Binding Sites on Fpn.
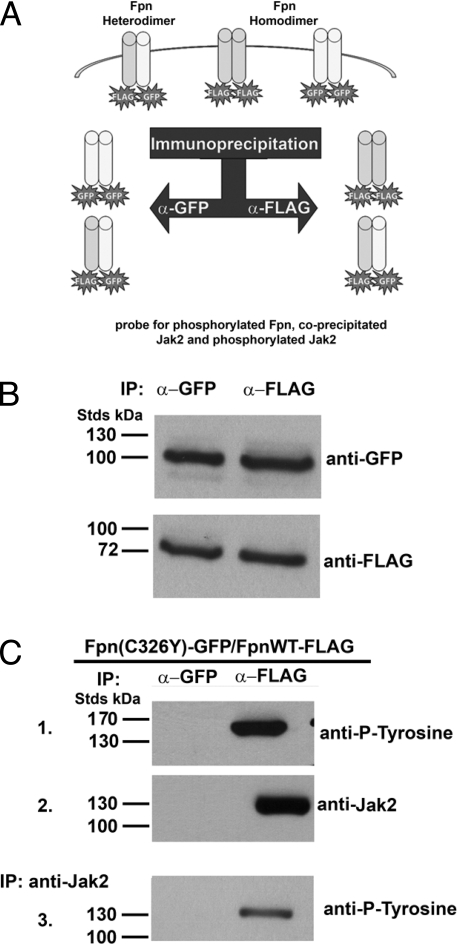
Fpn-linked hemochromatosis is transmitted as a dominant trait because Fpn is a dimer, based on size exclusion chromatography (13) and crosslinking studies (14). The product of the mutant allele participates in dimer formation and the behavior of the mutant Fpn affects the behavior of WT/mutant heterodimers. To determine if Jak2-mediated Fpn phosphorylation required the participation of both Fpn monomers, we used a known human mutant Fpn. The C326Y Fpn mutation results in the loss of hepcidin binding (15) and hepcidin resistant iron-overload disease (16). The hepcidin-binding domain of Fpn can be recapitulated in a chemically synthesized peptide of 19 aa, indicating that hepcidin can bind to monomeric Fpn (15). To determine if a single hepcidin binding site on dimeric Fpn is sufficient for Jak2 binding, cells were cotransfected with plasmids expressing WT Fpn-FLAG and Fpn(C326Y)-GFP. Transfected cells were incubated with hepcidin and samples immunoprecipitated with either anti-FLAG or anti-GFP antibody and the immunoprecipitates were then examined for Jak2 and for Jak2 activation (Fig. 5A). FLAG antibodies immunoprecipitated Fpn-FLAG and Fpn-GFP. Similarly, GFP antibodies immunoprecipitated Fpn-GFP and Fpn-FLAG (Fig. 5B). The Fpn-FLAG immunoprecipitate showed Fpn-phosphorylation and bound Jak2 (Fig. 5C). (Phosphorylated Fpn migrates slower than expected in SDS/PAGE; ref, 5). Samples were run under nonreducing conditions because reduction and heat treatment results in an inability to detect Fpn). Eluates from the Fpn-FLAG immunoprecipitation were then immunoprecipitated with an antibody specific for Jak2 and that immunoprecipitate was probed for tyrosine phosphorylation of Jak2 (Fig. 5C). The Jak2 immunoprecipitate showed phosphorylated Jak2, demonstrating that Jak2 was activated. Anti-GFP antibody immunoprecipitated Fpn(C326Y)-GFP homodimers and Fpn(C326Y)-GFP/Fpn-FLAG heterodimers (Fig. 5C). Jak2 was not found associated with the GFP specific immunoprecipitated Fpn. Lack of bound Jak2 demonstrates that both monomers of Fpn must bind hepcidin for Jak2 to bind to Fpn.
Fig. 5.
Two hepcidin binding sites on the Fpn dimer are required for Jak2 binding. (A) Schematic of experimental design. Cells were transfected with plasmids expressing Fpn-FLAG and mutant Fpn-GFP. Cells were incubated in the presence or absence of hepcidin and then immunoprecipitate with either anti-FLAG or anti-GFP antibodies. The immunoprecipitates were then analyzed for Fpn, phospho-Fpn, Jak2, and phospho-Jak2. (B) HEK293T cells were transiently transfected with plasmids containing WT Fpn-FLAG and Fpn(C326Y)-GFP. Cells were incubated in the presence of 1 μg/ml hepcidin for 30 min, solubilized, and immunoprecipitated with rabbit anti-GFP or anti-FLAG antibodies. The immunoprecipitates were analyzed by Western blot using anti-FLAG or anti-GFP antibodies. (C) Immunoprecipitated samples were analyzed by Western blot using mouse anti-phosphotyrosine (1) or rabbit anti-Jak2 followed by a peroxidase-conjugated goat anti-mouse or goat anti-rabbit IgG (2). A second immunoprecipitation was performed with rabbit anti-Jak2 and the immunoprecipitation samples were analyzed by Western blot using mouse anti-phosphotyrosine followed by a peroxidase-conjugated goat anti-mouse IgG (3).
We used a similar approach to determine if both Fpn monomers are required to participate in Jak2 binding and phosphorylation following hepcidin binding. Cells cotransfected with Fpn-FLAG and either Fpn(Y302–303F)-GFP or Fpn(N144H)-GFP were incubated with hepcidin and cell lysates immunoprecipitated with either anti-FLAG or anti-GFP. Immunoprecipitation of WT Fpn-FLAG homodimers showed the presence of phosphorylated Fpn, Jak2, and phosphorylated Jak2 (Fig. 6A Anti-FLAG). Immunoprecipitated Fpn(Y302–303F)-GFP or Fpn(N144H)-GFP homodimers and Fpn(Y302–303F)-GFP or Fpn(N144H)-GFP/Fpn-FLAG heterodimers coprecipitated Jak2 but Fpn was not phosphorylated nor was Jak2 phosphorylated (Fig. 6 A and B Anti-GFP). These results show Jak2 activation and Fpn phosphorylation require 2 functionally competent Fpn monomers, suggesting that Jak2 activation is highly cooperative. The highly cooperative nature of Jak2 activation provides a molecular explanation for the dominant transmission of hepcidin resistant iron overload disease.
Fig. 6.
Hepcidin-mediated activation of Fpn bound Jak2 is highly cooperative. (A) HEK293T cells were transiently transfected with WT Fpn-FLAG and Fpn(Y302–303F)-GFP expressed under a CMV promoter. Cells were incubated in the presence or absence of 1 μg/ml hepcidin for 30 min, solubilized, and immunoprecipitated with rabbit anti-GFP or anti-FLAG antibodies as in Fig. 5. Immunoprecipitated samples were analyzed by Western blot using mouse anti-phosphotyrosine (1) or rabbit anti-Jak2 (2) followed by a peroxidase-conjugated goat anti-mouse or goat anti-rabbit IgG. A second immunoprecipitation was performed as in (A) and Jak2 phosphorylation assessed using mouse anti-phosphotyrosine followed by a peroxidase-conjugated goat anti-mouse IgG (panel 3). (B) HEK293T cells were transiently transfected with WT Fpn-FLAG and Fpn(N144H)-GFP expressed under a CMV promoter. Cells were incubated in the presence or absence of 1 μg/ml hepcidin for 30 min, solubilized, and immunoprecipitated with rabbit anti-GFP or anti-FLAG antibodies. Immunoprecipitated samples were analyzed by Western blots using mouse anti-phosphotyrosine (1) or rabbit anti-Jak2 (2) followed by a peroxidase-conjugated goat anti-mouse or goat anti-rabbit IgG. A second immunoprecipitation was performed as in (A) and Jak2 phosphorylation assessed using mouse anti-phosphotyrosine followed by a peroxidase-conjugated goat anti-mouse IgG (3).
Discussion
Hepcidin, first identified as an antimicrobial peptide, regulates iron metabolism through its ability to down-regulate Fpn (1). We show that Jak2 is the kinase that phosphorylates Fpn in response to hepcidin, thereby identifying the molecular basis of Fpn down-regulation. The binding and activation of Jak2 to cytokine and growth factor receptors following ligand administration is well recognized (11, 17). Fpn differs from cytokine and growth factor receptors as it is a polytopic membrane protein and not a single transmembrane protein. The binding of Jak2 to polytopic proteins, however, is not without precedent as Jak2 binds to G protein coupled receptors such as the cholecystokinin receptor (18), angiotensin II receptor (19), and thyroid-stimulating hormone receptor (20). There is also precedent for phosphorylation of plasma membrane ion channels by Jak2. Jak2 phosphorylation of transmembrane ion channels has been shown to regulate the activity of the Na+, K+ Cl− transporter and the Na+/H+ exchanger (21, 22). Jak2-mediated phosphorylation of these transporters is usually found as a secondary consequence of a ligand-induced receptor event. For example, activation of the prolactin receptor leads to Jak2 phosphorylation of the Na+, K+ Cl− transporter (23). To our knowledge the binding of hepcidin to Fpn resulting in the downregulation of Fpn is the first instance of a ligand binding directly inducing Jak2-mediated phosphorylation of an ion channel.
We demonstrated that Jak2 is required for hepcidin-mediated Fpn internalization using both RNAi and Jak2 mutant cells. We also showed that hepcidin-induced Jak2 binding to Fpn occurred in cell lines transfected with Fpn expressing plasmids and in macrophages. Macrophages are responsible for most of the iron exported into plasma. Studies on the binding of Jak2 to cytokine and growth factor receptors have shown that Jak2 binds to a multimeric receptor or induces the dimerization of monomeric receptors (24). Dimerization is critical for Jak2 activation as receptor bound Jak2 molecules reciprocally activate each other before phosphorylating the receptor to which they are bound. Previously, we showed that Fpn is a dimer and that Fpn missense mutants participate in dimer formation with WT Fpn (13, 14, 25). The finding that Fpn is a dimer provides an explanation for the dominant transmission of Fpn-linked hemochromatosis. All reported human Fpn mutations are missense mutations (26). In mice, the one reported example of Fpn-linked iron overload disease is a missense mutation (25). Mice that are heterozygous for a targeted gene deletion in Fpn show no phenotype (27). Mutations in Fpn lead to either of 2 phenotypes: (i) iron overload in macrophages and low serum transferrin saturation due to mutant Fpns that are not targeted to the cell surface or are transport incompetent; (ii) hepatocyte iron overload and high serum transferrin saturation due to mutant Fpns that are unable to respond to hepcidin (13, 16, 28, 29). Here we show that hepcidin resistance of Fpn can occur when one of the monomers is unable to bind hepcidin (Fig. 7). Alternatively, hepcidin resistance can result from mutations that permit Fpn to bind hepcidin, which promotes the binding of Jak2, but Jak2 is unable to be activated. The inability of mutant WT Fpn heterodimers to be phosphorylated results in their retention on the cell surface and continued iron export into plasma. The highly cooperative nature of Jak2-mediated Fpn phosphorylation provides a mechanistic explanation for the dominant inheritance of hepcidin resistant Fpn disease.
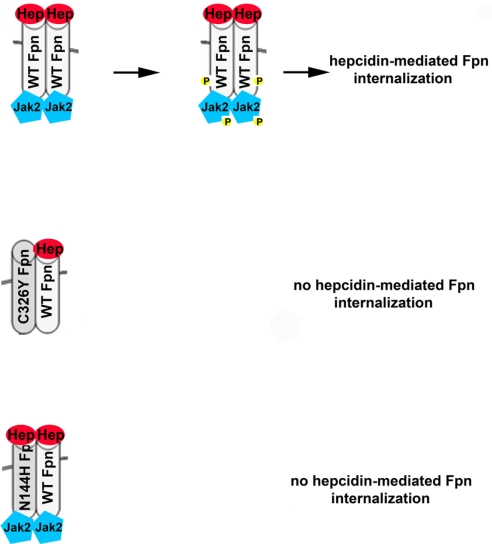
Fig. 7.
Model for hepcidin-resistant Fpn internalization. Fpn is a dimer and both monomers must bind hepcidin for Jak2 to bind to Fpn. Once Jak2 is bound, it is phosphorylated and then phosphorylates Fpn, which is the signal for Fpn internalization. Missense mutants of Fpn can participate in Fpn dimer formation. An inability to bind hepcidin (FpnC326Y) in one monomer will prevent Jak2 binding to a WT mutant heterodimer, resulting in retention of Fpn on the cell surface. Mutant Fpn that can bind hepcidin (FpnN144H) may lead to Jak2 binding; however, Jak2 is not activated and will not phosphorylate the WT mutant heterodimer. This results in retention of Fpn on the cell surface and continued iron export.
Methods
Cells and Media.
Human embryonic kidney HEK293T cells were maintained in DMEM with 10% FBS and transfected with Fpn-EGFP-N1 or Fpn(mutations)-EGFP-N1, CMV-Fpn-FLAG, Dynamin-EGFP, DynaminK44A-EGFP, or DMT1-EGFP-N1 by using Nucleofector technology (Amaxa) according to the manufacturer's directions. Human fibrosarcoma 2C4 and γ2A cells were maintained in DMEM with 10% FBS and transfected using Nucleofector technology. HEK293T Fpn is a stable cell line in which Fpn-GFP expression is regulated by the ecdysone promoter, which has been described previously (1). Mouse bone marrow macrophages, isolated from femurs, were grown in RPMI 1640 with 20% equine serum for 4 days and adherent cells were further cultured in RPMI 1640 with 20% FBS and 30% L cell-conditioned medium. Cells were then incubated in RPMI 1640 with 20% FBS for 24 h before experimental manipulation. Cells were iron loaded by addition of ferric ammonium citrate (FAC) (10 μM iron) for 18 h.
Small Interfering RNA (siRNA) Transfection.
siRNA oligonucleotide pools matching selected regions of human or mouse JAK2 and nonspecific oligonucleotide pools were obtained from Dharmacon RNA Technologies. HEK293T cells or mouse bone marrow macrophages were transfected with siRNA oligonucleotides at a final concentration of 100 nM using OligofectAMINE reagent (Invitrogen).
Immunofluorescence.
Cells were fixed with 3.7% formaldehyde in PBS (pH 7.2), permeabilized in PBS containing 1% BSA and 0.1% saponin, and incubated with rabbit anti-Fpn (1:100) for 60 min at room temperature, followed by Alexa 594 conjugated goat anti-rabbit antibody (1:750; Molecular Probes) for 60 min at room temperature. Cells were visualized using an epifluorescence microscope (Olympus Inc.) with a 100X oil immersion objective. Images were acquired using Magnafire analysis software (Optronix).
Other Procedures.
Immunoprecipitation of Fpn-GFP or Fpn-FLAG was performed as described previously (5) using protein A/G resin (Santa Cruz Biotechnology) and rabbit anti-Fpn or anti-FLAG M2 affinity gel (Sigma-Aldrich). To determine if Fpn bound Jak2 is phosphorylated, the Fpn immunoprecipitate was further immunoprecipitated using rabbit anti-Jak2. The immunoprecipitate was heat denatured in the presence of β-2 mercaptoethanol. These conditions result in a denatured Fpn that does not enter a polyacrylamide gel (5), thus permitting the specific detection of phosphorylated Jak2. Immunoprecipitation of phosphotyrosine was performed using mouse anti-phosphotyrosine antibody (1:500; Calbiochem) and protein A/G resin (Santa Cruz Biotechnology). Immunoprecipitation of Jak2 was performed using rabbit anti-Jak2 (1:250; Cell Signaling). Western analysis was performed using either mouse anti-FLAG antibody (1:10,000; Sigma-Aldrich), rabbit anti-Fpn (1:1000), mouse anti-tubulin (1:1000; GeneTex), mouse anti-phosphotyrosine clone 16F4 (1:500; Calbiochem), or mouse anti-Jak2 (1:1000; Cell Signaling), followed by either peroxidase-conjugated goat anti-mouse Ig IgG (1:10,000; Jackson ImmunoResearch Laboratories) or peroxidase-conjugated goat anti-rabbit IgG (1:10,000; Jackson ImmunoResearch Labs). All Western blots were normalized for total protein concentration using the bicinchoninic acid assay (Pierce Chemical). All experiments were performed a minimum of 3 times.
Acknowledgments.
The authors express their appreciation to members of the Kaplan lab for critically reading the manuscript. This work is supported by National Institutes of Health Grant DK070947 to J.K. and Center of Excellence in Molecular Hematology Grant SP30 DK072437 to J.K.
Footnotes
The authors declare no conflict of interest.
References
- 1.Nemeth E, et al. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science. 2004;306:2090–2093. doi: 10.1126/science.1104742. [DOI] [PubMed] [Google Scholar]
- 2.Nemeth E, Ganz T. Regulation of iron metabolism by hepcidin. Annu Rev Nutr. 2006;26:323–342. doi: 10.1146/annurev.nutr.26.061505.111303. [DOI] [PubMed] [Google Scholar]
- 3.Verga Falzacappa MV, Muckenthaler MU. Hepcidin: Iron-hormone and anti-microbial peptide. Gene. 2005;364:37–44. doi: 10.1016/j.gene.2005.07.020. [DOI] [PubMed] [Google Scholar]
- 4.Beutler E. Hemochromatosis: genetics and pathophysiology. Annu Rev Med. 2006;57:331–347. doi: 10.1146/annurev.med.57.121304.131310. [DOI] [PubMed] [Google Scholar]
- 5.De Domenico I, et al. The molecular mechanism of hepcidin-mediated ferroportin down-regulation. Mol Biol Cell. 2007;18:2569–2578. doi: 10.1091/mbc.E07-01-0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Watling D, et al. Complementation by the protein tyrosine kinase JAK2 of a mutant cell line defective in the interferon-gamma signal transduction pathway. Nature. 1993;366:166–170. doi: 10.1038/366166a0. [DOI] [PubMed] [Google Scholar]
- 7.Delaby C, Pilard N, Goncalves AS, Beaumont C, Canonne-Hergaux F. Presence of the iron exporter ferroportin at the plasma membrane of macrophages is enhanced by iron loading and down-regulated by hepcidin. Blood. 2005;106:3979–3984. doi: 10.1182/blood-2005-06-2398. [DOI] [PubMed] [Google Scholar]
- 8.Knutson MD, Oukka M, Koss LM, Aydemir F, Wessling-Resnick M. Iron release from macrophages after erythrophagocytosis is up-regulated by ferroportin 1 overexpression and down-regulated by hepcidin. Proc Natl Acad Sci USA. 2005;102:1324–1328. doi: 10.1073/pnas.0409409102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Domenico I, Vaughn M, Yoon D, Kushner JP, Ward DM, Kaplan J. Zebrafish as a model for defining the functional impact of mammalian ferroportin mutations. Blood. 2007;110:3780–3783. doi: 10.1182/blood-2007-07-100248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ihle JN, Kerr IM. Jaks and Stats in signaling by the cytokine receptor superfamily. Trends Genet. 1995;11:69–74. doi: 10.1016/s0168-9525(00)89000-9. [DOI] [PubMed] [Google Scholar]
- 11.Carter-Su C, King AP, Argetsinger LS, Smit LS, Vanderkuur J, Campbell GS. Signalling pathway of GH. Endocr J. 1996;43(Suppl):S65–S70. doi: 10.1507/endocrj.43.suppl_s65. [DOI] [PubMed] [Google Scholar]
- 12.Damke H, Baba T, Warnock DE, Schmid SL. Induction of mutant dynamin specifically blocks endocytic coated vesicle formation. J Cell Biol. 1994;127:915–934. doi: 10.1083/jcb.127.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Domenico I, et al. The molecular basis of ferroportin-linked hemochromatosis. Proc Natl Acad Sci USA. 2005;102:8955–8960. doi: 10.1073/pnas.0503804102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Domenico I, Ward DM, Musci G, Kaplan J. Evidence for the multimeric structure of ferroportin. Blood. 2007;109:2205–2209. doi: 10.1182/blood-2006-06-032516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Domenico I, et al. The hepcidin-binding site on ferroportin is evolutionarily conserved. Cell Metab. 2008;8:146–156. doi: 10.1016/j.cmet.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 16.Schimanski LM, et al. In vitro functional analysis of human ferroportin (FPN) and hemochromatosis-associated FPN mutations. Blood. 2005;105:4096–4102. doi: 10.1182/blood-2004-11-4502. [DOI] [PubMed] [Google Scholar]
- 17.Darnell JE, Jr, Kerr IM, Stark GR. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science. 1994;264:1415–1421. doi: 10.1126/science.8197455. [DOI] [PubMed] [Google Scholar]
- 18.Ferrand A, et al. A novel mechanism for JAK2 activation by a G protein-coupled receptor, the CCK2R: Implication of this signaling pathway in pancreatic tumor models. J Biol Chem. 2005;280:10710–10715. doi: 10.1074/jbc.M413309200. [DOI] [PubMed] [Google Scholar]
- 19.Marrero MB, et al. Direct stimulation of Jak/STAT pathway by the angiotensin II AT1 receptor. Nature. 1995;375:247–250. doi: 10.1038/375247a0. [DOI] [PubMed] [Google Scholar]
- 20.Park ES, et al. Involvement of JAK/STAT (Janus kinase/signal transducer and activator of transcription) in the thyrotropin signaling pathway. Mol Endocrinol. 2000;14:662–670. doi: 10.1210/mend.14.5.0458. [DOI] [PubMed] [Google Scholar]
- 21.Garnovskaya MN, et al. Mitogen-induced activation of Na+/H+ exchange in vascular smooth muscle cells involves janus kinase 2 and Ca2+/calmodulin. Biochemistry. 2003;42:7178–7187. doi: 10.1021/bi034563+. [DOI] [PubMed] [Google Scholar]
- 22.Mukhin YV, et al. Bradykinin B2 receptors activate Na+/H+ exchange in mIMCD-3 cells via Janus kinase 2 and Ca2+/calmodulin. J Biol Chem. 2001;276:17339–17346. doi: 10.1074/jbc.M010834200. [DOI] [PubMed] [Google Scholar]
- 23.Selvaraj NG, Omi E, Gibori G, Rao MC. Janus kinase 2 (JAK2) regulates prolactin-mediated chloride transport in mouse mammary epithelial cells through tyrosine phosphorylation of Na+-K+-2Cl− cotransporter. Mol Endocrinol. 2000;12:2054–2065. doi: 10.1210/mend.14.12.0568. [DOI] [PubMed] [Google Scholar]
- 24.Rodriguez-Frade JM, Mellado M, Martinez AC. Chemokine receptor dimerization: Two are better than one. Trends Immunol. 2001;22:612–617. doi: 10.1016/s1471-4906(01)02036-1. [DOI] [PubMed] [Google Scholar]
- 25.Zohn IE, et al. The flatiron mutation in mouse ferroportin acts as a dominant negative to cause ferroportin disease. Blood. 2007;109:4174–4180. doi: 10.1182/blood-2007-01-066068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.De Domenico I, Ward DM, Musci G, Kaplan J. Iron overload due to mutations in ferroportin. Haematologica. 2006;91:92–95. [PMC free article] [PubMed] [Google Scholar]
- 27.Donovan A, et al. The iron exporter ferroportin/Slc40a1 is essential for iron homeostasis. Cell Metab. 2005;1:191–200. doi: 10.1016/j.cmet.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 28.Drakesmith H, et al. Resistance to hepcidin is conferred by hemochromatosis-associated mutations of ferroportin. Blood. 2005;106:1092–1097. doi: 10.1182/blood-2005-02-0561. [DOI] [PubMed] [Google Scholar]
- 29.Liu XB, Yang F, Haile DJ. Functional consequences of ferroportin 1 mutations. Blood Cells Mol Dis. 2005;35:33–46. doi: 10.1016/j.bcmd.2005.04.005. [DOI] [PubMed] [Google Scholar]