Abstract
In light of recent discoveries of many new species of poorly-studied organisms, we examine the biodiversity of mammals, a well known “charismatic” group. Many assume that nearly all mammal species are known to scientists. We demonstrate that this assumption is incorrect. Since 1993, 408 new mammalian species have been described, ≈10% of the previously known fauna. Some 60% of these are “cryptic” species, but 40% are large and distinctive. A substantial number persist only in areas undergoing rapid habitat destruction. Our findings suggest global animal and plant species diversity is badly underestimated even in well studied taxa. This implies even greater threats to ecosystem services and human well-being than previously assumed, and an increased need to explore, understand, and conserve Earth's living resources.
Keywords: biodiversity, extinction, new mammals
Today biology is in “a new age of discovery” (1). That age is characterized by the uncovering of vast new elements of biodiversity, which are the fundamental building blocks of ecosystems, and thus the provision of ecosystem goods and services. There are thousands of examples of unexpected discoveries of new taxa across broad taxonomic and geographic spectra, from extremophile bacteria in Yellowstone geysers to whole new ecosystems in the Pacific Ocean hydrothermal vents (2, 3). For example, the Census of Marine Life program has uncovered hundreds of new species (4). Similarly, recent work has shown that a “species” of skipper butterfly, Astraptes fulgerator was actually a complex of 10 species with distinct life histories, and that 16 species of “generalist” tropical parasitoid tachinid flies were actually 73 evolutionary lineages (as indicated by mitochondrial DNA barcoding) including many lineages specialized to attack different hosts (5, 6).
These findings are of much more than academic interest. Most of the focus in conservation has been on trying to preserve as much of species diversity as possible (7, 8). Although the equally critical need for population preservation is now recognized (9, 10), the diversity of species remains crucial as a source of populations that can assume more distinct ecological roles (e.g., as generalist or specialist predators) in a rapidly changing world. Previously unrecognized genetic diversity must therefore be evaluated so that biologists have some idea of what they must strive to preserve, and how to deploy their limited resources to reduce biodiversity loss.
Here, we evaluate discoveries of new species of mammals, an especially well-studied group. We first give the methods by which new mammalian diversity has been discovered. Then we review the taxonomic affiliations, range size, and patterns of geographic distribution of mammal species described since a comprehensive 1993 checklist (11). Finally, we discuss the significance of these findings for the status of biodiversity in general, the problems of maintaining it, and thus of the ecosystem services that depend upon that diversity.
What are the ways in which additional mammal diversity has been uncovered? We started with a thorough search for new species of mammals and created maps for all new species except for marine ones, from the literature (SI Appendix). Global patterns of species distribution were done using 10,000-km2 (2) grid cells, similar to our previous studies (10, 12, 13). The new mammal species we found were of three types. The first was morphologically distinct species found in previously poorly surveyed areas. The second, the result of using molecular genetic techniques, was discoveries that the geographic range of a well-known organism was actually the combined ranges of two or more cryptic species—one's not easily recognized by morphological features. The third type consists of species that had been considered subspecies and were newly elevated to specific status (again, often as the result of molecular genetic discoveries). Two of the most prominent recent cases involved giving specific status to populations of forest elephants in central Africa and orangutans in Borneo (14).
In this article we will deal only with the first two cases—if the third were considered we would be dealing with >1000 “new” species. We did not map new species of marine mammals, which include whales and dolphins. Even 250 years after taxonomists started formally naming new mammals, 408 new species (excluding those elevated subspecies), have been documented in the last 15 years, a surprisingly large number considering <4,800 mammal species had been described at the beginning of that period. The discoveries include 18 new genera such as a large bovid (Pseudoryx), a rodent (Cuscomys), a bat (Xeronycteris), and a primate (Rungwecebus), and a living representative of Diatomyidae, a family considered extinct for 11 million years (Fig. 1 and SI Appendix). The new species belong to 18 mammalian orders (Table 1). The newly-discovered species varied in size from a 3-g shrew-tenrec (Microgale jobihely) to the 100-kg soala antelope (Pseudoryx nghetinhensis), and include some remarkable creatures such as a pygmy sloth (Bradypus pygmaeus) from a Panamanian island, a “giant” muntjac (Megamuntiacus vuquangensis) from Vietnam, a white titi monkey (Callithrix mauesi) from a river near Manaus in Brazil, and the Solomons islands monkey-faced bat (Pteralopex taki). The number of new species among orders was not random, i.e., related to the order's total species richness. It was higher than expected for Primates, Chiroptera, Rodentia, and all orders that used to belong to marsupials; in contrast, it was less than expected in Soricomorpha, Artiodactyla, and Carnivora (χ2 goodnes of fit between expected and observed speciess richness order; X(2) = 40.32, df = 12, P < 0.001).
Fig. 1.
Examples of new species of mammals discovered since 1993. From top left to bottom right, Rungwecebus kipunji (Copyright 2006, Tim Davenport/World Conservation Society). Cuscomys ashanika [Reproduced with permission from Emmons (SI Appendix) (Copyright 1999, American Museum of Natural History)]. Bradypus pygmaeus (Copyright 2007, Bill Haycher/National Geographic Society). Mirza zaza (Copyright 2006, David Haring/Duke Lemur Center). Cebus queirozi [Reproduced with permission from Pontes et al. (SI Appendix) (Copyright 2006, Magnolia Press)]. Rhyncocyon udzunwensis [Reproduced with permission from Rovero et al. (ref. 17) (Copyright 2007, The Zoological Society of London)]. Macrotarsomys petteri [Reproduced with permission from Goodman and Saorimalala (SI Appendix) (Copyright 2005, Biological Society of Washington)]. Laonastes aenigmamus (Copyright 2007, David Redfield/Florida State University). Scotophilus marovaza [Reproduced with permission from Goldman et al. (SI Appendix) (Copyright 2006, Polish Academy of Sciences)]. Microgale jenkinsae [Reproduced with permission from Goldman et al. (ref. 18) (Copyright 2006, The Zoological Society of London)].
Table 1.
Taxonomic composition of the new species of mammals (excluding marine species) discovered since 1993
| Order | Families with new species | Genera with new species | New species | New species with restricted distribution | New species probably at at risk of extinction |
|---|---|---|---|---|---|
| Afrosoricida | 2 | 2 | 12 | 8 | 2 |
| Artiodactyla | 5 | 9 | 11** | 7 | 1 |
| Carnivora | 1 | 2 | 2* | 2 | 2 |
| Macroscelidae | 1 | 1 | 1 | 1 | 1 |
| Chiroptera | 8 | 44 | 94* | 75 | 6 |
| Cingulata | 1 | 1 | 1 | 1 | 0 |
| Dasyuromorpha | 1 | 4 | 6* | 2 | 0 |
| Didelphimorphia | 2 | 5 | 8* | 8 | 0 |
| Diprodontia | 2 | 6 | 11* | 11 | 2 |
| Erinaceomorpha | 1 | 1 | 1 | 1 | 0 |
| Lagomorpha | 2 | 3 | 5 | 3 | 0 |
| Monotremata | 1 | 1 | 1 | 1 | 0 |
| Paucituberculata | 1 | 1 | 1* | 1 | 1 |
| Peramelemorphia | 1 | 1 | 2* | 2 | 0 |
| Pilosa | 1 | 1 | 1 | 1 | 0 |
| Primates | 9 | 16 | 55* | 51 | 10 |
| Rodentia | 16 | 87 | 174* | 29 | 4 |
| Soricomorpha | 2 | 9 | 22** | 17 | 2 |
| TOTAL | 57 | 195 | 408 | 221 | 34 |
There are new taxa up to the family levels. Some orders have either more (*) or fewer (**) new species than expected by their species richness.
The discovery of some of these species has generated considerable interest within the scientific community. For example, both the recently described rodent species from the family Diatomyidae and genus Cuscomys were already known from paleontological and prehistoric remains, respectively. This is an instance of the “Lazarus effect” (15)—in which an organism known only from fossils is discovered alive. Remarkably, the diatomid species (Laonastes aenigmamus) and a new rabbit species (Nesolagus timminsi) were first discovered being sold as food in a market in a Laotian village (15, 16). It appears that exploration of new regions has been the main factor for the discovery of as much as 40% of the new species, such as the pygmy deer (Muntiacus putaoensis) in Bhutan, the Arunachal macaque (Macaca muzala) from the Himalaya foothills of northeast India, the Amazonian basin monkeys, and most of the new Philippines species (SI Appendix). The exploration of new regions has been based on both the use of either new techniques such as camera-traps, which were the first indication that there was a new giant elephant shrew (Rhynchocyon udewensis) in Tanzania (17), or traditional techniques, such as pitfall traps, which have yielded specimens of 8 new species of shrew-tenrecs from Madagascar since 1988 (18). Molecular techniques have revealed cryptic species across many orders. For bats and galago monkeys, discriminating among echolocation signals and vocalizations respectively have been key to identifying cryptic species (SI Appendix).
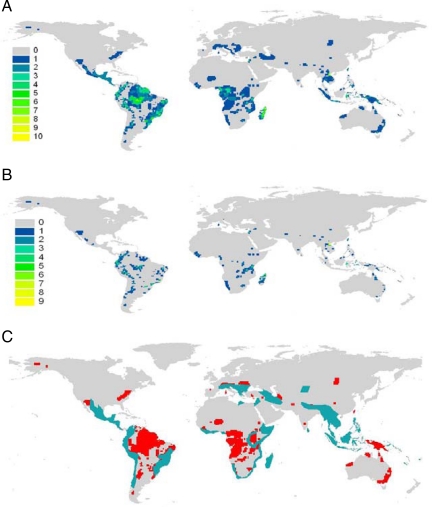
The patterns of distribution of new species are shown in Fig. 2, based on a global grid of some 17,000 10,000-km2 (2) terrestrial cells. The number of new species in a single cell varied from 1 to 10. New species have been discovered on all continents except Antarctica, with the majority in South America and Asia (SI Appendix). In the Americas, cells with one or two new species occur in temperate regions of Alaska, the eastern U.S., Chile, and Argentina, whereas cells with two species or more have been found throughout tropical and semitropical regions in Mexico and Central America, eastern Colombia, Peru and Ecuador, the Amazon basin, and the Atlantic forests of Brazil. Most new species on this continent are bats and primates.
Fig. 2.
Patterns of distribution in new species of mammals. (A) Species richness, n = 408. (B) Restricted-range species, n = 221. (C) Cells (in red) with new species located outside hotspots [in blue, sensu Myers (13)].
In Africa, most new species have been discovered in tropical regions, but some species have been found in arid regions in Western Sahara and Namibia; discoveries have been concentrated in eastern tropical forests of west Africa and the Congo Basin, from Liberia to Angola, the eastern mountains of Somalia, Kenya, and Tanzania, and Madagascar, where up to 3 new species have been discovered in some cells. Surprisingly, several new species have been discovered in Europe, mostly around the Mediterranean basin. New species in Asia are concentrated in the Malayan Peninsula, Indonesia, and New Guinea. The number of new species discovered in Philippines is rather remarkable.
On average these species had ranges of ≈87,000 km2 (2), significantly smaller compared with an average land mammal range of 400,000 km2 (2) (P < 0.0001). Indeed, 81% of the new species have very restricted ranges [i.e., <10,000 km2 (2)] (Fig. 2), which make them more prone to extinction. Interestingly, the distribution of newly discovered mammals often includes large areas not considered biodiversity hotspots (Fig. 3), which further indicates that conservation strategies to supplement the focus on hotspots are required (13, 19). Also interesting, and unexpected, is that the new mammal species were larger than average (P < 0.0001). This is primarily because few of the newly discovered species were either bats or rodents.
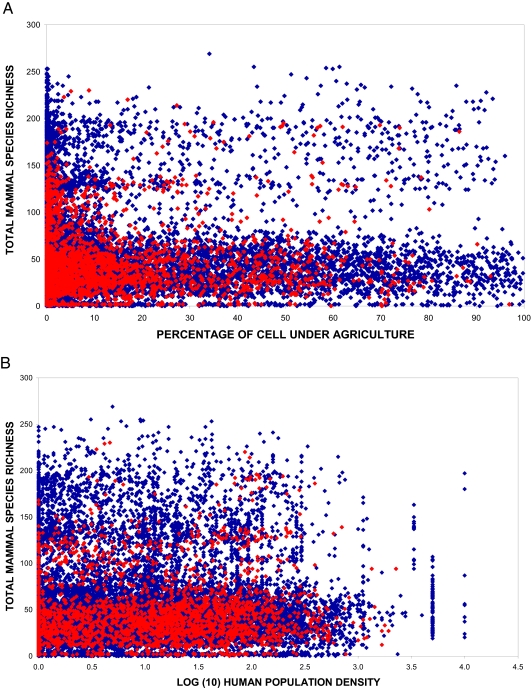
Fig. 3.
Anthropogenic threat in cells either with (red) or without (blue) new species of mammals measured by the percentage of the cell under agriculture (A) and its human population density (B).
Although most (61%, 1640) of the cells where new species have been found have relatively little anthropogenic threat, measured as both the area of the cell under agriculture and human population, 24% of the cells are located in cells with >10% of their land area under agriculture, including 12% of cells with >50% of agriculture (Fig. 3A). In contrast, most (46%) cells are in regions with low human population density [< 10 individuals per square kilometer (2)]; however, >20% are found in regions with relatively high human populations (Fig. 3B), indicating higher vulnerability. A very interesting example is the mammalian fauna discovered in a limestone karts outcrop in the the Kammaouan province, in the Lao People's Democratic Republic, which included a new family and 6 species, in a region completely isolated by agriculture (15).
The discoveries of new mammals are hardly unique (20, 21). Our analysis supports the anecdotal conclusions from butterflies, flies, and other organisms mentioned above. It suggests that other prominent taxa (e.g., birds and reptiles), and more obscure groups, likely contain many more species than are currently described. This could amount to millions of species and other distinct entities, greatly expanding estimates of the diversity of the living elements of Earth's natural capital (22), to even perhaps hundreds of millions of species. In addition, because 12% of Earth's land surface is used for crop agriculture, 25% is grazed by livestock, 2% has been paved or built on, 30% is exploited in other ways (23), our results suggest that many more unheralded organisms in all groups have likely recently gone extinct without being noticed. That implies that the levels of species extinction overall have been grossly underestimated. Thus, the situation is likely even worse than indicated by the steady rise of endangerment in the IUCN mammal statistics (8). Although it is common for estimates of total current plant and animal biodiversity to be in the tens of millions (24), those estimates are largely based on rates of discovery of morphologically defined species found in traditional surveys.
The problem of cryptic biodiversity, and the incompleteness of inventories of even charismatic organisms, is not usually considered. This is especially likely because the species now being discovered, as illustrated by mammals, tend to have limited distributions. For instance, the golden capuchin monkey (Cebus queirozi) was described in 2006, and is known to occur in a 200 ha remnant forest patch, isolated by sugar cane plantations (25). Similarly, the Solomon Islands flying fox (Pteralopex taki) was described in 2002 from 3 islands, and was already extinct on one of them (26). The lemur genus Microcebus, thought to consist of two species in 1982, has now been shown to comprise ≈13 cryptic species (27). It, of course, may have once contained many other cryptic species, all of which went extinct unheralded. This seems likely, considering the massive deforestation that has occurred on Madagascar and the inconspicuous character of many lemurs.
Population loss is also largely unrecorded, except when a well-defined subspecies goes extinct, as in the case of the satyrine butterfly Cercyonis sthenele sthenele that famously disappeared in the 1880s from San Francisco sand dune habitats (28) or the more recent loss of the Caspian, Balinese, and Javan tiger subspecies (Panthera tigris virgata, P. t. balica, P. t. sondaica) and the well-publicized near extinctions of the Asian cheetah (Acinonyx jubatus venaticus) and Florida panther (Puma concolor coryi). In short, there has probably been substantial cryptic loss of population biodiversity over much of the planet even in well-studied groups (10).
Several commentators have suggested that the discovery of “new species” is problematic for conservation—especially “taxonomic inflation” (raising of subspecies to specific status and uncovering of cryptic species) (29). We and others disagree (30). There is little need to focus on taxonomic rank when what needs to be preserved are the numbers and diversity of biological entities. For example, it is important to know that most tachinid flies in Costa Rica are host specialists. Whether they are counted as “good species” or “mitochondrial lineages” makes no scientific difference. Conserving one of those tachinid lineages, for instance, may preserve a crucial biological control agent. The key thing is that in an ideal world we should conserve all such units, regardless of appellation, keeping the loss rate not significantly above the “background” rate.
Many newly discovered entities may supply previously unrecognized ecosystem services. For example, a recent study has shown that the abundance of a hantavirus-prone rodent species and hantavirus infection rates are negatively correlated with the number of native rodent species in Panamanian tropical forests (31). Loss of such native taxa can thus potentially have negative effects on human health and welfare. Furthermore, the role of large mammals in regulating the trophic and architectural properties of ecosystems has become even clearer with the recent investigations of the impacts of large herbivores (32). Such results underscore the often-neglected point that conserving biodiversity over broad areas is essential to maintaining ecological function and critical ecosystem services (7, 9, 10).
However, no one is in a position to decide the full conservation value of any species, charismatic or not, let alone the other more or less distinct entities now being revealed. This moves the “rivet popper hypothesis” to a new level (33). Scientists know that there is some functional redundancy in the species composition of most ecosystems (34). However, the level of that redundancy may be generally overrated, as research on the buffering of ecosystem processes by diversity demonstrates (35).
In response to these problems, what should be the strategy of conservation biologists? It goes without saying that they should try to preserve as many genetically distinct species as possible. It is also crucial that the number and diversity of populations—many of which are clearly more genetically and ecologically differentiated than previously thought—and the ecosystem services they provide, also be preserved and, where possible, restored. The whole issue of triage needs to be revisited—triage decisions may be required, but they will involve vast scientific, socioeconomic, and political uncertainties. Also vexed will be issues of “where to draw the line” (because most individuals are genetically distinct and we can not preserve everything) (36). The more diversity that is discovered the more urgent becomes putting additional resources into understanding and finding ways to conserving it. The insufficiency to date of ethical and esthetic arguments for preserving biodiversity means that ecosystem service based approaches, typified by countryside biogeography and the Natural Capital Project, must be expanded (37). This is especially the case in the face of increasing threats to virtually all organisms, which are experiencing rapid climate, land conversion, and extensive toxic pollution—threats that now extend to areas previously considered protected, of marginal value, or remote.
Finding the political will to attain such goals will not be easy, but the survival of civilization may well hang on a cultural evolutionary sea change, and how much of societies resources get allocated to the task. Considering the complexity and uncertainty of the relationships between biodiversity and the delivery of ecosystem services, conservation decisions should include a very large precautionary principle bias toward protection of as many of our living companions as possible.
Supplementary Material
Acknowledgments.
We thank our friends Peter Raven, Rob Pringle, and Watt Ward for helpful comments on a previous draft of the manuscript; Ana Davidson and Navjot Sodhi for reviewing and helping us to improve the manuscript; Irma Salazar, Silvia Surhig, Pablo Ortega, and Regina Ceballos for kindly helping us with data and GIS analyses; and Tim Davenport, Louise Emmons, Bill Haycher, David Haringm, David Redfield, Francisco Rovero, Achille Raselimanana, and Steve M. Goodman for lending us their photographs. This work was supported by grants from Dirección General de Asuntos del Personal Académico of the Universidad Nacional Autónoma de Mexico, Comisión nacional para el conocimiento y uso de la biodiversidad (Mexico), and the Mertz Gilmore Foundation.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0812419106/DCSupplemental.
References
- 1.Donoghue MJ, Alverson WS. A new age of discovery. Ann Missouri Botanical Gardens. 2000;87:110–126. [Google Scholar]
- 2.Coriss JB. Submarine thermal springs on the Galápagos Rift. Science. 1979;203:1073. doi: 10.1126/science.203.4385.1073. [DOI] [PubMed] [Google Scholar]
- 3.Yooseph S, et al . The Sorcerer II: Global ocean sampling expedition: Expanding the universe of protein families. PLoS Biol. 2007;5:e16. doi: 10.1371/journal.pbio.0050016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Census of Marine Life Portal. [Accessed August 20, 2008];2008 www.coml.org/medres/highlights2006/AnnHigh06_low_res_revised.pdf.
- 5.Hebert PDN, et al. Ten species in one: DNA barcoding reveals cryptic species in the neotropical skipper butterfly Astraptes fulgerator. Proc Natl Acad Sci USA. 2004;101:14812–14817. doi: 10.1073/pnas.0406166101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith MA, Wood DM, Janzen DH, Hallwachs W, Hebert PDN. DNA barcodes affirm that 16 species of apparently generalist tropical parasitoid flies (Diptera, Tachinidae) are not all generalists. Proc Natl Acad Sci USA. 2007;104:4967–4972. doi: 10.1073/pnas.0700050104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ricketts TH, et al. Pinpointing and preventing imminent extinctions. Proc Natl Acad Sci USA. 2005;51:18497–18501. doi: 10.1073/pnas.0509060102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.International Union for the Conservation of Nature and Natural Resources. The 2008 IUCN Red List of Threatened Species. Gland, Switzerland: IUCN; 2008. [Google Scholar]
- 9.Hughes JB, Daily GC, Ehrlich PR. Population diversity: Its extent and extinction. Science. 1997;278:689–692. doi: 10.1126/science.278.5338.689. [DOI] [PubMed] [Google Scholar]
- 10.Ceballos G, Ehrlich PR. Mammal population losses and the extinction crisis. Science. 2002;296:904–907. doi: 10.1126/science.1069349. [DOI] [PubMed] [Google Scholar]
- 11.Wilson DE, Reeder DAM. Mammal Species of the World: A Taxonomic and Geographic Perspective. 2nd and 3rd Eds. Baltimore: Johns Hopkins Univ Press; 1993. [Google Scholar]
- 12.Ceballos G, Ehrlich PR, Soberón J, Salazar I, Fay JP. Global mammal conservation: What must we manage? Science. 2005;309:603–607. doi: 10.1126/science.1114015. [DOI] [PubMed] [Google Scholar]
- 13.Ceballos G, Ehrlich PR. Global biodiversity hotspots and conservation: Insights from mammal distributions. Proc Natl Acad Sci USA. 2006;103:19374–19379. doi: 10.1073/pnas.0609334103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roca AL, Georgiadis N, Pecon-Slattery J, O'Brien SJ. Genetic evidence for two species of elephant in Africa. Science. 2001;293:1473–1477. doi: 10.1126/science.1059936. [DOI] [PubMed] [Google Scholar]
- 15.Dawson MR, Marivaux L, Li C, Beard KC, Metais G. Laonastes and the “Lazarus Effect” in recent mammals. Science. 2006;311:1456–1458. doi: 10.1126/science.1124187. [DOI] [PubMed] [Google Scholar]
- 16.Averianov AO, Abramov AV, Tikhonov AN. A new species of Nesolagus (Lagomorpha, Leporidae) from Vietnam with osteological description. Contributions Zool Inst St. Pettersburg. 2000;3:1–22. [Google Scholar]
- 17.Rovero F, et al. A new species of giant sengi or elephant-shrew (genus Rhynchocyon) highlights the exceptional biodiversity of the Udzungwa Mountains of Tanzania. J Zool. 2008;274:126–133. [Google Scholar]
- 18.Goodman SM, Raxworthy CJ, Maminirima CP, Olson LE. A new species of shrew tenrec (Microgale jobihely) from northern Madagascar. J Zool. 2006;270:384–398. [Google Scholar]
- 19.Orme CD, et al. Global hotspots of species richness are not congruent with endemism or threat. Nature. 2005;436:1016–1019. doi: 10.1038/nature03850. [DOI] [PubMed] [Google Scholar]
- 20.Reeder DAM, Helgen KM, Wilson DE. Global trends and biases in new mammal species discoveries. Occas Pap Mus Texas Tech Univ. 2007;269:1–35. [Google Scholar]
- 21.Kholer J, Vieites DR, Bonett RM. New amphibians and global conservation: A boost in species discoveries in a highly endangered vertebrate group. Bioscience. 2005;55:693–696. [Google Scholar]
- 22.Ehrlich PR, Wilson EO. Biodiversity studies: Science and policy. Science. 1991;253:758–762. doi: 10.1126/science.253.5021.758. [DOI] [PubMed] [Google Scholar]
- 23.Ehrlich PR, Ehrlich AH. The Dominant Animal: Human Evolution and the Environment. Washington, D.C.: Island; 2008. [Google Scholar]
- 24.Erwin TR. How many species are there? Conservation Biol. 1991;5:330–333. [Google Scholar]
- 25.Mendes Pontes AR, Malta A, Asfora PH. A new species of capuchin monkey, genus Cebus Erxleben (Cebidae, Primates): Found at the very brink of extinction in the Pernambuco Endemism Centre. Zootaxa. 2006;1200:1–12. [Google Scholar]
- 26.Helgen KM. Systematics of the Pacific monkey-faced bats (Chiroptera: Pteropodidae), with a new species of Pteralopex and a new Fijian genus. Systemat Biodiversity. 2005;3:433–453. [Google Scholar]
- 27.Tattersall I. Paleoanthropology: The last half-century. Evol Anthropol. 2000;16:12–23. [Google Scholar]
- 28.Scott JA. The Butterflies of North America: A Natural History and Field Guide. Stanford, CA: Stanford Univ Press; 1986. [Google Scholar]
- 29.Isaac NJB, Mallet J, Mace GM. Taxonomic inflation: Its influence on macroecology and conservation. Trends Ecol Evol. 2004;19:464–469. doi: 10.1016/j.tree.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 30.Agapow PM, Sluys R. The reality of taxonomic change. Trends Ecol Evol. 2005;20:278–280. doi: 10.1016/j.tree.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 31.Suzan G, et al. Epidemiological considerations of rodent community composition in fragmented landscapes in Panama. J Mammal. 2008;89:684–690. [Google Scholar]
- 32.Pringle RM, Young TP, Rubenstein DI, McCauley DJ. Herbivore-initiated interaction cascades and their modulation by productivity in an African savanna. Proc Natl Acad Sci USA. 2007;104:193–197. doi: 10.1073/pnas.0609840104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ehrlich PR, Ehrlich AH. Extinction: The Causes and Consequences of the Disappearance of Species. New York: Random House; 1981. [Google Scholar]
- 34.Ehrlich PR, Walker B. Rivets and redundancy. BioScience. 1998;48:387. [Google Scholar]
- 35.Hobbs RJ, Yates , Mooney HA. Long-term data reveal complex dynamics in grassland in relation to climate and disturbance. Ecol Monogr. 2007;77:545–568. [Google Scholar]
- 36.Kareiva P, Levin S. The Importance of Species: Perspectives on Expendability and Triage. Princeton, NJ: Princeton Univ Press; 2002. [Google Scholar]
- 37.Turner RK, Daily GC. The ecosystem services framework and natural capital conservation. Environ Res Econ. 2008;39:25–35. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.