Abstract
Disorders affecting mitochondria, including those that directly affect the respiratory chain function or result from abnormalities in branched amino acid metabolism (organic acidemias), have been shown to be associated with impaired redox balance. Almost all of the evidence underlying this conclusion has been obtained from studies on patient biopsies or animal models. Since the glutathione (iGSH) system provides the main protection against oxidative damage, we hypothesized that untreated oxidative stress in individuals with mitochondrial dysfunction would result in chronic iGSH deficiency. We confirm this hypothesis here in studies using high-dimensional flow cytometry (Hi-D FACS) and biochemical analysis of freshly obtained blood samples from patients with mitochondrial disorders or organic acidemias. T lymphocyte subsets, monocytes and neutrophils from organic acidemia and mitochondrial patients who were not on antioxidant supplements showed low iGSH levels, whereas similar subjects on antioxidant supplements showed normal iGSH. Measures of iROS levels in blood were insufficient to reveal the chronic oxidative stress in untreated patients. Patients with organic acidemias showed elevated plasma protein carbonyls, while plasma samples from all patients tested showed hypocitrullinemia. These findings indicate that measurements of iGSH in leukocytes may be a particularly useful biomarker to detect redox imbalance in mitochondrial disorders and organic acidemias, thus providing a relatively non-invasive means to monitor disease status and response to therapies. Furthermore, studies here suggest that antioxidant therapy may be useful for relieving the chronic oxidative stress that otherwise occurs in patients with mitochondrial dysfunction.
Keywords: organic acidemia, mitochondrial disorders
Mitochondrial disorders in aggregates have an incidence in the adult population of ≈1/8,500 and, therefore, are relatively common inborn errors of metabolism (1). These conditions may affect any organ system, either in isolation or in any combination, resulting in significant morbidity and mortality. Dysfunction of the mitochondrial respiratory chain decreases ATP production, as well as increases generation of intracellular reactive oxygen species (iROS) and reactive nitrogen species (iRNS), which are also byproducts of mitochondrial oxidative phosphorylation (OXPHOS) under normal conditions (2). Respiratory chain abnormalities have been documented in organic acidemia patients, such as methylmalonic acidemia (MMA) and propionic acidemia (PA), a knockout mouse model of MMA, and other animal models exposed to acids typically produced in excess in organic acidemias (3–5). The precise mechanism of respiratory chain impairment in organic acidemias is unknown, although impaired OXPHOS, generation of free radicals, and decreased iGSH are likely contributors to disease pathogenesis (3, 4, 6).
Intracellular reduced glutathione (iGSH) protects against oxidative damage, but is transformed in the process to its oxidized form (GSSG) (7). Because individuals with mitochondrial disease and organic acidemias generate an increased amount of iROS, it is likely that the GSH system in such instances is stressed to a higher degree than in individuals with normal mitochondrial function, resulting in deficiency of GSH and possibly its precursor cysteine. In support of this theory, GSH deficiency has been detected in a heterozygous manganese superoxide dismutase (MnSOD) knockout mouse model and more recently in a mut MMA mouse model (4, 8). Conversely, γ-glutamyltranspeptidase-deficient knockout mice, which are characterized by chronic GSH deficiency, have impaired mitochondrial respiratory chain function (9). In times of metabolic crisis, iROS production is likely increased, which could lead to rapid depletion of iGSH stores and subsequently diminished cellular capacity to detoxify these intermediates. Such a situation may explain why individuals with genetic disorders that affect mitochondrial function or iGSH homeostasis rapidly worsen in times of intercurrent catabolic illness that may result in overproduction of oxidants.
Although the association of mitochondrial dysfunction with oxidative stress has been clearly established (2), surprisingly few reports have examined this relationship directly in blood samples from patients with mitochondrial disease or other disorders associated with impaired respiratory chain function such as organic acidemias (10–12). Despite the growing list of identified mitochondrial disorders, as well as an increasing appreciation of the role mitochondrial dysfunction plays in the pathogenesis of diseases associated with advancing age (such as type 2 diabetes, cancer, and neurodegenerative disorders), relatively few diagnostic and therapeutic monitoring tools are available to physicians caring for individuals who have mitochondrial disease. Furthermore, the assessment of respiratory chain function after muscle biopsy, a commonly used but invasive diagnostic procedure, is often insensitive and unreliable (13). With these considerations in mind, we used high-dimensional flow cytometry (Hi-D FACS) to analyze leukocyte subsets from blood obtained from individuals with mitochondrial disorders and organic acidemias, hypothesizing that increased iROS generation in these conditions would result in low iGSH levels. We found that in patients with disorders that affect mitochondrial respiratory chain function iGSH levels were indeed low in T lymphocyte subsets, monocytes, and neutrophils, but not B lymphocytes. Such measurements may serve as potential biomarkers for mitochondrial disorders and organic acidemias, allowing for relatively non-invasive monitoring of disease status and response to therapies.
Results
Mitochondrial Disorders and Organic Acidemias Are Associated with Glutathione Deficiency.
To assess the redox (1) status of patients with disorders affecting mitochondria we measured levels of iGSH and iROS in peripheral blood leukocytes; and 2) plasma protein carbonyl levels. Our results show that mitochondrial disorders and organic acidemias result in iGSH deficiency and a significant increase in plasma carbonyl content (Figs. 1–3).
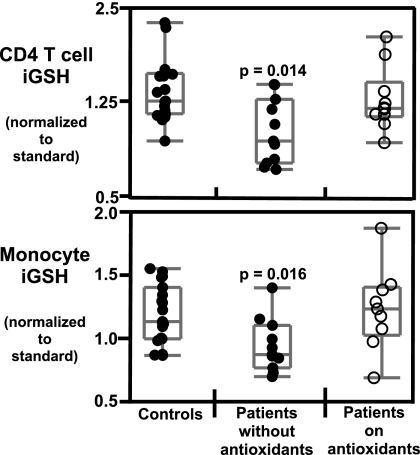
Fig. 1.
iGSH levels are lower in patients with mitochondrial disorders. iGSH levels were measured by the MCB assay on whole blood and analyzed by Hi-D FACS within 3 h of staining (see Materials and Methods). iGSH values are normalized to iGSH levels of a standard PBMC preparation stained and analyzed at the same time as patient samples. Top panel, iGSH levels in CD4 T cells; bottom panel, iGSH levels in monocytes. Statistical significance was determined by Wilcoxon/Kruskal Wallis non-parametric test. Each point represents a single sample. Adult controls (solid circles, n = 21); subjects not on antioxidant supplements (solid circles, n = 10); and subjects on antioxidant supplements (open circles, n = 11).
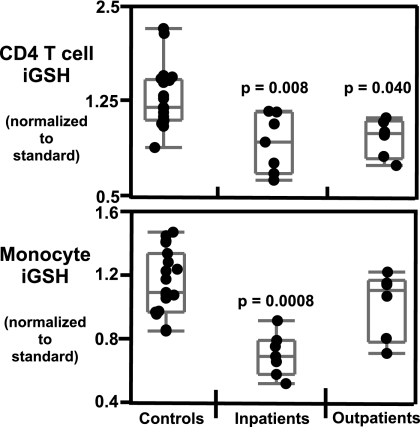
Fig. 2.
iGSH levels are lower in patients with organic acidemias. iGSH levels were measured by the MCB assay on whole blood and analyzed by Hi-D FACS within 3 h of staining (see Materials and Methods). iGSH levels are normalized to iGSH levels of a standard PBMC preparation stained and analyzed at the same time as patient samples. Top panel, iGSH levels in CD4 T cells; bottom panel, iGSH levels in monocytes. Statistical significance was determined by Wilcoxon/Kruskal Wallis non-parametric test. Each point represents a sample. Two subjects had 2 repeat measurements, and 1 subject had 3 repeat measurements. Adult controls (n = 21); inpatient subjects during an acute episode (n = 7); and outpatient subjects while clinically stable (n = 6).
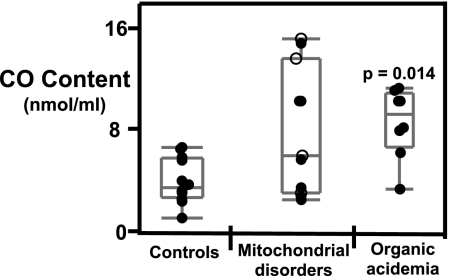
Fig. 3.
Plasma protein carbonyl levels are increased in subjects with organic acidemias. Plasma carbonyl levels were measured in 100 μL of platelet-free plasma as described in Materials and Methods. Statistical significance was determined by Wilcoxon/Kruskal Wallis non-parametric test for ranked sums using JMP software. Each point represents a single subject. Solid circles represent subjects not on antioxidants and open circles represent subjects on antioxidants. Adult controls (n = 10): subjects with mitochondrial disorders (n = 12); and subjects with organic acidemias (n = 8).
iGSH Levels in Mitochondrial Disorders.
Our study population consisted of 20 patients with either definite or probable mitochondrial disorders classified according to the criteria described in Materials and Methods. Ten subjects were not taking antioxidants at the time of assay, while 11 were supplemented with 1 or more antioxidants such as vitamin C, vitamin E, and coenzyme Q10 (see Table S1). One subject underwent 2 blood draws and started antioxidant supplements after the first draw. For data analysis we divided the patient cohort into 2 groups based on antioxidant status.
Levels of iGSH in CD4 T cells (P = 0.014), CD8 T cells (P = 0.005), monocytes (P = 0.016), and neutrophils (P = 0.044) were significantly lower in patients with mitochondrial disorders who were not taking antioxidants compared to healthy controls (Fig. 1 and Fig. S1). Subjects on antioxidant supplements were not significantly different in their iGSH levels compared to healthy controls.
iGSH in Organic Acidemias.
The organic acidemia cohort included patients with MMA, PA, and isovaleric acidemia (IVA). Of the 13 blood measurements in this cohort, 6 were obtained during routine outpatient clinic visits, while the patients were clinically well, and 7 were obtained during hospitalization for an acute metabolic crisis (see Table S1). One subject was taking vitamin C at the time of sample collection. For data analysis we divided the patients into 2 groups, inpatients (n = 7) and outpatients (n = 6). iGSH levels in CD4 T cells (P = 0.008), CD8 T cells (P = 0.003), monocytes (P = 0.0008), and neutrophils (P = 0.0006) are significantly lower in inpatients with organic acidemias as compared to healthy controls (Fig. 2 and Fig. S1). Lower GSH levels were detected only in CD4 T cells (P = 0.040) and CD8 T cells (P = 0.045) in outpatients. No significant reduction in iGSH levels was detected in B cells.
iROS Levels Are Not Elevated in Blood Cells in Diseases Affecting Mitochondria.
We did not detect significant overall differences in the basal levels of iROS between patient cohorts (mitochondrial disorders and organic acidemias) and healthy controls. However, 1 18-year-old female patient with thymidine kinase 2 deficiency and 1 20-year-old male with MELAS showed high levels of iROS.
Plasma Protein Carbonyl Content Is Elevated in Organic Acidemias.
Protein carbonyl levels in plasma, another marker for oxidative damage, were measured. Because of restrictions in the availability of plasma, only select samples were assayed for protein carbonyl levels (controls, n = 10; mitochondrial disorders, n = 12; organic acidemias, n = 8). Plasma from organic acidemia patients showed significantly higher levels of protein carbonyls (P = 0.014) as compared to healthy controls (Fig. 3). Plasma from patients with mitochondrial disorders, as a whole, did not show significantly higher levels of protein carbonyls, although 4 out of 10 samples showed elevated plasma carbonyl levels (Fig. 3).
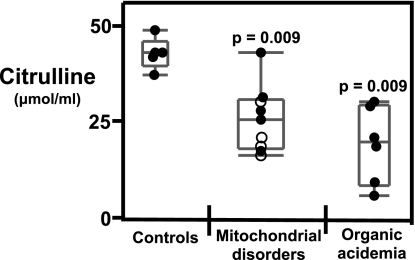
Mitochondrial Disorders and Organic Acidemias Are Associated with Lower Citrulline Levels in Plasma.
Forty standard and non-standard amino acids and their derivatives in the plasma of subjects with mitochondrial disorders and organic acidemias were assayed. Significantly lower citrulline levels were found in plasma in patients with mitochondrial disorders and organic acidemias (P = 0.009) (Fig. 4). Essential amino acids, particularly the branched chain amino acids valine, isoleucine, and leucine, were not significantly different in the patient cohorts as compared to healthy controls indicating no overall nutritional deficiency.
Fig. 4.
Mitochondrial disorders and organic acidemias are associated with hypocitrullinemia. Citrulline levels were measured in platelet-free plasma as described in Materials and Methods. Statistical significance was determined by Wilcoxon/Kruskal Wallis non-parametric test for ranked sums using JMP software. Each point represents a single subject. Solid circles represent subjects not on antioxidants and open circles represent subjects on antioxidants. Adults controls (n = 5): subjects with mitochondrial disorders n = 8; subjects with organic acidemias (n = 6).
Discussion
Studies here demonstrate low iGSH levels in blood cells from patients with disorders affecting mitochondrial function caused either by direct inhibition of the respiratory chain or by aberrant metabolism of branched chain amino acids. The low levels of this key intracellular antioxidant clearly indicate that these patients suffer from systemic oxidative stress, even during times of relatively good health. Our studies also demonstrate that iGSH levels are normal in mitochondrial patients taking antioxidants, suggesting that such supplementation may ameliorate some of the effects of impaired redox balance caused by disorders that affect mitochondrial respiratory chain function.
As we have shown, iGSH levels in CD4 and CD8 T lymphocytes, neutrophils and monocytes are decreased in individuals with mitochondrial disorders or organic acidemias who are not on antioxidant supplements. Interestingly, iGSH levels measured for patients and controls in B lymphocytes are equivalent, irrespective of the antioxidant status of the patients. The reasons underlying the difference between B cells and other blood cells in this respect are unclear.
Although we detected significantly decreased cellular iGSH levels, we did not detect a concomitant increase in iROS levels in blood samples. This could be due to the extremely transient nature of iROS, making their detection difficult in clinical settings. However, iROS production could be inferred from the observed decrease in iGSH, thus making iGSH measurement a more stable index of cellular redox status (14). Measurements of plasma amino acid levels did not reveal any significant changes in branched-chain amino acid levels in these patients, suggesting that nutritional insufficiency is less likely to be a major contributing factor for low iGSH levels. Since GSH is the main antioxidant in mammalian cells, a decrease in its intracellular levels, regardless of the mechanism, indicates chronic oxidative stress in patients with mitochondrial dysfunction.
There is strong theoretical rationale and previous experimental evidence suggesting that redox imbalance plays a major role in the pathogenic effects seen in patients with mitochondrial disease (2). The most widely accepted mechanism of chronic oxidative stress pathogenesis involves generation of oxidative metabolites (iROS, iRNS, and other free radicals) that deplete cellular antioxidant stores, leading to protein, lipid, and DNA damage. Numerous in vitro studies have shown that inhibition of respiratory chain complexes results in elevated levels of ROS within the mitochondrial matrix, ultimately leading to oxidative stress (15). These reports are supported by studies documenting increased production of ROS, decreased GSH, a compensatory increase in antioxidant enzymes, and elevated lipid hydroperoxide levels in blood and biopsy samples from a variety of mitochondrial disorders (10, 16, 17). Two reports on chronic progressive external ophthalmoplegia (CPEO) demonstrated low GSH levels in plasma and erythrocytes, higher levels of ROS, and a compensatory increase in antioxidant enzyme in muscle fibroblasts (10, 17).
Histochemical and immunohistochemical studies on muscle biopsies have shown that mitochondrial disorders caused by point mutations or deletions in mtDNA lead to an induction of antioxidant enzymes, possibly to counter chronic oxidative stress (16). Our study further supports the hypotheses that (i) mitochondrial diseases are associated with chronic oxidative stress and (ii) systemic levels of oxidative stress are reflected in peripheral blood GSH levels, making such measurements a potentially useful and non-invasive assay to routinely monitor redox imbalance.
Patients taking antioxidant supplements did not show decreased iGSH levels. This important observation lends support to the relatively common practice of treating mitochondrial disorders using a variety of antioxidants (1). Oxidative stress (iGSH depletion) further inhibits respiratory chain function, thus initiating a vicious cycle that ultimately increases the chances of accumulation of new mutations in mtDNA (8, 18). Further clinical studies are needed to determine whether the observed improvement of cellular iGSH levels in patients taking antioxidant supplementation is a general phenomenon, or applies to only a subset of mitochondrial disease patients. This study does not address which of the antioxidants or combination of antioxidants may be most active, nor what dose is optimal to achieve the observed effects. However, these results lay the foundation for a prospective study, with blinded and cross-over design, to address such questions.
Our results indicate that MMA, PA, and IVA patients have decreased iGSH. This observation supports a previous report of blood total glutathione deficiency in a 7-year-old boy with MMA during a metabolic crisis. This child responded favorably to high dose ascorbate supplementation, which the authors suggested replaced the antioxidant activity of glutathione (12). Other organic acidemias have not been studied in this manner. Nevertheless, studies in animal models have demonstrated a clear link between organic acid metabolites and oxidative stress-induced mitochondrial dysfunction (3, 4, 19). Our findings also lend support to evidence of increased ROS production and mitochondrial impairment found in animal studies and fibroblasts or liver samples obtained from organic acidemia patients (5, 6, 19).
Consistent with the idea of increased oxidative damage in organic acidemias, we have detected high levels of protein carbonyls in plasma from these patients. Protein carbonyls are well-established biomarkers of oxidative protein damage in vivo in various diseases (20). Although protein carbonyls are primarily caused by ROS-mediated protein damage, high protein carbonyl content in patients with organic acidemias may also be secondary to elevated levels of reactive aldehyde intermediates of organic acids in the blood.
We detected hypocitrullenimia in plasma from mitochondrial and organic acidemia subjects. Hypocitrullinemia has also been reported in some individuals with Reye syndrome, MELAS, and NARP (21–23). Low levels of plasma citrulline are a classic biochemical hallmark of proximal urea cycle disorders. In addressing the relationship between citrulline levels and mitochondrial function, it has been postulated that primary deficiencies in OXPHOS result in decreased citrulline synthesis via secondary impairment of carbamyl phosphate synthetase I, an early urea cycle enzyme that plays a key role in citrulline synthesis (23), or by inhibiting production of the citrulline precursor Δ-1-pyrroline carboxylate through inhibition of proline oxidase (22).
There is increasing interest in identifying biomarkers of oxidative stress in human disease. ROS generation due to mitochondrial dysfunction likely plays a role in multiple disorders, including diabetes, atherosclerosis, neurodegenerative diseases, hypoxic-ischemic encephalopathy, autism, retinopathy of prematurity, and cancer (20, 24). Our results indicate that iGSH measured by Hi-D FACS may be a useful biomarker for assessing degree of mitochondrial impairment and even response to therapy in mitochondrial and organic acidemia patients.
At present, antioxidant supplements are often given to mitochondrial patients without the ability to monitor therapeutic response. However, antioxidant supplementation has not been widely used for the management of organic acidemia patients. Given the significantly low iGSH levels detected in patients with organic acidemias, especially during acute illness, there may be a role for such supplementation in these patients as well. Even with optimal metabolic control, organic acidemia patients often demonstrate significant mental retardation. Some patients have had so-called “metabolic strokes,” i.e., injury to deep gray matter structures, but many without obvious brain imaging abnormalities also display cognitive impairment (25). Because iGSH deficiency likely plays a role in pathogenesis of neurodegenerative diseases, it is also reasonable to speculate that therapies that improve redox imbalance may be beneficial for cognitive and neurologic outcome in organic acidemias and mitochondrial disorders.
In this observational study, iGSH deficiency was demonstrated in patients with disorders characterized by mitochondrial dysfunction who were not taking antioxidants, despite the heterogeneous nature of the subject population. However, subjects on antioxidants did not show detectable iGSH deficiency. Although these findings need to be confirmed in a larger cohort of patients, results presented here show that iGSH measurement represents an important initial step toward a rational assessment of therapeutic response, and even the development of individualized treatment regimens, in disorders that affect mitochondrial function. Moreover, these findings provide the foundation to embark on further studies focusing on the relationship of factors such as age, specific diagnosis, disease severity, clinical status, and treatment to iGSH deficiency in mitochondrial disease.
Methods
Materials.
All monoclonal antibodies (either purified or preconjugated to fluorochromes) were procured from Becton Dickinson Biosciences (BDB). PE and Allophycocyanin were obtained from Prozyme. Monochlorobimane (MCB), Dihydrorhodamine 123 (DHR123), and RPMI medium 1640 were obtained from Invitrogen. Probenecid and other fine chemicals were obtained from Sigma Aldrich. Protein Carbonyl assay kit was procured from Cayman Chemicals.
Human Subjects.
Twenty-nine subjects were included in the study, including 9 with organic acidemias (6 MMA mut0, 2 IVA, 1 PA) and 20 with mitochondrial disorders [4 tRNALeu3243A>G, 4 complex I deficiency, 2 complex IV deficiency, 2 combined complex I/III deficiency, 1 combined complex I/IV deficiency (tRNALeu3243A>T), 1 combined complex II/III deficiency, 1 complex III deficiency, 1 mtDNA deletion syndrome, 1 mtDNA depletion syndrome (TK2 deficiency), and 3 with undefined disease but with clinical features including Leigh syndrome or multiorgan system involvement, and biochemical findings consistent with mitochondrial disease]. Organic acidemia diagnoses were established by urine organic acid analysis; MMA patients further underwent complementation studies on cultured skin fibroblasts (Dr. David Rosenblatt, McGill University, Montreal, Canada). The organic acidemia cohort was further classified by clinical status as either inpatient (acutely ill) or outpatient (clinically stable). Mitochondrial disease was diagnosed based on clinical signs and symptoms, as well as standard biochemical and molecular analyses (e.g., muscle or skin fibroblast respiratory chain activities and mitochondrial DNA or nuclear DNA sequencing). Subjects were classified as having definite (n = 11) or probable (n = 9) mitochondrial disease based on diagnostic criteria defined by Bernier et al. (26). For data analysis the mitochondrial disease cohort was classified according to whether or not subjects were taking pharmacologic doses of supplements with antioxidant activity (e.g., ascorbate, vitamin E, α-lipoic acid, coenzyme Q10) at the time of sample collection. Antioxidant supplementation was not standardized. All controls were adults and not age matched. Samples were collected after informed consent. The Stanford University Institutional Review Board approved all study protocols.
Sample Collection and Preparation.
Peripheral blood (1–5 mL) from patients and healthy volunteers was collected by venipuncture into heparanized tubes (Vacutainer BDB). Blood was processed as previously described (27). In brief, blood was centrifuged at 400 × g and the plasma fraction was collected for further processing. The remaining cellular fraction was washed with DPBS-EDTA (DPBS containing 2.5 mM EDTA) and finally resuspended in bimane medium in a volume equivalent to the original volume of blood collected. The plasma fraction was further centrifuged for 10 min at 3,000 × g to remove the platelets and the resulting platelet-free plasma was aliquoted and stored at -80 °C for amino acid and protein carbonyl assays.
FACS Assays for Intracellular Redox Status.
The intracellular redox state of peripheral blood leukocytes was determined by FACS assays for iGSH and iROS according to Atkuri et al. (28). In brief, separate aliquots of cells were stained with 40 μM monochlorobimane (for iGSH) or 1 μM DHR123 (for iROS) for 20 min in staining media (RPMI medium 1640, 4% FCS and 2.5 mM probenecid) at room temperature. The reaction was quenched with excess chilled staining media. The cells were then centrifuged and resuspended in staining media for further processing for Hi-D FACS. iGSH levels were expressed as median fluorescence intensity (MFI) of intracellular GS-bimane adducts.
High-Dimensional (Hi-D) FACS Analysis.
Fifty microliters of washed cellular fraction was stained with different cocktails of fluorochrome-conjugated antibodies [CD3, CD4, CD8, CD14, CD16, CD19, CD45, CD235 (glycophorin)] prepared in our laboratory or obtained from BD-PharMingen. Surface staining was performed as described (29, 30). Hi-D FACS data were collected on a modified BD FACStar with Moflo electronics (Cytomation, MO) or BD FACSAria (BD). Flowjo (Treestar) software was used for fluorescence compensation and analysis. See Fig. S2 for cell gating scheme.
iGSH Levels Normalization to Correct for Experimental Variation.
iGSH levels were expressed relative to iGSH levels measured in the same experiment for a standard PBMC preparation. The standard PBMC preparation was isolated by Ficoll gradient centrifugation from a 500-mL blood sample from a healthy individual, aliquoted, and maintained in liquid nitrogen until immediately before use. Aliquots of the same standard were used for all normalizations carried out in this study.
Plasma Protein Carbonyl Assay.
Plasma protein carbonyls were assayed according to the protocol provided by the manufacturer (Cayman Chemicals; catalog # 10005020).
Amino Acid Analysis of Plasma.
Plasma amino acids were measured by ninhydrin derivatization followed by spectrometric detection with S-aminoethylcystine as an internal standard as described by Spackman et al. (31).
Statistical Analysis.
Analyses of FACS data, was performed using FlowJo software (Treestar). Statistical analyses were performed with the JMP statistical software package (SAS Institute).
Supplementary Material
Acknowledgments.
We thank the following members of the Herzenberg Laboratory (Genetics Department, Stanford University School of Medicine): Bahram Aram and Glenn Smith for excellent and devoted technical support; and John J. Mantovani for administrative help, including the preparation of the manuscript. We thank Takeshi Fukuhara (VA Hospital) for help with measurements with protein carbonyls. We thank Vicki Sweet, RN, PNP; Andrea Kwan, MS; Elizabeth Hadley, RN; and Daphne Nayyar, RN for coordinating the patient sample collection. This work was generously supported by our community, the Lucile Packard Foundation For Children's Health, and by grants from the United Mitochondrial Disease Foundation, the Lucile Packard Children's Fund, and the Arline and Pete Harman Scholarship.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0813409106/DCSupplemental.
References
- 1.Chinnery PF, Turnbull DM. Epidemiology and treatment of mitochondrial disorders. Am J Med Genet. 2001;106:94–101. doi: 10.1002/ajmg.1426. [DOI] [PubMed] [Google Scholar]
- 2.Wallace DC. Mitochondrial diseases in man and mouse. Science. 1999;283:1482–1488. doi: 10.1126/science.283.5407.1482. [DOI] [PubMed] [Google Scholar]
- 3.Fontella FU, et al. Propionic and L-methylmalonic acids induce oxidative stress in brain of young rats. Neuroreport. 2000;11:541–544. doi: 10.1097/00001756-200002280-00023. [DOI] [PubMed] [Google Scholar]
- 4.Chandler RJ, et al. Mitochondrial dysfunction in mut methylmalonic acidemia. FASEB J. 2008 doi: 10.1096/fj.08-121848. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mardach R, Verity MA, Cederbaum SD. Clinical, pathological, and biochemical studies in a patient with propionic acidemia and fatal cardiomyopathy. Mol Genet Metab. 2005;85:286–290. doi: 10.1016/j.ymgme.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 6.Richard E, Alvarez-Barrientos A, Perez B, Desviat LR, Ugarte M. Methylmalonic acidaemia leads to increased production of reactive oxygen species and induction of apoptosis through the mitochondrial/caspase pathway. J Pathol. 2007;213:453–461. doi: 10.1002/path.2248. [DOI] [PubMed] [Google Scholar]
- 7.Schafer FQ, Buettner GR. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic Biol Med. 2001;30:1191–1212. doi: 10.1016/s0891-5849(01)00480-4. [DOI] [PubMed] [Google Scholar]
- 8.Williams MD, et al. Increased oxidative damage is correlated to altered mitochondrial function in heterozygous manganese superoxide dismutase knockout mice. J Biol Chem. 1998;273:28510–28515. doi: 10.1074/jbc.273.43.28510. [DOI] [PubMed] [Google Scholar]
- 9.Will Y, et al. gamma-glutamyltranspeptidase-deficient knockout mice as a model to study the relationship between glutathione status, mitochondrial function, and cellular function. Hepatology. 2000;32:740–749. doi: 10.1053/jhep.2000.17913. [DOI] [PubMed] [Google Scholar]
- 10.Piccolo G, et al. Biological markers of oxidative stress in mitochondrial myopathies with progressive external ophthalmoplegia. J Neurol Sci. 1991;105:57–60. doi: 10.1016/0022-510x(91)90118-q. [DOI] [PubMed] [Google Scholar]
- 11.Piemonte F, et al. Glutathione in blood of patients with Friedreich's ataxia. Eur J Clin Invest. 2001;31:1007–1011. doi: 10.1046/j.1365-2362.2001.00922.x. [DOI] [PubMed] [Google Scholar]
- 12.Treacy E, et al. Glutathione deficiency as a complication of methylmalonic acidemia: response to high doses of ascorbate. J Pediatr. 1996;129:445–448. doi: 10.1016/s0022-3476(96)70080-x. [DOI] [PubMed] [Google Scholar]
- 13.Thorburn DR, Smeitink J. Diagnosis of mitochondrial disorders: Clinical and biochemical approach. J Inherit Metab Dis. 2001;24:312–316. doi: 10.1023/a:1010347808082. [DOI] [PubMed] [Google Scholar]
- 14.Pastore A, Federici G, Bertini E, Piemonte F. Analysis of glutathione: Implication in redox and detoxification. Clin Chim Acta. 2003;333:19–39. doi: 10.1016/s0009-8981(03)00200-6. [DOI] [PubMed] [Google Scholar]
- 15.Turrens JF. Superoxide production by the mitochondrial respiratory chain. Biosci Rep. 1997;17:3–8. doi: 10.1023/a:1027374931887. [DOI] [PubMed] [Google Scholar]
- 16.Filosto M, et al. Antioxidant agents have a different expression pattern in muscle fibers of patients with mitochondrial diseases. Acta Neuropathol (Berl) 2002;103:215–220. doi: 10.1007/s004010100455. [DOI] [PubMed] [Google Scholar]
- 17.Lu CY, Wang EK, Lee HC, Tsay HJ, Wei YH. Increased expression of manganese-superoxide dismutase in fibroblasts of patients with CPEO syndrome. Mol Genet Metab. 2003;80:321–329. doi: 10.1016/j.ymgme.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 18.Jha N, et al. Glutathione depletion in PC12 results in selective inhibition of mitochondrial complex I activity. Implications for Parkinson's disease. J Biol Chem. 2000;275:26096–26101. doi: 10.1074/jbc.M000120200. [DOI] [PubMed] [Google Scholar]
- 19.Hayasaka K, et al. Comparison of cytosolic and mitochondrial enzyme alterations in the livers of propionic or methylmalonic acidemia: A reduction of cytochrome oxidase activity. Tohoku J Exp Med. 1982;137:329–334. doi: 10.1620/tjem.137.329. [DOI] [PubMed] [Google Scholar]
- 20.Dalle-Donne I, Rossi R, Colombo R, Giustarini D, Milzani A. Biomarkers of oxidative damage in human disease. Clin Chem. 2006;52:601–623. doi: 10.1373/clinchem.2005.061408. [DOI] [PubMed] [Google Scholar]
- 21.Schiff GM. Reye's syndrome. Annu Rev Med. 1976;27:447–452. doi: 10.1146/annurev.me.27.020176.002311. [DOI] [PubMed] [Google Scholar]
- 22.Naini A, et al. Hypocitrullinemia in patients with MELAS: An insight into the “MELAS paradox. J Neurol Sci. 2005;229–230:187–193. doi: 10.1016/j.jns.2004.11.026. [DOI] [PubMed] [Google Scholar]
- 23.Parfait B, et al. The neurogenic weakness, ataxia, and retinitis pigmentosa (NARP) syndrome mtDNA mutation (T8993G) triggers muscle ATPase deficiency and hypocitrullinaemia. Eur J Pediatr. 1999;158:55–58. doi: 10.1007/s004310051009. [DOI] [PubMed] [Google Scholar]
- 24.Enns GM. The contribution of mitochondria to common disorders. Mol Genet Metab. 2003;80:11–26. doi: 10.1016/j.ymgme.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 25.Heidenreich R, et al. Acute extrapyramidal syndrome in methylmalonic acidemia: “Metabolic stroke” involving the globus pallidus. J Pediatr. 1988;113:1022–1027. doi: 10.1016/s0022-3476(88)80574-2. [DOI] [PubMed] [Google Scholar]
- 26.Bernier FP, et al. Diagnostic criteria for respiratory chain disorders in adults and children. Neurology. 2002;59:1406–1411. doi: 10.1212/01.wnl.0000033795.17156.00. [DOI] [PubMed] [Google Scholar]
- 27.Tirouvanziam R, et al. High-dose oral N-acetylcysteine, a glutathione prodrug, modulates inflammation in cystic fibrosis. Proc Natl Acad Sci USA. 2006;103:4628–4633. doi: 10.1073/pnas.0511304103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Atkuri KR, Herzenberg LA, Niemi AK, Cowan T, Herzenberg LA. Importance of culturing primary lymphocytes at physiological oxygen levels. Proc Natl Acad Sci USA. 2007;104:4547–4552. doi: 10.1073/pnas.0611732104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.De Rosa SC, Roederer M. Eleven-color flow cytometry. A powerful tool for elucidation of the complex immune system. Clin Lab Med. 2001;21:697–712. [PubMed] [Google Scholar]
- 30.Sahaf B, Heydari K, Herzenberg LA. Lymphocyte surface thiol levels. Proc Natl Acad Sci USA. 2003;100:4001–4005. doi: 10.1073/pnas.2628032100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Spackman DH, Moore S, Stein WH. Automatic recording apparatus for use in the chromatography of amino acids. Fed Proc. 1958;17:1107–1115. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.