Abstract
Autoimmune diseases tend to be chronic and progressive, but how these responses are sustained is not clear. One cell type that might contribute to autoimmunity is the cytotoxic T lymphocyte (CTL), which, as a consequence of causing tissue destruction and production of cytokines, could provide a sustained supply of antigen and inflammatory signals for dendritic cells to maintain immune stimulation. Here we examined whether such CTL-mediated tissue damage alone could provide antigen in the right context to recruit immune effectors and sustain autoimmunity. We show that while CTL-mediated tissue damage caused the release of self-antigens that stimulated the proliferation of naive autoreactive CD8+ T cells, such responses failed to precipitate disease and, instead, led to deletional tolerance. These findings indicate that despite the capacity of CTLs to produce inflammatory cytokines and to cause tissue damage, their responses are not sustaining, but instead favor induction of self-tolerance.
Keywords: antigen presentation, apoptosis, autoimmunity, CTL, T cell
To induce a robust adaptive immune response, antigens must be encountered in the context of an activating stimulus (1). Extensive evidence suggests that this stimulus can take the form of pathogen-associated molecular patterns (2) or “danger” signals associated with tissue damage caused by infection (3). As autoimmune diseases represent adaptive responses to self, it has long been speculated as to the nature of the activating stimuli required for initiation and maintenance of autoimmunity. While chronic infections may provide such a long-term source of activating stimuli, it is also feasible that autoimmune progression is driven by ongoing T cell-mediated tissue damage and production of inflammatory mediators.
In the NOD mouse, CD8+ T cells are important for the initial phase of autoimmunity (4–6), raising the question of whether such cytotoxic T lymphocyte (CTL)-mediated cellular destruction of islets is sufficient to maintain autoantigen presentation and effector T cell generation in the draining lymph node. CTLs are known to cause target cell apoptosis, and this death can supply antigen for cross-presentation in the draining lymph node (7). However, whether apoptotic islets cells produced in this manner are immunogenic or tolerogenic is open to speculation as apoptotic cells delivered alone have been shown to induce T cell tolerance in some cases (8, 9) and immunity in others (10, 11). There is evidence that apoptotic cells can impair dendritic cell (DC) priming capacity (12, 13), but codelivery of apoptotic cells with adjuvant signals has been demonstrated to induce T cell immunity in vivo (9, 14, 15).
In this study, we questioned whether antigens released during CTL-mediated tissue destruction would be immunogenic and hence able to drive chronic autoimmune pathology. While CTL-mediated tissue destruction will release self-antigens through target cell apoptosis, this cell death may be accompanied by the production of CTL derived inflammatory cytokines such as TNFα and IFN-γ (16) or with the release of inflammatory components of the degraded extracellular matrix such as heparan sulfate and hyaluronan oligosaccharides (17, 18). Depending on the extent of tissue destruction, secondary necrosis may also accompany CTL damage, leading to the release of intracellular components of potential immunogenicity (19–21). Collectively, these signals may be sufficient to overcome the immunosuppressive nature of apoptotic material and drive T cell priming against any released antigens.
Using 2 transgenic mouse lines where model self-antigens are released from tissues only in the context of CTL-mediated destruction, we show that self-antigens released by CTL killing not only fail to precipitate autoimmune disease, but also actively lead to deletional tolerance. These findings have important implications for maintaining the integrity of the immune system and illustrate a process by which autoimmunity is limited in healthy individuals.
Results
CTL Damage Induces Antigen Release.
To investigate the immunogenicity of antigens released by CTL-mediated tissue destruction, we used a C57BL/6 (B6) transgenic mouse line called RIP-OVAlo that exhibits pancreatic islet-specific, low-level expression of the neo-self antigen ovalbumin (OVA) (22). Under normal circumstances RIP-OVAlo mice do not express sufficient OVA for presentation to naive OVA-specific transgenic CD8+ T (OT-I) cells, although activated OVA-specific CTL effectors can recognize and destroy their islets (7). Such destruction releases OVA for presentation within the draining pancreatic lymph node, allowing activation of naive T cells (7) (illustrated in Fig. 1A). This model has provided us with an approach to address whether presentation of tissue antigens released by CTL damage initiates self-sustaining CTL-mediated autoimmune destruction or acts to maintain self-tolerance.
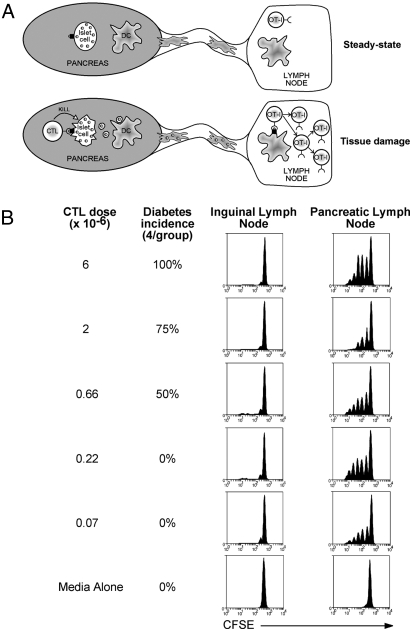
Fig. 1.
Low-dose cytotoxic T lymphocytes (CTLs) induce ovalbumin (OVA) presentation without inducing diabetes. (A) The RIP-OVAlo system. In the steady state, insufficient amounts of OVA (small, shaded circles) are captured by antigen-presenting cells (APCs) to cause proliferation of OVA-specific CD8+ T cells (OT-I cells) (Upper). However, the islets still express enough MHC I-OVA peptide on their surface to be targeted by OVA-specific CTLs and this killing event is able to initiate presentation to OT-I cells in the draining pancreatic lymph node (Lower). (B) RIP-OVAlo mice were given graded doses of in vitro activated OT-I CTL intravenously (i.v.) and were monitored for diabetes over a 3-week period to determine the diabetes incidence. In separate experiments, RIP-OVAlo mice injected with equivalent doses of activated CTL were also injected with 2 × 106 CFSE-labeled OT-I cells i.v. 4 days later and then a further 60 h later proliferation was examined by flow cytometry of cells from the draining pancreatic and nondraining inguinal lymph node. Representative data from 3 independent experiments are shown.
To examine the effect of CTL-mediated antigen release on the progression of autoimmunity, it was first necessary to determine a CTL dose that would destroy enough RIP-OVAlo β islet cells to initiate OVA presentation in the lymph node, but would not cause overt diabetes, which can be lethal. Injection of graded doses of in vitro activated OT-I CTLs into RIP-OVAlo mice revealed that at least 0.66 × 106 CTLs were required to induce diabetes (Fig. 1B). It has previously been shown that CTL damage in RIP-OVAlo mice induces OVA presentation between 4 and 7 days after CTL injection (7). To measure OVA presentation for the above CTL doses, CTL-treated mice were given carboxyfluorescein diacetate succinimidyl ester (CFSE)-labeled, naive OT-I cells 4 days after CTL injection. Sixty hours later, the proliferation of these naive cells was determined. Proliferation was seen in the pancreatic lymph node at all CTL doses examined (but not in the absence of CTLs), indicating that subdiabetogenic CTL doses were still capable of inducing enough tissue damage to initiate presentation (Fig. 1B). As proliferation induced by doses of ≤0.07 × 106 CTLs was variable (data not shown), 0.22 × 106 CTLs were used to induce damage in subsequent experiments. This low dose of CTLs caused histologically detectable islet damage and infiltration [supporting information (SI) Fig. S1].
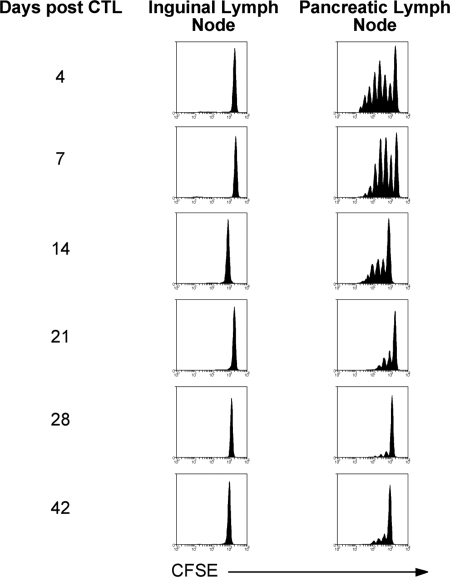
To determine the duration of presentation initiated by CTL damage, mice were treated with CTL and then left for various times before assessment of OVA presentation. While there was vigorous proliferation of OT-I cells up to 1 week after CTL injection, proliferation began to subside by 2 weeks and was barely detectable at 4 weeks (Fig. 2). Presentation after this time was very weak, but reproducible, perhaps reflecting ongoing minor damage or antigen persistence within the lymph node. Overall, however, the bulk of presentation induced by CTL damage was transient.
Fig. 2.
CTL-mediated islet damage initiates transient OVA presentation. RIP-OVAlo mice were injected i.v. with 0.22 × 106 CTLs and then left for 4, 7, 14, 21, 28, or 42 days before being injected i.v. with 2 × 106 CFSE-labeled OT-I cells. Proliferation was examined by flow cytometry in the draining pancreatic and nondraining inguinal lymph node 60 h after CFSE OT-I injection. Representative data from 3 independent experiments are shown.
Dendritic Cells Cross-Present Released Antigen.
We next wished to examine whether presentation was mediated by a bone marrow-derived cell. To do this, bm1→RIP-OVAlo bone marrow chimeras were generated. bm1 mice have a mutant Kb gene that is incapable of presenting OVA257–264 to OT-I cells (23). Thus, in bm1→RIP-OVAlo bone marrow chimeras, all bone marrow-derived antigen presenting cells (APCs) will be incapable of presenting OVA257–264 but their islet cells will still maintain this capacity. OT-I CTLs were introduced into these chimeras to cause damage and then the proliferation of naive CFSE-labeled OT-I cells was examined 4 days later. No proliferation was seen in the draining pancreatic lymph nodes, indicating that direct presentation by islet cells was incapable of inducing naive OT-I proliferation (Fig. 3A). Thus, tissue damage-associated presentation in RIP-OVAlo mice required OVA uptake and cross-presentation by bone marrow-derived APCs.
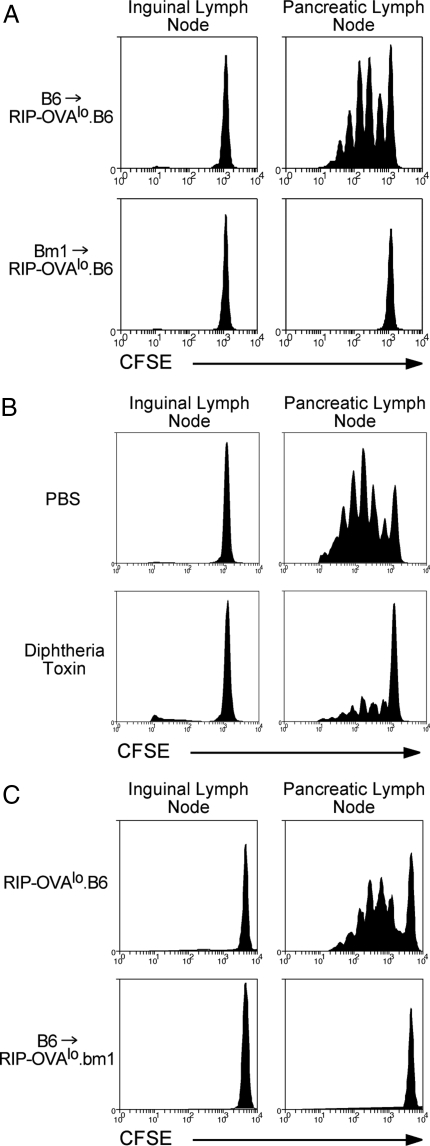
Fig. 3.
CTL-mediated islet damage initiates cross-presentation by DCs. (A) bm1→RIP-OVAlo and control B6→RIP-OVAlo bone marrow chimeras were injected i.v. with 0.22 × 106 CTLs and then 4 days later were injected i.v. with 2 × 106 CFSE-labeled OT-I cells. Proliferation was examined by flow cytometry of the draining pancreatic and nondraining inguinal lymph node cells 60 h after injection of CFSE OT-I cells. Representative data from 3 independent experiments are shown. (B) CD11cDTR→RIP-OVAlo chimeras were given 0.22 × 106 CTLs i.v. and then injected i.p. with either PBS (PBS) or 100 ng diphtheria toxin in PBS on days 1, 3, and 6 postinjection of CTLs. On day 4 after CTL injection the mice were given 2 × 106 CFSE-labeled naive OT-I cells i.v. and their proliferation was examined as in A. Representative data from 2 independent experiments are shown. (C) B6→RIP-OVAlo.bm1 bone marrow chimeras and control RIP-OVAlo.B6 mice were given 107 CTLs i.v. followed by 2 × 106 CFSE-labeled OT-I cells i.v. 4 days later. Proliferation was examined as in A. Representative data from 2 independent experiments are shown.
Bone marrow-derived APCs include macrophages, B cells, and dendritic cells. Nevertheless, previous studies have demonstrated that DCs are the predominant cell type that presents islet-derived self-antigens (24–27). To determine whether the cells responsible for presentation here were DCs, CD11c-DTR→RIP-OVAlo bone marrow chimeras were generated. CD11c-DTR mice express the diphtheria toxin receptor under the control of the CD11c promoter and, hence, diphtheria toxin treatment results in DC depletion (28). CD11c-DTR→RIP-OVAlo chimeras were given CTLs followed by diphtheria toxin to deplete the CD11c+ cells. This substantially reduced naive OT-I proliferation, indicating that presentation was primarily mediated by DCs (Fig. 3B) and that control of proliferating OT-I cell fate is largely attributable to DCs.
It remained possible that increased OVA presentation upon CTL treatment was not caused by CTL-mediated release of islet antigens for DC access. Instead, the CTLs could be directly activating DCs within the lymph node to upregulate cross-presentation of small amounts of OVA captured in the steady state. To show that OVA presentation required tissue damage, B6→RIP-OVAlo.bm1 chimeras were generated. In these mice, tissue damage cannot be initiated as the islet cells express the mutant Kbm1 molecule, but CTL can still potentially interact with DCs. Even when these mice were given a high dose (107) of CTLs no presentation was seen, demonstrating that tissue damage was required to initiate presentation (Fig. 3C). Together, the above findings indicated that CTL damage induced OVA uptake and presentation by DCs.
When DCs receive immunogenic signals and are activated to prime T cells, they usually undergo maturation and upregulate cell surface molecules such as MHCII, CD86, and CD40. To ascertain whether inflammation associated with CTL damage caused DCs to mature, DCs from the pancreatic lymph nodes were isolated and phenotyped. Surprisingly, CTL damage was capable of inducing only a slight upregulation of CD86 and MHCII and moderate upregulation of CD40 on pancreatic lymph node DCs (Fig. S2). Thus, tissue disruption generated by CTLs had only a marginal impact on DC maturity.
OVA Release by CTL Killing Induces OT-I Deletion.
It remained possible that, despite a negligible shift in maturity, the presenting DCs were still capable of promoting naive T cell priming. It was thus important to elucidate the fate of autoreactive T cells responding to this antigen. One of the hallmarks of CD8+ T cells undergoing tolerance induction is their inability to produce the cytokine IFN-γ (29). We thus first examined IFN-γ production by OT-I cells proliferating in CTL-treated RIP-OVAlo mice. Surprisingly, in contrast to OT-I cells proliferating in the established RIP-OVAhi model of cross-tolerance (22), OT-I cells proliferating in CTL-treated RIP-OVAlo mice were able to produce IFN-γ upon peptide restimulation at similar proportions to cells from OVA-primed mice (Fig. S3). This initially suggested that OT-I cells proliferating in response to islet antigens released by CTL killing were primed. Nevertheless, T cells can pass through an effector phase during tolerance induction (30), meaning that the ability of these cells to initiate autoimmunity needed to be tracked to accurately determine fate. To further follow the fate of the naive OT-I cells, RIP-OVAlo mice were injected i.v. with either a low dose of Ly5.1+-activated CTLs (to cause damage) or media alone and then 4 days later injected with 5 × 106 naive Ly5.2+ OT-I cells. These mice were monitored for diabetes over a 4-week period and no diabetic mice were detected in either the CTL-treated mice (0/19) or the media control group (0/18). This implied that despite proliferating and acquiring effector functions, naive OT-I cells were incapable of triggering autoimmune pathology. Thus, the net outcome of the T cell response to antigen released by CTL killing was not overtly immunogenic and implied tolerogenic control of the response of naive T cells.
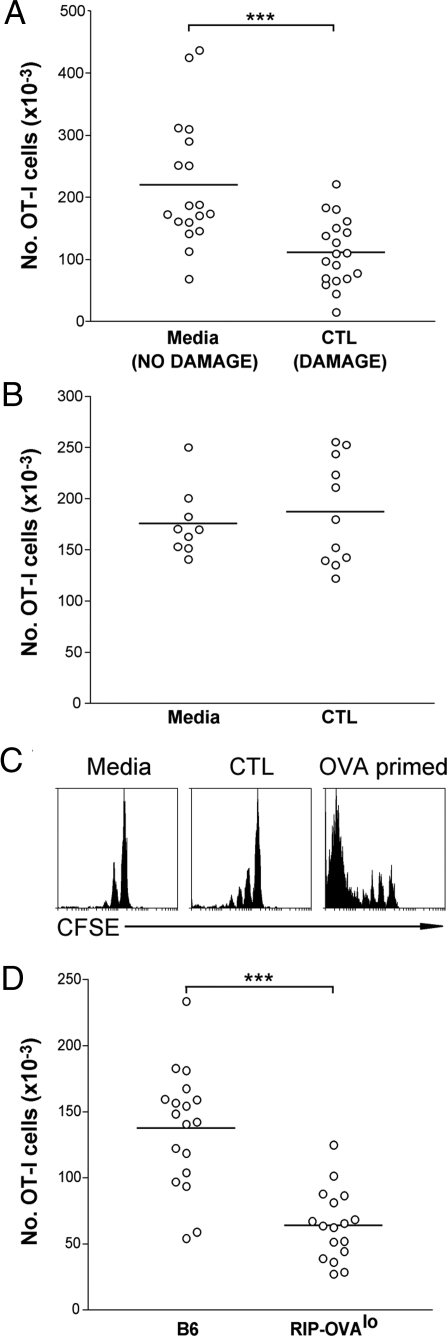
To further delineate the fate of the responding OT-I cells, the number of Ly5.2+ OT-I cells (derived from the population of transferred naive OT-I cells) remaining within the lymphoid compartment (spleen and lymph nodes) was quantitated 4 weeks after naive OT-I injection. A significant (P < 0.001) reduction of ≈50% in the number of transferred naive Ly5.2+ OT-I cells in mice that received CTL relative to those given media alone was shown (Fig. 4A). No such reduction was seen when nontransgenic B6 hosts were used (Fig. 4B). These data suggested that OVA released by CTL-mediated tissue damage induced deletional tolerance of antigen-specific T cells, a conclusion further supported by assessing the deletion of OT-I cells relative to T cells of an irrelevant specificity (Fig. S4 A and B).
Fig. 4.
OVA released by CTL-mediated islet destruction induces deletion of naive OVA-specific cells. (A) RIP-OVAlo mice were given either media (NO DAMAGE) or 0.22 × 106 Ly5.1+ CTLs (DAMAGE) i.v. followed by 5 × 106 Ly5.2+ naive OT-I cells i.v. 4 days later. Four weeks after naive OT-I injection, the number of Ly5.1− OVA-tetramer+ cells (derived from the original naive Ly5.2+ OT-I cells) remaining in the spleen and lymph nodes of the mice was determined by flow cytometry. Pooled data are shown from 5 independent experiments. ***, P < 0.001. Circles represent individual mice and the bar represents the average. (B) An experiment was performed as in A except nontransgenic B6 mice were used as recipient mice instead of RIP-OVAlo mice. Pooled data are shown from 2 independent experiments. (C) A similar experiment to that performed in A was undertaken, with RIP-OVAlo mice given media alone (Left) or activated CTL (Middle), except that naive OT-I cells were labeled with CFSE and their proliferation was assessed after 4 weeks. As a positive control (Right), B6 mice were injected i.v. with 5 × 106 naive CFSE-labeled OT-I cells followed by 2 × 107 OVA-coated splenocytes (46) with 1 μg LPS (OVA primed) and analyzed after 4 weeks. Representative data from 11 mice per group collected over 2 independent experiments are shown. (D) B6 or RIP-OVAlo mice were given 0.22 × 106 CTLs (i.v.). Four weeks after CTL injection, the number of CD8+OVA-tetramer+ cells remaining in the spleen and lymph nodes of the mice was determined by flow cytometry. Pooled data are shown from 3 independent experiments. ***, P < 0.001. Circles represent individual mice and the bar represents the average.
To determine whether more complete deletion could be achieved with a lower number of cells, similar experiments were performed with transfer of 5-fold fewer naive OT-I cells. Again, no mice developed diabetes and, in this case, there was a slight increase in the proportion of deleted cells after 4 weeks (60%, Fig. S4C). When cells surviving deletion, as in Fig. 4A, were phenotyped, it was found that they were antigen inexperienced. They showed limited division (Fig. 4C) and expressed naive levels of CD44 (Fig. S5). Furthermore, the undeleted cells were capable of proliferating in response to in vitro restimulation (Fig. S6), indicating that the residual cells were not anergic. Thus, surviving cells were naive, suggesting they had simply not yet encountered antigen, most likely due to the transient presentation seen in this system. Collectively, these data implied that virtually all naive OT-I cells that encountered and responded to released antigen were deleted and that the mechanism of tolerance in this system was deletion and not anergy.
OVA-Specific CTLs Are Selectively Deleted in RIP-OVAlo Mice.
To this point, we had clearly shown that antigen released by CTL-mediated tissue destruction induced naive OT-I cell deletion, but were yet to determine the fate of those activated CTLs introduced into the RIP-OVAlo mice to mediate β cell killing. However, the transient nature of presentation (Fig. 2) suggested that these CTLs might also be undergoing tolerance induction. To directly measure the fate of activated CTLs within RIP-OVAlo mice, these mice and nontransgenic B6 controls were injected with 0.22 × 106 CTLs and then left for 4 weeks. As before, none of the B6 (0/18) or RIP-OVAlo (0/17) mice developed diabetes over this period. When the number of OT-I CTLs remaining in the lymphoid compartment of each mouse was quantitated, there was a significant (P < 0.0001), ≈60% reduction in the number of CTLs recovered from RIP-OVAlo mice relative to B6 controls (Fig. 4D). Thus, the CTLs themselves are deleted in the presence of their autoantigen.
These Findings Extend to Other Antigens and Tissues.
Thus far, we have shown that deletional tolerance is induced following presentation of OVA released from the islets by CTL-mediated damage. To determine whether this extended to other models of CTL-mediated tissue damage, we generated mice expressing the herpes simplex virus glycoprotein B (gB) in keratinocytes and examined the response of gB-specific CD8+ T cells from the gBT-I transgenic line (31). Similar to RIP-OVAlo mice, there was no presentation of gB to naive CD8+ T cells in the skin-draining lymph nodes of K14-gB mice (Fig. 5A). However, upon transfer of activated gB-specific CTLs that could mediate skin damage, gB was released from the skin and presented in the skin-draining lymph nodes (Fig. 5A). Examination of the fate of naive gBT-I cells responding to released antigens showed that like OT-I cells in RIP-OVAlo mice, such presentation of released tissue antigen led to deletion of responding naive gB-specific CD8+ T cells in K14-gB mice (Fig. 5B).
Fig. 5.
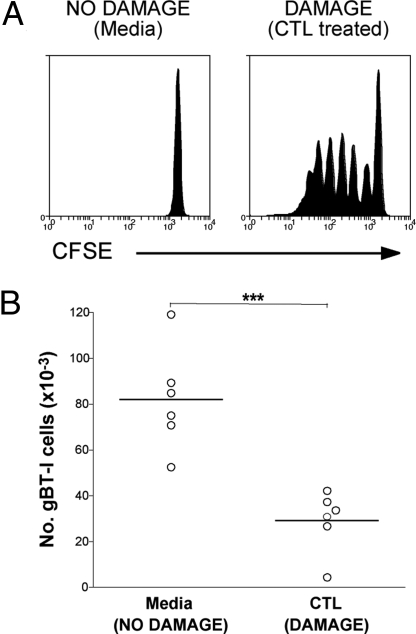
gB antigen released from the skin of K14-gB mice by CTL killing induces naive gBT-I deletion. (A) K14-gB mice were given either 7.5 × 106 in vitro activated gBT-I CTL (DAMAGE) or media (NO DAMAGE) i.v. and then 7 days later were injected i.v. with 2 × 106 CFSE-labeled Ly5.1+ naive gBT-I cells. Proliferation was examined by flow cytometry of the pooled skin draining lymph node cells 60 h after injection of CFSE gBT-I cells. Representative data from 7 independent experiments are shown. (B) K14-gB mice were given either media (NO DAMAGE) or 7.5 × 106 Ly5.2+ CTLs (DAMAGE) i.v. followed by 2 × 106 Ly5.1+ naive gBT-I cells i.v. 4 days later. Six weeks after naive gBT-I injection, the number of Ly5.1+ CD8+ cells (derived from the original naive Ly5.1+ gBT-I cells) remaining in the spleen and lymph nodes of the mice was determined by flow cytometry. Pooled data are shown from 2 independent experiments. ***, P < 0.001. Circles represent individual mice and the bar represents the average.
Discussion
While it was proposed 10 years ago that autoreactive CTL responses would be self-limiting (3), our data represent the first experimental proof of this idea. We show that, under normal circumstances, tissue damage caused by CTLs releases self-antigens that cause deletion of naive autoreactive CD8+ T cells.
While most studies of tolerance focus on the mechanisms that prevent priming of naive autoreactive T cells in the steady state, we examined whether a preexisting autoimmune CTL response would provide sufficient adjuvant signals to drive its own expansion. It is clear from our data that, if the initial autoimmune response is of great enough magnitude (>220,000 CTLs in the RIP-OVAlo system), then it can directly cause overt tissue damage and autoimmune pathology. However, we clearly demonstrate that a more modest autoreactive CTL response will self-limit by tolerizing any other self-reactive cells that respond to released antigen. This likely occurs because the signals generated by an autoreactive CTL response appear incapable of overriding the tolerogenic effect of apoptotic cell death. CTL-driven inflammation obviously has some impact upon tolerance induction as OT-I cells responding in the context of a preexisting CTL response acquire the ability to produce IFN-γ before death. This is in direct contrast to cells responding to self-antigens in the steady state, which generally are defective in IFN-γ production (29) (Fig. S3). However, the inability of these cells to precipitate diabetes suggests that they are deleted before they cause pathology. As such, our data demonstrate that, in healthy individuals, an autoreactive CTL response in isolation is incapable of providing the signals to sustain chronic autoimmunity. Sustained adjuvant signals will likely be required to break this negative feedback loop, as single doses of adjuvant are incapable of preventing deletion (32).
One question that arises from this study is how infection might influence the fate of naive T cells responding to released antigens. It is possible that inflammatory signals associated with infection will circumvent the deletion process and lead to development of fully fledged autoreactive CTLs. If so, these CTLs could then act much like those transferred here and cause some degree of tissue damage. Once infection is cleared, however, further damage would revert to presentation of antigen in a tolerogenic manner, leading to deletion of any newly arising cells and thus preventing sustained autoimmunity. The fate of those activated CTLs recruited during infection would likely also be deletion as was seen for those CTLs transferred into RIP-OVAlo mice (Fig. 4D).
Given the large body of evidence supporting the adjuvant effect of tissue damage (11, 15, 17–21), it is surprising that CTL-mediated tissue destruction has such a minimal immunological impact. Moreover, the CTLs themselves should produce inflammatory cytokines, which might be expected to promote immunity. The recent demonstration that indirect activation of DCs by inflammatory cytokines was insufficient to promote T cell priming (33) might in part explain why inflammatory signals produced by CTLs do not themselves promote further CTL priming. However, it is interesting that other aspects of CTL-driven tissue disruption did not promote autoimmune progression. We can only speculate that, while the tissue damage signals generated by an autoreactive CTL response may synergize with other adjuvants to augment priming during infection, they are clearly insufficient to promote priming in its absence.
One potential explanation for the lack of autoimmunity caused by naive OT-I cells exposed to released OVA is that naive CD8+ T cells require help for effective priming (34–37) but help is limiting. In a preliminary experiment, we found that cotransferring large numbers of naive OT-II helper cells (2 × 106) rarely led to diabetes induction (1 in 4 mice). While this result suggests that help can potentiate autoimmunity, we have to consider that the situation in the absence of added OT-II cells is more physiologically relevant. When islet cells are destroyed by CTLs, all their endogenous antigens are released (including natural islet proteins and transgenically expressed OVA) and presentation of these antigens on MHC II should recruit any helpers available in a normal repertoire. If helpers to endogenous islet antigens are available, then they have already contributed their potential to help in our studies (Figs. 4 and 5), and if they are not available, then this is the normal situation and the fate of the naive OT-I CD8+ T cells is a reflection of what would happen to endogenous CD8+ T cells. In other words, the deletional fate of responding naive OT-I cells in our RIP-OVAlo model reflects what would happen to naive CD8+ T cells specific for released islet antigens in B6 mice.
Despite our finding that autoreactive CTL responses are self-limiting, there is evidence that autoreactive CD4+ T cell responses can be self-sustaining (38). This is exemplified by the phenomenon of epitope spreading, where immunization against one MHC II-restricted self-peptide is sufficient to induce responses to other MHC II-restricted self-peptides (39). Interestingly, there is a paucity of such data for CD8+ T cells, which tends to support our conclusions. Nevertheless, MHC I-restricted epitope spreading has been reported for responses to some tumors (40–42). Our findings suggest that such responses should occur only if the tumor itself is able to supply sustained adjuvant signals, which could be the case for some tumors. However, there is much evidence to suggest that tumors generally promote a tolerogenic environment (43), suggesting adjuvant production by tumors is likely to be rare. Our studies thus imply that the self-limiting nature of autoreactive CTL responses described here would hamper the induction of sustained CTL responses to tumors after therapeutic vaccination, a pitfall that may be overcome by sustained adjuvant treatment after tumor vaccination.
Materials and Methods
Mice.
All mice were bred and maintained at The Walter and Eliza Hall Institute for Medical Research. Transgenic OT-I (44), RIP-OVAlo (7), gBT-I (31), and CD11cDTR (28) mice have been described previously. C57BL/6, B6.SJL-PtprcaPep3b/BoyJ (Ly5.1+), and B6.C-H2bm1/By (bm1) mice were purchased from Jackson Laboratories and maintained at The Walter and Eliza Hall Institute for Medical Research. All animal experimentation was performed in accordance with institutional guidelines and the Melbourne Health Animal Ethics Committee, which granted permission for this study.
In Vitro Activation of OT-I Cells.
A total of 2 × 107 OT-I splenocytes were cultured for 5 days at 37 °C, 5% CO2 in 30 mL of medium [mouse tonicity RPMI1640, 10 units/mL recombinant human interleukin 2 (Peprotech), 0.03 μg/mL lipopolysaccharide (LPS) (Escherichia coli, 0111:B4, Difco), 10% FCS, 2 mM L-glutamine, 5 × 10−5 M 2-mercaptoethanol, and antibiotics] with 2 × 107 irradiated (1500 cGy) B6 splenocytes previously coated for 1 h at 2 × 107 cells/mL with 1 μg/mL OVA257–264 peptide.
Bone Marrow Chimeras.
Bone marrow chimeras were generated as previously described (45) except that recipients were given between 2.5 and 5.0 × 106 bone marrow cells and mice were left to reconstitute for 6–8 weeks. Where indicated, chimeras were given 100 ng of diphtheria toxin (provided by A. Lew, The Walter and Eliza Hall Institutes) i.p. on the indicated days while control mice were given PBS.
CFSE Labeling, Adoptive Transfer, and FACS Analysis.
CD8+ T cells were enriched from spleen and lymph nodes by generating single-cell suspensions and incubating the cells with monoclonal Abs against Mac-1 (M1/70), macrophages (F4/80), red blood cells (Ter119), Gr1 (RB6–8C5), MHC class II (M5/114), and CD4 (GK1.5) on ice for 30 min. The Ab-labeled cells were removed by anti-rat IgG-coupled magnetic beads (QIAGEN). Purity at this point was typically 85–90% CD8+ cells. CFSE labeling was performed by labeling cells in PBS [containing 0.1% BSA (Sigma-Aldrich)] with 5 μM CFSE (Invitrogen) at 37 °C as described previously (45). Flow cytometry analysis was performed on a FACScan, FACSCalibur, or BD-LSR (BD Biosciences) instrument. Antibodies used for flow cytometry were CD44, CD8, CD45.1(Ly5.1), Vβ8.1/8.2, IFN-γ (BD Biosciences), CD11c (N418), CD86 (GL1), MHCII (M5/114), and CD40 (FGK45.5) (provided by K. Shortman, The Walter and Eliza Hall Institute for Medical Research, Victoria, Australia). H-2Kb-OVA257–264-tetramer was provided by A. Brooks (Department of Microbiology and Immunology, University of Melbourne, Victoria, Australia). For deletion experiments mice were given 5 × 106 or 1 × 106 enriched CD8+ T cells 4 days after CTL or media treatment. Four weeks after T cell transfer, spleen and lymph nodes were harvested from recipient mice and transferred T cell numbers were determined by flow cytometry with Sphero beads (BD Biosciences) as described previously (45). OVA-coated splenocytes were prepared as described previously (46) and injected i.v. along with 1 μg LPS.
In Vitro Restimulation of T Cells.
Bead-enriched CD8+ T cells from RIP-OVAlo mice were CFSE labeled and the percentage of CD8+Ly5.1+ cells was determined. A total of 106 CD8+ cells from each mouse were added to a well in a 96-well plate and serially diluted. A total of 106 peptide pulsed B6 stimulators prepared as for CTLs were added to each well to a final volume of 200 μL. Proliferation was determined by CFSE dilution after 60 h at 37 °C, 5% CO2. For intracellular cytokine staining assays, RIP-OVAhi mice, RIP-OVAlo mice (pretreated with 0.22 × 106 CTL 4 days earlier), and B6 mice primed with OVA-coated splenocytes and LPS (as described above) were given 2 × 106 CFSE-labeled OT-I cells (i.v.). Sixty hours later, cells were isolated from the pancreatic lymph nodes (RIP-OVAhi and RIP-OVAlo mice) or spleen (OVA-primed B6 mice) and restimulated with 1 μg/mL OVA257–264 peptide in the presence of 5 μg/mL Brefeldin A. Cells were then fixed at room temperature with 1% formaldehyde, stained for IFN-γ in the presence of 0.3% saponin on ice, washed, and analyzed by flow cytometry.
Dendritic Cell Enrichment.
Dendritic cell enrichment was performed as described (47).
Diabetes.
Mice were given in vitro activated OT-I CTLs (i.v.). Recipient mice were monitored for diabetes, by urine glucose testing, from day 1 after transfer. Animals were monitored for 3 weeks and were considered diabetic after 2 consecutive days with readings ≥55 mmol/L.
Statistical Analysis.
All graphing and statistical analyses were performed using the Prism graphing program (Version 3.0, GraphPad software). P values were calculated using a 2-tailed unpaired T test (Fig. S2B, Fig. S4B, and Fig. 5B) or a 2-tailed Mann–Whitney test when data failed normality tests (Fig. 4 A and D and Fig. S4C).
Supplementary Material
Acknowledgments.
We thank J. Langley, F. Kupresanin, M. Camilleri, A. Bradfield, K. Jordan, and L. Mackiewicz for their technical assistance. We also thank A. Lew, K. Shortman, and A. Brooks for kindly supplying reagents. This work was supported by the National Health and Medical Research Council (Australia), Wellcome Trust (United Kingdom) (G.T.B.), the Howard Hughes Medical Institute (G.T.B. and W.R.H), the Juvenile Diabetes Research Foundation (R.M.S.), and the Australian Government's Co-operative Research Centre Program (I.A.P.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0810427106/DCSupplemental.
References
- 1.Williams MA, Bevan MJ. Effector and memory CTL differentiation. Annu Rev Immunol. 2006;25:171–192. doi: 10.1146/annurev.immunol.25.022106.141548. [DOI] [PubMed] [Google Scholar]
- 2.Janeway CA, Jr, Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- 3.Matzinger P. Tolerance, danger, and the extended family. Annu Rev Immunol. 1994;12:991–1045. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- 4.Katz J, Benoist C, Mathis D. Major histocompatibility complex class I molecules are required for the development of insulitis in non-obese diabetic mice. Eur J Immunol. 1993;23:3358–3360. doi: 10.1002/eji.1830231244. [DOI] [PubMed] [Google Scholar]
- 5.Serreze DV, Leiter EH, Christianson GJ, Greiner D, Roopenian DC. Major histocompatibility complex class I-deficient NOD-B2mnull mice are diabetes and insulitis resistant. Diabetes. 1994;43:505–509. doi: 10.2337/diab.43.3.505. [DOI] [PubMed] [Google Scholar]
- 6.Wicker LS, et al. Beta 2-microglobulin-deficient NOD mice do not develop insulitis or diabetes. Diabetes. 1994;43:500–504. doi: 10.2337/diab.43.3.500. [DOI] [PubMed] [Google Scholar]
- 7.Kurts C, Miller JF, Subramaniam RM, Carbone FR, Heath WR. Major histocompatibility complex class I-restricted cross-presentation is biased towards high dose antigens and those released during cellular destruction. J Exp Med. 1998;188:409–414. doi: 10.1084/jem.188.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Albert ML, Jegathesan M, Darnell RB. Dendritic cell maturation is required for the cross-tolerization of CD8+ T cells. Nat Immunol. 2001;2:1010–1017. doi: 10.1038/ni722. [DOI] [PubMed] [Google Scholar]
- 9.Liu K, et al. Immune tolerance after delivery of dying cells to dendritic cells in situ. J Exp Med. 2002;196:1091–1097. doi: 10.1084/jem.20021215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang MK, et al. Apoptotic cells with oxidation-specific epitopes are immunogenic and proinflammatory. J Exp Med. 2004;200:1359–1370. doi: 10.1084/jem.20031763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Janssen E, et al. Efficient T cell activation via a Toll-Interleukin 1 receptor-independent pathway. Immunity. 2006;24:787–799. doi: 10.1016/j.immuni.2006.03.024. [DOI] [PubMed] [Google Scholar]
- 12.Urban BC, Willcox N, Roberts DJ. A role for CD36 in the regulation of dendritic cell function. Proc Natl Acad Sci USA. 2001;98:8750–8755. doi: 10.1073/pnas.151028698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stuart LM, et al. Inhibitory effects of apoptotic cell ingestion upon endotoxin-driven myeloid dendritic cell maturation. J Immunol. 2002;168:1627–1635. doi: 10.4049/jimmunol.168.4.1627. [DOI] [PubMed] [Google Scholar]
- 14.Albert ML, Sauter B, Bhardwaj N. Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature. 1998;392:86–89. doi: 10.1038/32183. [DOI] [PubMed] [Google Scholar]
- 15.Feng H, Zeng Y, Graner MW, Katsanis E. Stressed apoptotic tumor cells stimulate dendritic cells and induce specific cytotoxic T cells. Blood. 2002;100:4108–4115. doi: 10.1182/blood-2002-05-1389. [DOI] [PubMed] [Google Scholar]
- 16.Wherry EJ, Ahmed R. Memory CD8 T-cell differentiation during viral infection. J Virol. 2004;78:5535–5545. doi: 10.1128/JVI.78.11.5535-5545.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson GB, Brunn GJ, Kodaira Y, Platt JL. Receptor-mediated monitoring of tissue well-being via detection of soluble heparan sulfate by Toll-like receptor 4. J Immunol. 2002;168:5233–5239. doi: 10.4049/jimmunol.168.10.5233. [DOI] [PubMed] [Google Scholar]
- 18.Termeer C, et al. Oligosaccharides of Hyaluronan activate dendritic cells via toll-like receptor 4. J Exp Med. 2002;195:99–111. doi: 10.1084/jem.20001858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gallucci S, Lolkema M, Matzinger P. Natural adjuvants: endogenous activators of dendritic cells. Nat Med. 1999;5:1249–1255. doi: 10.1038/15200. [DOI] [PubMed] [Google Scholar]
- 20.Shi Y, Zheng W, Rock KL. Cell injury releases endogenous adjuvants that stimulate cytotoxic T cell responses. Proc Natl Acad Sci USA. 2000;97:14590–14595. doi: 10.1073/pnas.260497597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shi Y, Evans JE, Rock KL. Molecular identification of a danger signal that alerts the immune system to dying cells. Nature. 2003;425:516–521. doi: 10.1038/nature01991. [DOI] [PubMed] [Google Scholar]
- 22.Kurts C, et al. CD8 T cell ignorance or tolerance to islet antigens depends on antigen dose. Proc Natl Acad Sci USA. 1999;96:12703–12707. doi: 10.1073/pnas.96.22.12703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nikolic ZJ, Carbone FR. The effect of mutations in the MHC class I peptide binding groove on the cytotoxic T lymphocyte recognition of the Kb-restricted ovalbumin determinant. Eur J Immunol. 1990;20:2431–2437. doi: 10.1002/eji.1830201111. [DOI] [PubMed] [Google Scholar]
- 24.Belz GT, et al. The CD8alpha(+) dendritic cell is responsible for inducing peripheral self-tolerance to tissue-associated antigens. J Exp Med. 2002;196:1099–1104. doi: 10.1084/jem.20020861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hugues S, et al. Tolerance to islet antigens and prevention from diabetes induced by limited apoptosis of pancreatic beta cells. Immunity. 2002;16:169–181. doi: 10.1016/s1074-7613(02)00273-x. [DOI] [PubMed] [Google Scholar]
- 26.Kurts C, Cannarile M, Klebba I, Brocker T. Dendritic cells are sufficient to cross-present self-antigens to CD8 T cells in vivo. J Immunol. 2001;166:1439–1442. doi: 10.4049/jimmunol.166.3.1439. [DOI] [PubMed] [Google Scholar]
- 27.Turley S, Poirot L, Hattori M, Benoist C, Mathis D. Physiological beta cell death triggers priming of self-reactive T cells by dendritic cells in a type-1 diabetes model. J Exp Med. 2003;198:1527–1537. doi: 10.1084/jem.20030966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jung S, et al. In vivo depletion of CD11c(+) dendritic cells abrogates priming of CD8(+) T cells by exogenous cell-associated antigens. Immunity. 2002;17:211–220. doi: 10.1016/s1074-7613(02)00365-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hernandez J, Aung S, Redmond WL, Sherman LA. Phenotypic and functional analysis of CD8(+) T cells undergoing peripheral deletion in response to cross-presentation of self-antigen. J Exp Med. 2001;194:707–717. doi: 10.1084/jem.194.6.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang CT, et al. CD4+ T cells pass through an effector phase during the process of in vivo tolerance induction. J Immunol. 2003;170:3945–3953. doi: 10.4049/jimmunol.170.8.3945. [DOI] [PubMed] [Google Scholar]
- 31.Mueller SN, Heath W, McLain JD, Carbone FR, Jones CM. Characterization of two TCR transgenic mouse lines specific for herpes simplex virus. Immunol Cell Biol. 2002;80:156–163. doi: 10.1046/j.1440-1711.2002.01071.x. [DOI] [PubMed] [Google Scholar]
- 32.Hamilton-Williams EE, et al. Cutting edge: TLR ligands are not sufficient to break cross-tolerance to self-antigens. J Immunol. 2005;174:1159–1163. doi: 10.4049/jimmunol.174.3.1159. [DOI] [PubMed] [Google Scholar]
- 33.Sporri R, Reis e Sousa C. Inflammatory mediators are insufficient for full dendritic cell activation and promote expansion of CD4(+) T cell populations lacking helper function. Nat Immunol. 2005;6:163–170. doi: 10.1038/ni1162. [DOI] [PubMed] [Google Scholar]
- 34.Bennett SR, et al. Help for cytotoxic-T-cell responses is mediated by CD40 signalling. Nature. 1998;393:478–480. doi: 10.1038/30996. [DOI] [PubMed] [Google Scholar]
- 35.Bennett SR, Carbone FR, Karamalis F, Miller JF, Heath WR. Induction of a CD8+ cytotoxic T lymphocyte response by cross-priming requires cognate CD4+ T cell help. J Exp Med. 1997;186:65–70. doi: 10.1084/jem.186.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ridge JP, Di Rosa F, Matzinger P. A conditioned dendritic cell can be a temporal bridge between a CD4+ T-helper and a T-killer cell. Nature. 1998;393:474–478. doi: 10.1038/30989. [DOI] [PubMed] [Google Scholar]
- 37.Schoenberger SP, Toes RE, van der Voort EI, Offringa R, Melief CJ. T-cell help for cytotoxic T lymphocytes is mediated by CD40-CD40L interactions. Nature. 1998;393:480–483. doi: 10.1038/31002. [DOI] [PubMed] [Google Scholar]
- 38.Vanderlugt CL, Miller SD. Epitope spreading in immune-mediated diseases: implications for immunotherapy. Nat Rev Immunol. 2002;2:85–95. doi: 10.1038/nri724. [DOI] [PubMed] [Google Scholar]
- 39.Lehmann PV, Forsthuber T, Miller A, Sercarz EE. Spreading of T-cell autoimmunity to cryptic determinants of an autoantigen. Nature. 1992;358:155–157. doi: 10.1038/358155a0. [DOI] [PubMed] [Google Scholar]
- 40.Brossart P, et al. Induction of cytotoxic T-lymphocyte responses in vivo after vaccinations with peptide-pulsed dendritic cells. Blood. 2000;96:3102–3108. [PubMed] [Google Scholar]
- 41.el-Shami K, et al. MHC class I-restricted epitope spreading in the context of tumor rejection following vaccination with a single immunodominant CTL epitope. Eur J Immunol. 1999;29:3295–3301. doi: 10.1002/(SICI)1521-4141(199910)29:10<3295::AID-IMMU3295>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 42.Markiewicz MA, Fallarino F, Ashikari A, Gajewski TF. Epitope spreading upon P815 tumor rejection triggered by vaccination with the single class I MHC-restricted peptide P1A. Int Immunol. 2001;13:625–632. doi: 10.1093/intimm/13.5.625. [DOI] [PubMed] [Google Scholar]
- 43.Munn DH, Mellor AL. The tumor-draining lymph node as an immune-privileged site. Immunol Rev. 2006;213:146–158. doi: 10.1111/j.1600-065X.2006.00444.x. [DOI] [PubMed] [Google Scholar]
- 44.Hogquist KA, et al. T cell receptor antagonist peptides induce positive selection. Cell. 1994;76:17–27. doi: 10.1016/0092-8674(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 45.Davey GM, et al. SOCS-1 regulates IL-15-driven homeostatic proliferation of antigen-naive CD8 T cells, limiting their autoimmune potential. J Exp Med. 2005;202:1099–1108. doi: 10.1084/jem.20050003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Carbone FR, Bevan MJ. Class I-restricted processing and presentation of exogenous cell-associated antigen in vivo. J Exp Med. 1990;171:377–387. doi: 10.1084/jem.171.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Henri S, et al. The dendritic cell populations of mouse lymph nodes. J Immunol. 2001;167:741–748. doi: 10.4049/jimmunol.167.2.741. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.