Abstract
Increasing evidence demonstrates that interleukin (IL)–32 is a pro-inflammatory cytokine, inducing IL-1α, IL-1β, IL-6, tumor necrosis factor (TNF)–α, and chemokines via nuclear factor (NF)–κB, p38 mitogen-activated protein kinase (MAPK), and activating protein (AP)-1 activation. Here we report that IL-32 is expressed and is also functional in human vascular endothelial cells (EC) of various origins. Compared with primary blood monocytes, high levels of IL-32 are constitutively produced in human umbilical vein EC (HUVEC), aortic macrovascular EC, and cardiac as well as pulmonary microvascular EC. At concentrations as low as 0.1 ng/ml, IL-1β stimulated IL-32 up to 15-fold over constitutive levels, whereas 10 ng/ml of TNFα or 100 ng/ml of lipopolysaccharide (LPS) were required to induce similar quantities of IL-32. IL-1β–induced IL-32 was reduced by inhibition of the IκB kinase-β/NF-κB and ERK pathways. In addition to IL-1β, pro-coagulant concentrations of thrombin or fresh platelets increased IL-32 protein up to 6-fold. IL-1β and thrombin induced an isoform-switch in steady-state mRNA levels from IL-32α/γ to β/ε. Adult EC responded in a similar fashion. To prove functionality, we silenced endogenous IL-32 with siRNA, decreasing intracellular IL-32 protein levels by 86%. The knockdown of IL-32 resulted in reduction of constitutive as well as IL-1β–induced intercellular adhesion molecule–1 (ICAM-1) (of 55% and 54%, respectively), IL-1α (of 62% and 43%), IL-6 (of 53% and 43%), and IL-8 (of 46% and 42%). In contrast, the anti-inflammatory/anti-coagulant CD141/thrombomodulin increased markedly when IL-32 was silenced. This study introduces IL-32 as a critical regulator of endothelial function, expanding the properties of this cytokine relevant to coagulation, endothelial inflammation, and atherosclerosis.
Keywords: Atherosclerosis, Coagulation, Inflammation
Interleukin (IL)–32 appears to play a role in inflammation by inducing IL-1β, IL-6, IL-8 (CXCL8), and tumor necrosis factor (TNF)–α via the p38MAPK, nuclear factor (NF)–κB, and activating protein (AP)-1 signal transduction pathways (1, 2). The sources of IL-32 include natural killer (NK) cells, T cells, monocytes, and epithelial cells. Although six isoforms, IL-32α to ζ, have been described (1, 3), any functional differences between these isoforms remain unknown. It is recognized, however, that IL-32α and IL-32γ are prominently expressed in human peripheral blood mononuclear cells (PBMC) stimulated with killed Mycobacterium tuberculosis (4). Overexpression of human IL-32β in mice increased the levels of IL-1β, IL-6, and TNFα, which was associated with a worsening of collagen-induced arthritis and sulfonic acid–mediated colitis (5). In addition, IL-32γ induced type I interferons and inhibited human immunodeficiency virus (HIV)–1 production in latently infected promonocytic U1 cells (2). In the latter report, it was demonstrated that knockdown of endogenous IL-32 by siRNA in HIV-1–infected PBMC resulted in a marked decrease in Th1 cytokines and chemokines (IL-12, IFNγ, IP-10 (CXCL10), I-TAC (CXCL11), MIP-1α/β (CCL3/4)), Th17 cytokines (IL-17, IL-23), as well as IL-1β, IL-6, TNFα, CD40L, and C5a. Th2 and anti-inflammatory cytokines were less affected. IL-32 also appears to possess intrinsic anti-viral activity due to induction of IFNα (2). Anti-viral activity of IL-32 was confirmed by another group (6): Infection with influenza A led to in vitro production of IL-32 and, similar to HIV-1, IL-32 inhibited influenza virus replication (7). Although the antiviral properties of IL-32 against this virus were mediated by COX-2, it remains unclear whether IL-32 directly inhibits influenza replication.
Immunohistochemical staining of IL-32 in pulmonary epithelial cells and alveolar macrophages revealed a correlation with disease severity in chronic obstructive pulmonary disease (8). In psoriatic lesions, capillary endothelial cells (EC) stained positive for IL-32 (11). In Crohn disease (9), ulcerative colitis (10), and rheumatoid arthritis, similar associations between IL-32 expression and disease severity have also been reported (12–14). In addition, increased levels of this cytokine have been demonstrated in bone marrow stromal cells from patients with myelodysplastic syndrome, where IL-32 appears to facilitate hematopoietic stem cell death (15).
In the present study, we investigated the role of IL-32 in EC and compared the production of IL-32 in human umbilical vein (HUVEC), aortic macrovascular (AEC), and cardiac as well as pulmonary microvascular EC (CMEC and PMEC, respectively). We were particularly interested in the effects of the EC activators thrombin, IL-1β, and LPS on expression of the splice variants of IL-32 mRNA as well as of the IL-32 protein. In addition, the effects of inhibiting the IκB kinase (IKK)-β/NF-κB and the ERK pathways were studied. Silencing of IL-32 with specific siRNA was used to establish the extent, if any, of a functional role for IL-32 in the production of intercellular adhesion molecule (ICAM)–1, CD141/thrombomodulin (TM), and pro-inflammatory cytokines.
Results
IL-32 Is Constitutively Expressed in HUVEC and Is Augmented by Thrombin, Platelets, IL-1β, and LPS.
To establish the presence of IL-32 in endothelial cells, we investigated IL-32 protein production in HUVEC by Western blotting. As shown in Fig. 1A, IL-32 protein was constitutively expressed in these cells. Production was markedly increased when HUVEC were stimulated with IL-1β or LPS, whereas this effect was less pronounced upon treatment with IFNγ.
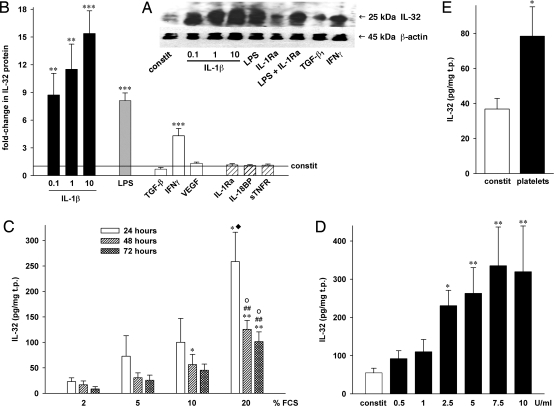
Fig. 1.
Expression and regulation of IL-32 in HUVEC. HUVEC were incubated with the indicated stimuli and/or inhibitors for 20 hours (A, B, D, E) or for the periods indicated (C). Concentrations (in ng/ml) were as follows: LPS, 100; TGF-β1, 20; IFNγ, 25; VEGF, 25; IL-1Ra, 10000; IL-18BP, 1000; sTNFR, 10000. (B) Mean fold-changes in IL-32 protein levels normalized to total protein (in mg) ± SEM; n = 9; *P < 0.05; **P < 0.01; and ***P < 0.001 for stimulated vs. untreated cultures. Normalized absolute IL-32 concentrations ranged from 28 to 269 pg/mg in controls and from 581 to 4025 pg/mg in IL-1β– (10 ng/ml) stimulated cells. (A) One representative of five independently performed Western blots of HUVEC cell lysates is shown. (C) HUVEC were cultured with different concentrations of FCS. Normalized absolute IL-32 levels ± SEM are shown; n = 5; *P < 0.05; and **P < 0.01 for 2% vs. 10 or 20%; ##P < 0.01 for 5% vs. 20%; oP < 0.05 for 10% vs. 20%; ♦, 20% at 24 hours vs. 20% at 72 hours. (D) IL-32 protein after incubation with the indicated concentrations of thrombin, mean ± SEM; n = 5; *P < 0.05; and **P < 0.01 for constitutive vs. thrombin. (E) Effect of incubation of HUVEC with 50 × 106 platelets on IL-32 is depicted. n = 3; *P < 0.05 for constitutive vs. platelets.
Next, we quantified the intracellular levels of IL-32 protein by a specific immunoassay (Fig. 1B). Although there was variability in different preparations of HUVEC, in general, constitutive levels ranged from 6 to 369 pg/mg total protein (t.p., not shown). The overall average in untreated HUVEC was 57 pg/mg. However, IL-32 production increased to more than 4000 pg/mg in IL-1β–stimulated cultures.
Stimulation with IL-1β resulted in a dose-dependent increase in IL-32 protein levels of more than 15-fold (Fig. 1B). Although this increase was observed at concentrations as low as 100 pg/ml (9-fold), a 1000 times higher concentration of LPS (100 ng/ml) induced a similar level of IL-32 (8-fold increase). Next, we incubated EC with the cytokine antagonists IL-1 receptor antagonist (IL-1Ra), IL-18 binding protein (IL-18BP), and soluble TNF receptor (sTNFR) to determine whether expression of IL-32 was dependent on intermediate production of IL-1, IL-18, or TNFα. However, there was no reduction in LPS-stimulated cells (data not shown), nor in constitutive protein levels of IL-32 (Fig. 1B).
IFNγ was also an inducer of IL-32 production (4-fold increase), whereas vascular endothelial growth factor (VEGF) was not. Treatment of EC with TGF-β1 resulted in a moderate decrease in IL-32 production, which did not reach significance.
As shown in Figs. 1C and 1D, we observed that increasing concentrations of fetal calf serum (FCS) as well as thrombin induced IL-32. Although the effect of FCS was dose dependent, with the greatest increase at 20% FCS (11-fold at 24 hours compared with 2% FCS), IL-32 levels did not increase with time. In fact, after reaching peak levels at 24 hours, protein synthesis waned and at 72 hours only 34–45% of IL-32 at 24 hours was present in the EC lysates. In these cultures, we also tested whether FCS-induced IL-32 was dependent on IL-1. In the presence of saturating concentrations of IL-1Ra, this was not the case (data not shown). Thrombin dose dependently stimulated HUVEC to synthesize IL-32 with a maximum increase of 6-fold at 7.5 U/ml (Fig. 1D). In addition, we incubated HUVEC with human platelets and also observed a 2-fold increase in IL-32 production (Fig. 1E).
To verify bioactivity of the stimuli (IL-1β, LPS, IFNγ, thrombin, platelets, TGF-β1) and the inhibitors (IL-1Ra, IL-18BP, sTNFR), we measured IL-1α, IL-6, and IL-8 in the cultures from which the figures were generated. Each of the stimuli was active and specifically inhibited by the antagonists (data not shown).
Expression and Regulation of IL-32 in AEC, CMEC, and PMEC.
Because HUVEC are a fetal cell population, we examined whether constitutive production of IL-32 and its regulation are similar in EC obtained from human adults. Indeed, constitutive IL-32 was found in the adult EC populations at levels comparable to HUVEC, although some differences were evident. Fig. 2 demonstrates that constitutive levels of IL-32 protein were lowest in PMEC (average of 4 pg/mg). Lysates of CMEC (21 pg/mg) and AEC (38 pg/mg) contained intermediate amounts of IL-32 compared with HUVEC, which averaged 57 pg/mg.
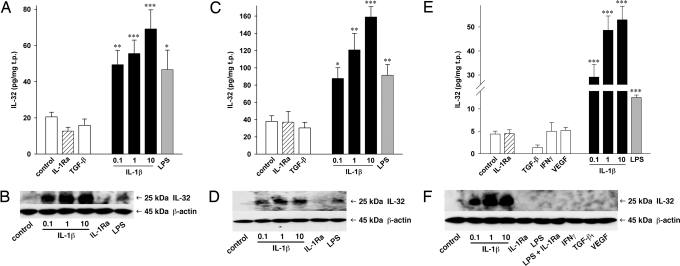
Fig. 2.
IL-32 production in EC of different origins. AEC (A, B), CMEC (C, D), and PMEC (E, F) were treated with the indicated stimuli (concentrations as in Fig. 1) or IL-1Ra (10 μg/ml) for 20 hours in the presence of 2% FCS. “Control” indicates constitutive production of IL-32. IL-1β concentrations are displayed in ng/ml. Cell lysates were harvested and IL-32 protein levels were determined. (A, C, E) The absolute IL-32 concentrations normalized to total protein are shown. Mean ± SEM; n = 4 for AEC and n = 8 for CMEC and PMEC; *P < 0.05; **P < 0.01; and ***P < 0.001 for treated cells vs. controls. (B, D, F) One of four blots resulting from independent experiments from each cell type is shown.
In each EC type, IL-1β was the most potent inducer of IL-32 synthesis. The increases triggered by 10 ng/ml of IL-1β ranged from 3- and 4-fold in CMEC and AEC, respectively, to 12-fold in PMEC. For comparison, the maximum increase in HUVEC was 15-fold (Fig. 1). A 100 ng/ml quantity of LPS and a 10 ng/ml quantity of TNFα (data not shown) also augmented production of IL-32, and the increases were comparable to 0.1 ng/ml of IL-1β. IFNγ, TGF-β1, and VEGF failed to induce IL-32 in adult ECs. The addition of IL-1Ra to the cultures did not affect LPS-induced or constitutive IL-32 production; thus LPS-induced IL-32 is independent of IL-1 activity.
Differential Regulation of Different IL-32 Isoforms in EC.
As expected from the increases in IL-32 protein after stimulation with IL-1β or thrombin, we observed an increase in total IL-32 mRNA. Whereas in HUVEC (Fig. 3A) and PMEC (data not shown) there was more than 20-fold more IL-32 mRNA 24 hours after stimulation with IL-1β, a maximal increase of 7-fold was present in CMEC (Fig. 3B). The maximum up-regulation after thrombin stimulation was about 6-fold and appeared to occur more slowly than after IL-1β.
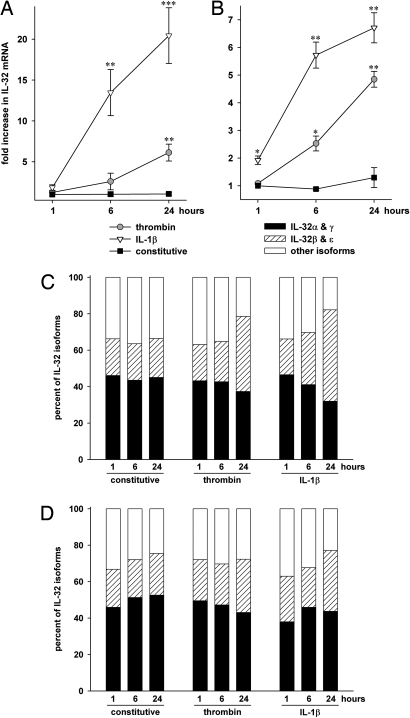
Fig. 3.
Expression and regulation of IL-32 mRNA isoforms in EC. Two hours after changing from growth- to stimulation medium, HUVEC (A, C) or CMEC (B, D) were stimulated with 10 ng/ml IL-1β or 7.5 U/ml thrombin for the indicated periods of time or were left untreated. Relative mRNA quantities were calculated using the ΔΔCT method. (A, B) Constitutive mRNA levels at 1 hour are defined as background. Mean fold increases ± SEM in all isoforms of IL-32 mRNA over background are shown; n = 4; *P < 0.05; **P < 0.01; and ***P < 0.001 for treated cells vs. controls. (C, D) The fractions of IL-32α/γ, β/ε, and other isoforms are depicted for each stimulation condition. The levels of all isoforms for each sample were set at 100%; n = 4.
To date, six different splice variants of the mRNA of IL-32, termed α, β, γ, δ, ε, and ζ, have been described. As demonstrated in Figs. 3C and 3D, the most prominently expressed isoforms in unstimulated EC are IL-32α and γ, which together account for 40% (in PMEC, data not shown) to 53% (in CMEC, Fig. 3D) of total IL-32 mRNA. IL-32β and ε are considerably less in untreated EC, ranging from 19% to 23%. However, the fraction of the latter isoforms markedly increased when the cells were stimulated with IL-1β for 24 hours. This change was most pronounced in HUVEC, where after stimulation with IL-1β or thrombin IL-32β and ε accounted for 50% and 41% of total IL-32 mRNA, respectively. The average increases in IL-32β and ε were 23-fold (after thrombin) and 69-fold (after IL-1β) compared with 7- and 20-fold for IL-32α and γ. Although this isoform switch also occurred in PMEC where the fraction of IL-32β and ε increased from 19% to 37%, it was less pronounced in CMEC (23% vs. 29% and 33%, Fig. 3D).
Silencing Endogenous IL-32 in HUVEC.
To demonstrate functionality of IL-32 in EC, we reduced endogenous IL-32 in HUVEC by specific siRNA. Supporting information (SI) Fig. S1 shows that constitutive and IL-1β–induced IL-32 protein levels were up to 86% lower by siRNA to IL-32 (siIL-32) compared with control cultures transfected with scrambled RNA. In general, the effects of 333 nM siRNA were not greater than 100 nM. Thus, with the exception of the cytokine studies (see Fig. 5), we conducted subsequent experiments with 100 nM siRNA.
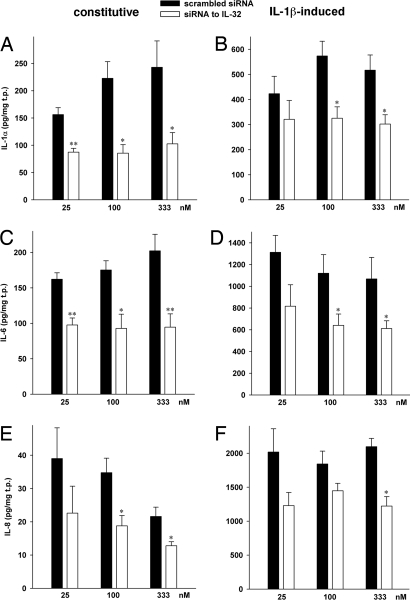
Fig. 5.
The effects of siIL-32 on cytokine levels in HUVEC. Cell lysates (A, B) or supernatants (C–F) from unstimulated (Left column) or IL-1β-treated (10 ng/ml, Right column) HUVEC transfected with concentration-matched pairs of either siIL-32 or scrambled siRNA were analyzed for protein levels of IL-1α (A, B), IL-6 (C, D), and IL-8 (E, F) after a 20-hour incubation period. IL-32 levels in these cultures are shown in Fig. S1 A and B. Means of normalized absolute cytokine concentrations ± SEM are shown; n = 10; *P < 0.05; and **P < 0.01 for siIL-32 compared with scrambled.
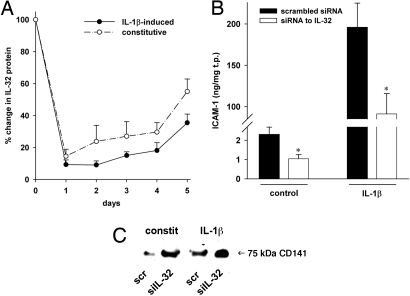
As shown in Fig. 4A, the siRNA-mediated knockdown was sustained several days. After 2 days, IL-32 protein levels were still at the same low levels as on day 1 in IL-1β–treated cells. This duration was also observed in unstimulated cells, as the recovery was slow (85% knockdown after 1 day, 76% after 2 days). On day 4, the reduction in IL-32 protein was still greater than 70% and 81% in unstimulated and IL-1β–transfected cells, respectively; a significant recovery of IL-32 production occurred only on day 5.
Fig. 4.
Knockdown of IL-32 and its effect on ICAM-1 and thrombomodulin/CD141. (A) After transfection and stimulation with 10 ng/ml IL-1β, EC were cultured for the indicated time periods and IL-32 was measured in cell lysates. The graph depicts mean percent changes in IL-32 protein levels comparing siIL-32-transfected to scrambled-transfected cells ± SEM; n = 3. (B, C) HUVEC transfected with 100 nmol/l of either siIL-32 or scrambled were stimulated with 10 ng/ml IL-1β for 20 hours (B) or 30 minutes (C) or left untreated. (B) ICAM-1 was assayed by ELISA in cell lysates. Means of normalized protein concentration ± SEM in ng/mg is shown; n = 6; *P < 0.05 for siIL-32 compared with scrambled. (C) One representative of four Western blots of HUVEC lysates is depicted. The degree of silencing in these lysates is shown in Fig. S1 A and B.
Endogenous IL-32 Is Functional in EC.
When endogenous IL-32 protein levels were reduced by siIL-32, we observed a reduction in constitutive (by 55%) as well as IL-1β–induced ICAM-1 production (by 54%) compared with scrambled-transfected cultures (Fig. 4B). In contrast, TM/CD141 increased considerably when IL-32 was silenced in both untreated and IL-1β–stimulated HUVEC (Fig. 4C).
In addition to these modulators of endothelial function, we measured the production of IL-1α, IL-6, and IL-8. As shown in Figs. 5A–5F, silencing of IL-32 had the greatest effect on IL-1α and IL-6, which revealed reductions of 62% and 53%, respectively, whereas IL-8 was reduced by up to 46%. For each of the three cytokines, the siIL-32–induced reduction was less pronounced in the presence of IL-1β, with maximal inhibition at 43% (Figs. 5B, 5D, and 5F).
IL-32 Is Mainly Induced via the IKK-β/NF-κB Pathway.
Because of the common Toll-IL-1-receptor (TIR) domain, IL-1 and LPS trigger similar intracellular signaling pathways. To elucidate which of these pathways are involved in the induction of IL-32 in EC, we preincubated HUVEC cultures with increasing concentrations of inhibitors of IKK-β and NF-κB as well as of the MAPkinases JNK, MEK (and thus indirectly ERK1/2/5), and p38 (SP600125, PD98059, and SB203580, respectively). For each inhibitor, we used a concentration close to its IC50 as a starting point. As shown in Fig. S2, IL-1β–induced IL-32 production was reduced in the presence of inhibitors of IKK-β (60%) or NF-κB (58%) activation. A comparison between the NF-κB and the IKK-β (the upstream kinase of NF-κB) inhibitors revealed that the concentrations needed to reduce IL-32 production were lower when the transcription factor itself was the target (IL-32 reductions at the lowest concentrations were 46% vs. 16%, respectively). Whereas the JNK and unexpectedly also the p38 MAPK inhibitors failed to inhibit IL-32, there was a moderate but significant effect when PD98059 was applied (40% reduction).
Although with regard to signaling pathway use the induction of IL-32 by LPS resembled that by IL-1β, the MEK/ERK inhibitor affected background IL-32 expression to a greater degree than IL-1β– or LPS-stimulated IL-32 in addition to the IKK-β and NF-κB inhibitors (data not shown).
Discussion
In this study, we report the contribution of IL-32 to endothelial cell function and hence to systemic inflammation. In contrast to IL-32 levels in PBMC (1, 2, 4), NK cells (1), lung epithelial A549 cells (1, 7), and HIV-1–infected U1 cells (a cell line derived from U937) (2), we observed impressively high levels of constitutive and IL-1β–inducible IL-32 in EC. LPS also stimulated IL-32, but 1000-fold higher concentrations were required to induce levels comparable to IL-1β. Unexpectedly, IL-1β and thrombin induced an isoform switch from IL-32α/γ to β/ε. It is unclear at the present time whether this change has functional implications, which may be important for the selection of the relevant isoform to block in vivo. Moreover, we observed a large fraction of non-α, -β, -γ, and -ε IL-32 mRNA. This may be an indication that the role of IL-32δ and ζ could be greater than previously assumed.
The importance of IL-1 in endothelial biology and vessel wall inflammation was first investigated by the studies of Mantovani et al. in 1985 (16). Since then, a growing body of evidence supports the concept that the endothelium is a primary target by which IL-1β exerts its pro-inflammatory effects in systemic inflammation (17–19). For instance, because the endothelium is the first compartment encountered by s.c. administered IL-1Ra, blocking IL-1 receptors on the endothelium with the antagonist results in a rapid and sustained reduction of disease severity in IL-1β–driven systemic inflammation (20). Moreover, IL-1 triggers the production of PGE, IL-6, IL-8, clotting factors, inhibitors of fibrinolysis, and adhesion molecules in the endothelium, each of which contributes to pathological conditions. For example, IL-1β–induced IL-6 stimulates hepatic acute phase protein synthesis (e.g., C-reactive protein [CRP]) and induces thrombocytosis (17). IL-1β also plays an aggravating role in the atherosclerotic process and in neointima formation. In the latter case, IL-1β stimulates vascular smooth muscle cell (VSMC) migration as well as cytokine production (21) and recruits monocytes to the injured site via up-regulation of adhesion molecules and chemokines. These immunocompetent cells in turn synthesize growth factors such as fibroblast growth factor (FGF), which promote uncontrolled EC and vascular smooth muscle cell (VSMC) proliferation (22). Furthermore, IL-1 has been suggested to be involved in the pathogenesis of pulmonary hypertension (23, 24) and chronic obstructive pulmonary disease (25).
The major impact of the present report is the unexpected finding that a new cytokine, IL-32, mediates a considerable component of the pro-coagulant, pro-inflammatory, and cytokine effects of IL-1β on EC. Namely, when IL-32 levels were reduced by approximately 80% by siRNA, IL-1β–stimulated production of ICAM-1, IL-1α, IL-6, and IL-8 decreased by up to 62%, whereas expression of CD141/TM increased. These results suggest that IL-32 may function as a major effector cytokine of IL-1 signals in the endothelium. This hypothesis is supported by the fact that concentrations of IL-1β as low as 0.1 ng/ml were highly effective in inducing IL-32, which in turn was produced at unprecedented concentrations by EC.
The role of endogenous IL-32 for constitutive as well as IL-1β–induced ICAM–1 and CD141/TM may be of pivotal importance to endothelial biology. ICAM-1 and its close relative VCAM-1 recruit circulating leukocytes to sites of inflammation by facilitating the adherence of these cells to the endothelium, which is followed by extravasation and chemokine-directed migration of these cells to injured tissues. Hence, ICAM-1 participates in the initiation and perpetuation of inflammation in virtually all tissues (26–28), including the endothelium itself and thus atherosclerosis (29, 30). ICAM-1 can also activate target cells (31, 32). Furthermore, ICAM-1 is involved in different aspects of tumor pathophysiology: whereas cells from some metastasizing cancers use ICAM-1 as a homing receptor (33), other tumors exhibit reduced surface expression of ICAM-1 on their blood vessels, thus limiting access of immune cells.
Thrombomodulin was discovered as an important cofactor in the activation of the major naturally occurring anti-coagulant protein C (PC) (34). After cleavage by the thrombin/TM/endothelial protein C receptor complex, PC has also been shown to exert a variety of anti-inflammatory (35, 36) and cytoprotective (36, 37) activities. However, TM also features thrombin- and PC-independent anti-inflammatory activities. In addition to sequestering pro-inflammatory thrombin (38), TM interferes with complement activation and alleviates arthritis (39). Moreover, mice lacking the lectin-like domain of TM exhibited reduced survival after endotoxin challenge as well as larger infarcts after ischemia/reperfusion. The latter was due to the lack of TM-mediated suppression of neutrophil adhesion to EC and of the activation of NF-κB and ERK1/2 (40). Low-expressing polymorphisms of the TM gene are associated with an increased risk of myocardial infarction and/or atherosclerosis (41, 42). With regard to the latter disease, retroviral delivery of TM to mechanically dilated femoral arteries ameliorated disease severity in a rabbit model (43). In addition, high levels of TM were associated with improved survival in several cancers (44).
By augmenting ICAM-1 but reducing TM, IL-32 likely affects major endothelial functions; leukocyte adhesion and activation are enhanced, whereas anti-inflammatory and anti-coagulant mechanisms, including the activation of PC, are impaired. We furthermore infer that IL-32 is a pathogenic factor in atherosclerosis. This notion is not only supported by the IL-32–mediated regulation of ICAM-1 and TM but also by the data regarding cytokine regulation, as synthesis of IL-1α, IL-6, and IL-8 was partly dependent on IL-32.
Each of these effector cytokines is well known to promote atherogenesis. For example, IL-6 contributes to thrombocytosis during systemic diseases; furthermore, one of the Framingham studies found a correlation between elevations in IL-6 and mortality (45). IL-8 has been identified as a culprit in progression of atherosclerosis along with several other chemokines, as these chemokines recruit immune cells to the endothelial intima during various stages of plaque development (46). The role of IL-1α in the endothelium is particularly interesting: radiation-treated mice transplanted with IL-1α−/− bone marrow showed decelerated development of the formation of atherosclerotic lesions (47). IL-1α promotes senescence in EC (48) and can regulate the migratory properties of these cells (49). In patients, higher levels of IL-1α and IL-1β produced by monocytes after percutaneous transluminal coronary angioplasty were associated with a higher risk of restenosis (50). Finally, each of these three cytokines is also relevant in other vascular diseases; for example, one report demonstrated elevated levels of IL-1α, IL-6, and IL-8 in samples from abdominal aortic aneurysms (51).
In summary, this study highlights IL-32 as a major player on the endothelial stage. Because IL-32 regulates constitutive as well as IL-1β–induced ICAM-1, TM, and pro-inflammatory cytokines in each of the EC types tested, this cytokine is likely to be relevant in atherosclerosis and coagulation, as well as in endothelial aspects of cancer and inflammation.
Materials and Methods
Reagents.
Please see SI Text.
Cell Culture.
AEC, CMEC, and PMEC were obtained from Lonza (Walkersville, MD). CMEC and PMEC were cultured in EGM-2MV (Lonza) with a final concentration of 5% FCS. AEC were grown in EGM-2 with a final concentration of 2% FCS.
The HUVEC were isolated from human umbilical cords (see SI Text) after informed consent was obtained from the parents. These experiments were approved by the Colorado Multiple Institutional Review Board. For stimulation of EC, the medium was changed to endothelial cell stimulation medium (M199 (Gibco) with 2% FCS, 10 ng/ml human acidic FGF (Peprotech), 1% penicillin/streptomycin (P/S), and 18 U/ml heparin).
Transfections.
HUVEC were detached, counted, centrifuged at 200 g for 12 minutes, and subjected to electroporation using the Amaxa HUVEC Nucleofector Kit and program U001. One cuvette contained 0.8 × 106 cells in 100 μl Nucleofector solution and 25–333 nM of either siIL-32 or scrambled. Immediately after electroporation, the cells were incubated in 300 μl prewarmed M199 for 5 minutes. Thereafter, 0.2 × 106 cells were transferred into gelatinized six-well plates to a final volume of 1 ml growth medium and allowed to recover overnight. On the next day, the medium was replaced with stimulation medium and the cells were stimulated.
Isolation of and Stimulation with Platelets.
Venous blood was drawn into Na-citrate tubes and centrifuged at 180 g at room temperature for 20 minutes without braking. The resulting plasma was transferred to a 15-ml tube and platelets were washed twice in PBS without Ca and Mg. Thereafter, platelets were resuspended with a repeater tip, counted, adjusted to 100 × 106/ml in stimulation medium, and added to EC cultures at a dilution of 1:2, resulting in a final concentration of 50 × 106/ml.
Electrochemiluminescence Assays, Enzyme-Linked Immunosorbent Assay, and Western Blot.
Please see SI Text.
RNA Isolation and Real-Time Polymerase Chain Reaction.
RNA was isolated using mirVana kits (Ambion, Austin, TX), followed by determination of RNA concentrations using a NanoDrop spectrophotometer (Thermo Scientific, Wilmington, DE). Reverse transcription and TaqMan real-time polymerase chain reactions (PCRs) were then performed using reagents (for details see SI Text) and devices by Applied Biosystems (Foster City, CA). Relative quantities were calculated by the ΔΔCT method (see SI Text).
Statistical Analysis.
Datasets (raw data) were first tested for normality by the Kolmogorov-Smirnov method and equal variance (p value to reject = 0.05) using the SigmaStat software (Systat Software Inc., San Jose, CA). Thereafter, these data were analyzed by the appropriate statistical test, using either the unpaired t test (α set at 0.05 in all cases), the Mann-Whitney rank sum test, or one-way analysis of variance (ANOVA).
Supplementary Material
Acknowledgments.
We are thankful for excellent technical assistance by Alaine Walborn and Hannah Oliver. This work was supported by National Institutes of Health grants AI-15614 and CA-04 6934 as well as by the Juvenile Diabetes Research Foundation grant 26–2008-893 (to C.A.D.); by funds provided by the V. Johnson Laboratory for obstructive lung disease research (to N.F.V.); and by the Deutsche Forschungsgemeinschaft grant 747/1–1 (to M.F.N.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0813334106/DCSupplemental.
References
- 1.Kim SH, et al. Interleukin-32: A cytokine and inducer of TNFalpha. Immunity. 2005;22:131–142. doi: 10.1016/j.immuni.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 2.Nold MF, et al. Endogenous IL-32 controls cytokine and HIV-1 production. J Immunol. 2008;181:557–565. doi: 10.4049/jimmunol.181.1.557. [DOI] [PubMed] [Google Scholar]
- 3.Goda C, et al. Involvement of IL-32 in activation-induced cell death in T cells. Int Immunol. 2006;18:233–240. doi: 10.1093/intimm/dxh339. [DOI] [PubMed] [Google Scholar]
- 4.Netea MG, et al. Mycobacterium tuberculosis induces interleukin-32 production through a caspase-1/IL-18/interferon-gamma-dependent mechanism. PLoS Med. 2006;3:e277. doi: 10.1371/journal.pmed.0030277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shoda H, et al. Interactions between IL-32 and tumor necrosis factor alpha contribute to the exacerbation of immune-inflammatory diseases. Arthritis Res Ther. 2006;8:R166. doi: 10.1186/ar2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rasool ST, et al. Increased level of IL-32 during human immunodeficiency virus infection suppresses HIV replication. Immunol Lett. 2008;117:161–167. doi: 10.1016/j.imlet.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 7.Li W, et al. Activation of interleukin-32 pro-inflammatory pathway in response to influenza A virus infection. PLoS ONE. 2008;3:e1985. doi: 10.1371/journal.pone.0001985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Calabrese F, et al. IL-32, a novel proinflammatory cytokine in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2008;178:894–901. doi: 10.1164/rccm.200804-646OC. [DOI] [PubMed] [Google Scholar]
- 9.Netea MG, et al. IL-32 synergizes with nucleotide oligomerization domain (NOD) 1 and NOD2 ligands for IL-1beta and IL-6 production through a caspase 1-dependent mechanism. Proc Natl Acad Sci USA. 2005;102:16309–16314. doi: 10.1073/pnas.0508237102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shioya M, et al. Epithelial overexpression of interleukin-32alpha in inflammatory bowel disease. Clin Exp Immunol. 2007;149:480–486. doi: 10.1111/j.1365-2249.2007.03439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dinarello CA, Kim SH. IL-32, a novel cytokine with a possible role in disease. Ann Rheum Dis. 2006;65(Suppl 3):iii61–iii64. doi: 10.1136/ard.2006.058511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cagnard N, et al. Interleukin-32, CCL2, PF4F1 and GFD10 are the only cytokine/chemokine genes differentially expressed by in vitro cultured rheumatoid and osteoarthritis fibroblast-like synoviocytes. Eur Cytokine Netw. 2005;16:289–292. [PubMed] [Google Scholar]
- 13.Edwards CJ, et al. Molecular profile of peripheral blood mononuclear cells from patients with rheumatoid arthritis. Mol Med. 2007;13:40–58. doi: 10.2119/2006-000056.Edwards. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Joosten LA, et al. IL-32, a proinflammatory cytokine in rheumatoid arthritis. Proc Natl Acad Sci USA. 2006;103:3298–3303. doi: 10.1073/pnas.0511233103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marcondes AM, et al. Dysregulation of IL-32 in myelodysplastic syndrome and chronic myelomonocytic leukemia modulates apoptosis and impairs NK function. Proc Natl Acad Sci USA. 2008;105:2865–2870. doi: 10.1073/pnas.0712391105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rossi V, et al. Prostacyclin synthesis induced in vascular cells by interleukin-1. Science. 1985;229:174–176. doi: 10.1126/science.2409598. [DOI] [PubMed] [Google Scholar]
- 17.Dinarello CA. Blocking IL-1 in systemic inflammation. J Exp Med. 2005;201:1355–1359. doi: 10.1084/jem.20050640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Introna M, et al. Pro- and anti-inflammatory cytokines: Interactions with vascular endothelium. Clin Exp Rheumatol. 1994;10(Suppl):S19–23. [PubMed] [Google Scholar]
- 19.Voelkel NF, Tuder R. Interleukin-1 receptor antagonist inhibits pulmonary hypertension induced by inflammation. Ann N Y Acad Sci. 1994;725:104–109. doi: 10.1111/j.1749-6632.1994.tb39794.x. [DOI] [PubMed] [Google Scholar]
- 20.Goldbach-Mansky R, et al. Neonatal-onset multisystem inflammatory disease responsive to interleukin-1beta inhibition. N Engl J Med. 2006;355:581–592. doi: 10.1056/NEJMoa055137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Loppnow H, et al. Platelet-derived interleukin-1 induces cytokine production, but not proliferation of human vascular smooth muscle cells. Blood. 1998;91:134–141. [PubMed] [Google Scholar]
- 22.Mandinov L, et al. Interleukin 1: The choreographer for the restenotic ballet. Thromb Haemost. 2003;90:369–371. doi: 10.1160/TH03-06-0340. [DOI] [PubMed] [Google Scholar]
- 23.Tuder RM, Groves B, Badesch DB, Voelkel NF. Exuberant endothelial cell growth and elements of inflammation are present in plexiform lesions of pulmonary hypertension. Am J Pathol. 1994;144:275–285. [PMC free article] [PubMed] [Google Scholar]
- 24.Voelkel NF, Tuder RM. Cellular and molecular mechanisms in the pathogenesis of severe pulmonary hypertension. Eur Respir J. 1995;8:2129–2138. doi: 10.1183/09031936.95.08122129. [DOI] [PubMed] [Google Scholar]
- 25.Voelkel NF, Cool CD. Pulmonary vascular involvement in chronic obstructive pulmonary disease. Eur Respir J. 2003;46(Suppl):28s–32s. doi: 10.1183/09031936.03.00000503. [DOI] [PubMed] [Google Scholar]
- 26.Cook-Mills JM, Deem TL. Active participation of endothelial cells in inflammation. J Leukoc Biol. 2005;77:487–495. doi: 10.1189/jlb.0904554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dinarello CA. The IL-1 family and inflammatory diseases. Clin Exp Rheumatol. 2002;20(5 Suppl 27):S1–13. [PubMed] [Google Scholar]
- 28.Dustin ML, et al. Induction by IL 1 and interferon-gamma: Tissue distribution, biochemistry, and function of a natural adherence molecule (ICAM-1) J Immunol. 1986;137:245–254. [PubMed] [Google Scholar]
- 29.Galkina E, Ley K. Vascular adhesion molecules in atherosclerosis. Arterioscler Thromb Vasc Biol. 2007;27:2292–2301. doi: 10.1161/ATVBAHA.107.149179. [DOI] [PubMed] [Google Scholar]
- 30.Steffens S, Mach F. Inflammation and atherosclerosis. Herz. 2004;29:741–748. doi: 10.1007/s00059-004-2634-9. [DOI] [PubMed] [Google Scholar]
- 31.Lebedeva T, Dustin ML, Sykulev Y. ICAM-1 co-stimulates target cells to facilitate antigen presentation. Curr Opin Immunol. 2005;17:251–258. doi: 10.1016/j.coi.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 32.Dinarello CA. Biologic basis for interleukin-1 in disease. Blood. 1996;87:2095–2147. [PubMed] [Google Scholar]
- 33.Griffioen AW. Anti-angiogenesis: Making the tumor vulnerable to the immune system. Cancer Immunol Immunother. 2008;57:1553–1558. doi: 10.1007/s00262-008-0524-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Esmon CT, Esmon NL, Harris KW. Complex formation between thrombin and thrombomodulin inhibits both thrombin-catalyzed fibrin formation and factor V activation. J Biol Chem. 1982;257:7944–7947. [PubMed] [Google Scholar]
- 35.Esmon CT. Inflammation and the activated protein C anticoagulant pathway. Semin Thromb Hemost. 2006;32(Suppl 1):49–60. doi: 10.1055/s-2006-939554. [DOI] [PubMed] [Google Scholar]
- 36.Nold MF, et al. Activated protein C downregulates p38 mitogen-activated protein kinase and improves clinical parameters in an in-vivo model of septic shock. Thromb Haemost. 2007;98:1118–1126. doi: 10.1160/th07-01-0052. [DOI] [PubMed] [Google Scholar]
- 37.Joyce DE, et al. Gene expression profile of antithrombotic protein c defines new mechanisms modulating inflammation and apoptosis. J Biol Chem. 2001;276:11199–11203. doi: 10.1074/jbc.C100017200. [DOI] [PubMed] [Google Scholar]
- 38.Van de Wouwer M, Conway EM. Novel functions of thrombomodulin in inflammation. Crit Care Med. 2004;32(5 Suppl):S254–S261. doi: 10.1097/01.ccm.0000128036.64448.9e. [DOI] [PubMed] [Google Scholar]
- 39.Van de Wouwer M, et al. The lectin-like domain of thrombomodulin interferes with complement activation and protects against arthritis. J Thromb Haemost. 2006;4:1813–1824. doi: 10.1111/j.1538-7836.2006.02033.x. [DOI] [PubMed] [Google Scholar]
- 40.Conway EM, et al. The lectin-like domain of thrombomodulin confers protection from neutrophil-mediated tissue damage by suppressing adhesion molecule expression via nuclear factor kappaB and mitogen-activated protein kinase pathways. J Exp Med. 2002;196:565–577. doi: 10.1084/jem.20020077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li YH, et al. Functional mutation in the promoter region of thrombomodulin gene in relation to carotid atherosclerosis. Atherosclerosis. 2001;154:713–719. doi: 10.1016/s0021-9150(00)00639-0. [DOI] [PubMed] [Google Scholar]
- 42.Li YH, et al. Synergistic effect of thrombomodulin promoter -33G/A polymorphism and smoking on the onset of acute myocardial infarction. Thromb Haemost. 2002;87:86–91. [PubMed] [Google Scholar]
- 43.Waugh JM, et al. Thrombomodulin overexpression to limit neointima formation. Circulation. 2000;102:332–337. doi: 10.1161/01.cir.102.3.332. [DOI] [PubMed] [Google Scholar]
- 44.Weiler H, Isermann BH. Thrombomodulin. J Thromb Haemost. 2003;1:1515–1524. doi: 10.1046/j.1538-7836.2003.00306.x. [DOI] [PubMed] [Google Scholar]
- 45.Roubenoff R, et al. Cytokines, insulin-like growth factor 1, sarcopenia, and mortality in very old community-dwelling men and women: The Framingham Heart Study. Am J Med. 2003;115:429–435. doi: 10.1016/j.amjmed.2003.05.001. [DOI] [PubMed] [Google Scholar]
- 46.Braunersreuther V, Mach F, Steffens S. The specific role of chemokines in atherosclerosis. Thromb Haemost. 2007;97:714–721. [PubMed] [Google Scholar]
- 47.Kamari Y, et al. Differential role and tissue specificity of interleukin-1alpha gene expression in atherogenesis and lipid metabolism. Atherosclerosis. 2007;195:31–38. doi: 10.1016/j.atherosclerosis.2006.11.026. [DOI] [PubMed] [Google Scholar]
- 48.Maier JA, Voulalas P, Roeder D, Maciag T. Extension of the life-span of human endothelial cells by an interleukin-1 alpha antisense oligomer. Science. 1990;249:1570–1574. doi: 10.1126/science.2218499. [DOI] [PubMed] [Google Scholar]
- 49.McMahon GA, et al. Intracellular precursor interleukin (IL)-1alpha, but not mature IL-1alpha, is able to regulate human endothelial cell migration in vitro. J Biol Chem. 1997;272:28202–28205. doi: 10.1074/jbc.272.45.28202. [DOI] [PubMed] [Google Scholar]
- 50.Tashiro H, et al. Role of cytokines in the pathogenesis of restenosis after percutaneous transluminal coronary angioplasty. Coron Artery Dis. 2001;12:107–113. doi: 10.1097/00019501-200103000-00004. [DOI] [PubMed] [Google Scholar]
- 51.Lindeman JH, et al. Enhanced expression and activation of pro-inflammatory transcription factors distinguish aneurysmal from atherosclerotic aorta: IL-6- and IL-8-dominated inflammatory responses prevail in the human aneurysm. Clin Sci (Lond) 2008;114:687–697. doi: 10.1042/CS20070352. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.