Abstract
Patients with sepsis have a marked defect in neutrophil migration. Here we identify a key role of Toll-like receptor 2 (TLR2) in the regulation of neutrophil migration and resistance during polymicrobial sepsis. We found that the expression of the chemokine receptor CXCR2 was dramatically down-regulated in circulating neutrophils from WT mice with severe sepsis, which correlates with reduced chemotaxis to CXCL2 in vitro and impaired migration into an infectious focus in vivo. TLR2 deficiency prevented the down-regulation of CXCR2 and failure of neutrophil migration. Moreover, TLR2−/− mice exhibited higher bacterial clearance, lower serum inflammatory cytokines, and improved survival rate during severe sepsis compared with WT mice. In vitro, the TLR2 agonist lipoteichoic acid (LTA) down-regulated CXCR2 expression and markedly inhibited the neutrophil chemotaxis and actin polymerization induced by CXCL2. Moreover, neutrophils activated ex vivo by LTA and adoptively transferred into naïve WT recipient mice displayed a significantly reduced competence to migrate toward thioglycolate-induced peritonitis. Finally, LTA enhanced the expression of G protein–coupled receptor kinases 2 (GRK2) in neutrophils; increased expression of GRK2 was seen in blood neutrophils from WT mice, but not TLR2−/− mice, with severe sepsis. Our findings identify an unexpected detrimental role of TLR2 in polymicrobial sepsis and suggest that inhibition of TLR2 signaling may improve survival from sepsis.
Keywords: chemokine receptor, neutrophil, sepsis, Toll-like receptor
Successful clearance of bacterial infection depends on efficient neutrophil migration into the infected tissues (1). We and others have demonstrated a marked defect in neutrophil recruitment into the infectious focus during severe experimental sepsis, followed by failure of local bacterial clearance and dissemination of infection, resulting in inappropriate systemic inflammation and increased mortality (2–4). A defect of neutrophil migration also has been described in human sepsis (5). In accordance with these experimental studies, the reduction of neutrophil chemotaxis to several chemotactic mediators has been associated with illness severity and organ damage (6, 7).
The Toll-like receptors (TLRs) have been identified as the key receptors that recognize conserved components of invading pathogens (8). Although signaling through TLRs has been implicated as an important element of host defense, a growing body of evidence indicates that these receptors also may play a role in the pathophysiology of sepsis. Recent studies using mice with single TLR deficiencies have demonstrated the relative importance of TLR4 and TLR9 in the pathophysiology of polymicrobial sepsis (9, 10). These findings have raised the possibility that stimulation of multiple TLRs is required for an overwhelming inflammatory response. In this context, no study has yet examined the role of TLR2 in polymicrobial sepsis.
In the present study, we provide evidence that the systemic activation of TLR2 contributes to mortality in mice subjected to severe cecal ligation and puncture (CLP), a model of acute polymicrobial septic peritonitis (11). We found that TLR2 signaling down-regulated the expression of the chemokine receptor CXCR2 on the surface of circulating neutrophils, impairing migration to the site of infection and affecting disease severity.
Results
TLR2 Deficiency Improves Survival in Polymicrobial Sepsis.
To explore the role of TLR2 in polymicrobial sepsis, we performed nonsevere (NS) and severe (S) septic peritonitis using CLP in WT and TLR2-deficient (TLR2−/−) mice. All of the WT and TLR2−/− mice subjected to NS-CLP survived for 7 days after CLP; however, whereas 100% of the WT mice subjected to S-CLP died within 2 days, the TLR2−/− mice had a significantly enhanced survival rate, with 40% surviving to day 2 and 35% surviving to the end of the observation period (Fig. 1A).
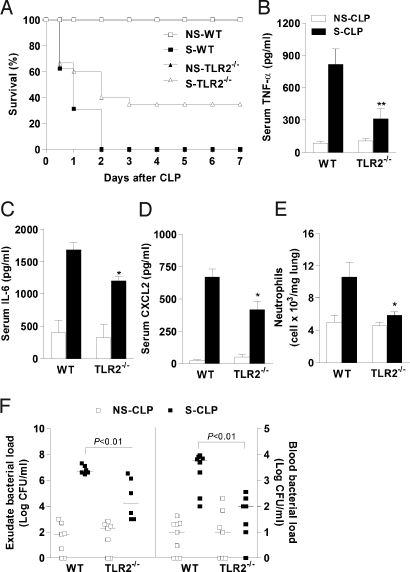
Fig. 1.
TLR2 deficiency improves survival during septic peritonitis. (A) Survival rate after NS-CLP or S-CLP in WT or TLR2−/− mice (n = 20). P < .05; log-rank test. (B–D) ELISA of TNF-α (B), IL-6 (C), and CXCL2 (D) in serum of WT mice (n = 10) and TLR2−/− mice (n = 10) 6 h after CLP. (E) Neutrophil sequestration in lung measured 6 h after CLP. Data are mean ± SEM. *P < .05, **P < .01 relative to WT S-CLP mice. (F) Colony-forming units in peritoneal exudate and blood 6 h after CLP. Horizontal bars represent median values, and squares represent individual mice (n = 6–8 each).
High levels of systemic inflammatory cytokines and neutrophil sequestration in the lung are markers and causative agents of poor prognosis in sepsis (12, 13). Six hours after S-CLP, the WT mice exhibited significant increases in serum TNF-α, IL-6, and CXCL2 levels (Fig. 1 B–D). They also demonstrated increased myeloperoxidase (MPO) activity (a quantitative measure of neutrophil sequestration) in the lung tissue compared with the mice subjected to NS-CLP (Fig. 1E). In contrast, these markers were substantially reduced in the TLR2−/− mice subjected to S-CLP, as were the numbers of bacterial cfu in peritoneal exudates and blood (Fig. 1F).
TLR2 Deficiency Prevents Impairment of Neutrophil Migration in Severe Polymicrobial Sepsis.
To investigate the mechanism underlying the improved survival in TLR2−/− mice, we evaluated neutrophil recruitment into the infectious focus. The WT mice with S-CLP exhibited impaired neutrophil migration, with 3-fold fewer neutrophils in the peritoneal cavity, compared with the WT and TLR2−/− mice subjected to NS-CLP (Fig. 2A). In contrast, the TLR2−/− mice with S-CLP demonstrated only modest reduced neutrophil migration, comparable to that in the WT and TLR2−/− NS-CLP mice. Moreover, using intravital microscopy to visualize leukocyte–endothelial cell interactions, we found that the impaired neutrophil migration in the WT S-CLP mice was associated with a reduced number of adherent leukocytes to the postcapillary venules of the mesentery. This reduction was not observed in the TLR2−/−S-CLP mice (Fig. 2B).
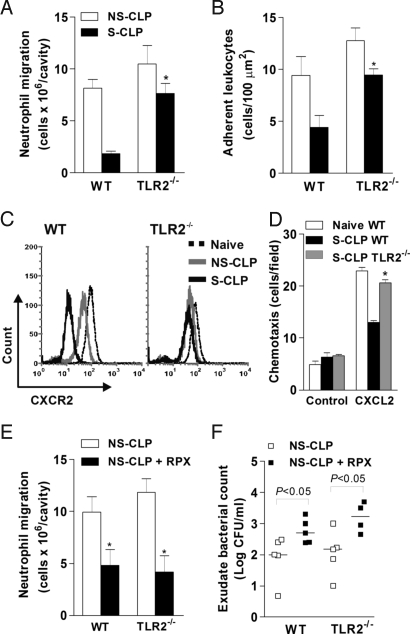
Fig. 2.
TLR2 deficiency prevents impairment of neutrophil migration. (A) Neutrophils in peritoneal exudate of WT and TLR2−/− mice 6 h after CLP (n = 15). (B) Adherent leukocytes in mesenteric venules 4 h after CLP as determined by intravital microscopy. (C) Flow cytometry of surface expression of CXCR2 on blood neutrophils of WT mice (Right) and TLR2−/− mice (Left) 2 h after CLP. (D) Chemotaxis of neutrophils to CXCL2 isolated from whole blood 2 h after CLP. (E and F) Neutrophil number (E) and cfu (F) in peritoneal exudate of WT and TLR2−/− mice treated with CXCR2 antagonist 6 h after NS-CLP (n = 10). Mice were injected i.v. with PBS or RTX (30 mg/kg) 30 min before CLP. Data are mean ± SEM. *P < .01 relative to WT S-CLP mice.
Neutrophil recruitment is a complex process involving adhesion molecules and inflammatory mediators. Interestingly, we found no significant differences in the levels of cytokines TNF-α and IL-6 and in chemokines CXCL1, CXCL2, and CCL2 in the peritoneal exudate of WT and TLR2−/− mice after CLP [supporting information (SI) Fig. S1]. Moreover, the number of neutrophils in the blood were comparable in the 2 groups (data not shown). Thus, it seems unlikely that a local difference in cytokine production or circulating neutrophil level can explain the increased neutrophil migration seen in the TLR2−/− mice.
Reduction of neutrophil migration is associated with decreased CXCR2 protein expression on the membrane of circulating neutrophils (14). To address a possible role of TLR2 in the regulation of CXCR2 expression, we performed a flow cytometry analysis. Two hours after S-CLP, CXCR2 expression on neutrophils was significantly reduced in the WT mice compared with the NS-CLP group or naïve mice (Fig. 2C), and this reduction was correlated with decreased chemotaxis to CXCL2 (30 ng/mL) (Fig. 2D). Notably, CXCR2 expression and chemotaxis to CXCL2 were significantly higher in neutrophils from TLR2−/− mice compared with those from WT mice subjected to S-CLP (Fig. 2 C and D). This suggests that the maintenance of CXCR2 expression may explain the increased neutrophil migration and enhanced bacterial clearance seen in the TLR2−/− mice. In support of this supposition, TLR2−/− mice that received the CXCR2 antagonist repertaxin (RPX) exhibited markedly reduced neutrophil migration and abrogation of the enhanced bacterial clearance (Fig. 2 E and F).
Systemic Injection of TLR2 Agonist Suppresses Neutrophil Migration via Down-Regulation of CXCR2.
We hypothesized that systemic activation of TLR2 down-regulates expression of CXCR2 on blood neutrophils, inhibiting their migration into the inflammatory site. To investigate our hypothesis, mice were given a single i.v. injection of the TLR2 agonist lipoteichoic acid (LTA), and after 2 h, surface CXCR2 expression and chemotaxis to CXCL2 in blood neutrophils were evaluated. The LTA injection induced a substantial reduction in the cell surface expression of CXCR2 on blood neutrophils (Fig. 3A), which was associated with decreased chemotaxis to CXCL2 (Fig. 3B).
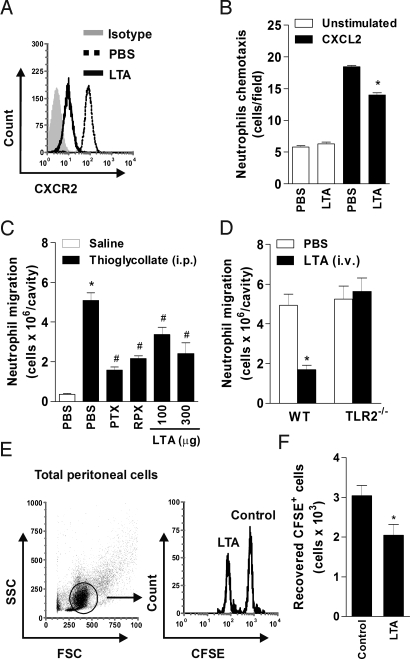
Fig. 3.
Systemic injection of TLR2 agonist suppresses neutrophil migration. (A) Flow cytometry of CXCR2 expression on blood neutrophils 2 h after i.v. injection of PBS or LTA (300 μg/mouse). (B) Chemotaxis of blood neutrophils to CXCL2 isolated 2 h after i.v. LTA injection. Data are mean ± SEM. *P < .001 relative to WT S-CLP mice. (C and D) Neutrophils in peritoneal exudate 4 h after i.p thioglycollate injection. (C) Mice were treated with PBS, PTX (4 μg/mouse), RPX (30 mg/kg), or LTA (100 and 300 μg/mouse) 30 min before thioglycollate injection. (D) WT and TLR2−/− mice were injected with either PBS or LTA (300 μg/mouse, i.v.) 30 min before thioglycollate injection (n = 8). Data are mean ± SEM. *P < .001 relative to the PBS group plus thioglycollate. (E and F) Adoptive transfer of ex vivo LTA-activated neutrophils. BM neutrophils were treated with LTA or untreated (control), labeled with 0.5 or 5.0 μM CSFE, respectively, and infused i.v. into WT recipient mice in equal numbers. Then thioglycollate was injected i.p., and the peritoneal cells were harvested 4 h later. (E) Representative flow cytometry results of recovered CFSE+ cells from an individual mouse (n = 5). (F) Counts of recovered CFSE+ cells. Data are mean ± SEM. *P < .05.
To examine whether i.v. injection of LTA also could affect neutrophil influx into inflammatory sites, we evaluated neutrophil migration into thioglycollate-induced peritonitis 4 h after LTA i.v. injection. We further confirmed the role of Gαi-coupled CXCR2 in neutrophil migration induced by thioglycollate, using the CXCR2 antagonist RPX (30 mg/kg) and pertussis toxin (PTX; 4 μg/mouse). Neutrophil recruitment to the peritoneum was reduced in mice pretreated with PTX or RPX compared with control PBS mice (Fig. 3C). Notably, significantly inhibited thioglycollate-induced neutrophil migration was observed after the LTA treatment (Fig. 3C). We confirmed that the inhibitory effects of LTA on neutrophil migration were mediated by TLR2 activation in the TLR2−/− mice (Fig. 3D). These findings indicate that systemic injection of LTA activates neutrophils in the circulation, promoting the down-regulation of surface CXCR2 expression and inhibiting neutrophil migration to the inflammatory site.
To investigate whether this effect of LTA is due to direct activation of neutrophils in the circulation, we incubated purified bone marrow (BM) neutrophils ex vivo in the presence or absence of LTA (10 μg/mL) for 1 h. We then labeled the LTA-treated and control cells with 0.5 or 5.0 μM of the intracellular fluorescent dye carboxyfluorescein succinimidyl ester (CFSE), respectively, to obtain CFSEhigh (control) and CFSElow (LTA-treated) populations (Fig. S2). A single-cell suspension, in a 1:1 ratio, was administered i.v. into recipient WT mice, followed immediately by the thioglycollate injection into the peritoneum. After 4 h, peritoneal cells were harvested, and CFSE-positive cells were analyzed by flow cytometry (Fig. 3E). As evidenced by the i.v. injection of LTA, neutrophils activated ex vivo with LTA migrated less efficiently into the peritoneal cavity compared with control neutrophils (Fig. 3F).
Direct Activation of TLR2 in Neutrophils Down-Regulates CXCR2 and Impairs Chemotaxis.
To address the direct effects of LTA and TLR2 signaling on surface CXCR2 expression of neutrophils, we incubated BM neutrophils with LTA for 1 h. Flow cytometry revealed that this LTA treatment reduced the surface expression of CXCR2 on neutrophils in a concentration-dependent manner compared with control cells (Fig. 4A). We confirmed that these changes in receptor expression were not due to changes in cellular viability, because the LTA-treated neutrophils had the same percentage of cell viability (Annexin V−/PI−) as the control cells (Fig. S3). Thus, we next determined the functional significance of the LTA-induced CXCR2 down-regulation by assessing chemotaxis and F-actin polymerization. We found a significant concentration- and time-dependent reduction in chemotaxis to CXCL2 in neutrophils treated with LTA (Fig. 4 B and C), and similar results for CXCL1 (data not shown). We confirmed the ability of CXCL2 to induce neutrophil chemotaxis through CXCR2, using antibodies to CXCR2 and the CXCR2 antagonist RPX (Fig. 4B). The decreased surface expression of CXCR2 by LTA was accompanied by reduced F-actin polymerization in response to CXCL2 (Fig. 4D).
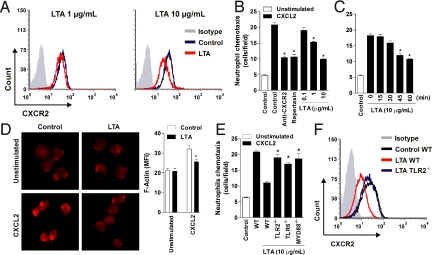
Fig. 4.
Direct activation of TLR2 impairs chemotaxis in neutrophils. (A) Flow cytometry of CXCR2 expression on BM neutrophils treated with 1 μg/mL (Right) or 10 μg/mL (Left) of LTA or untreated (control) for 1 h. (B and C) Chemotaxis of BM neutrophils to CXCL2. (B) Neutrophils treated with anti-CXCR2 antibody, RPX (30 mg/kg), or LTA (0.1, 1, or 10 μg/mL) for 1 h. (C) Neutrophils treated with LTA (10 μg/mL) for different times, as indicated. Data are mean ± SEM. *P < .001 relative to the control group plus CXCL2. (D) F-actin polymerization of BM neutrophils to CXCL2 after LTA treatment. Representative images from 3 independent experiments are shown (Left). F-actin polymerization was quantified by mean fluorescence intensity (Right). *P < .001 relative to the control group plus CXCL2. (E) Chemotaxis of WT, TLR2−/−, TLR6−/−, and Myd88−/− BM neutrophils to CXCL2 after LTA treatment. (F) Flow cytometry of CXCR2 expression on WT and TLR2−/− BM neutrophils after LTA treatment.
To address the role of TLR2 signaling in mediating the effects of LTA, we isolated BM neutrophils from WT, TLR2−/−, TLR6−/−, and MyD88−/− mice, incubated them with LTA (10 μg/mL), and evaluated the chemotaxis to CXCL2. The LTA-induced reduction of chemotaxis to CXCL2 in WT neutrophils was reversed in neutrophils lacking TLR2, TLR6, or MyD88 (Fig. 4E). The down-regulation of surface CXCR2 expression induced by LTA was inhibited in neutrophils from the TLR2−/− mice (Fig. 4F). In accordance with these findings, we confirmed significant TLR2 expression in WT BM neutrophils (Fig. S4).
TLR2 Activation Up-Regulates GRK2 Expression in Neutrophils.
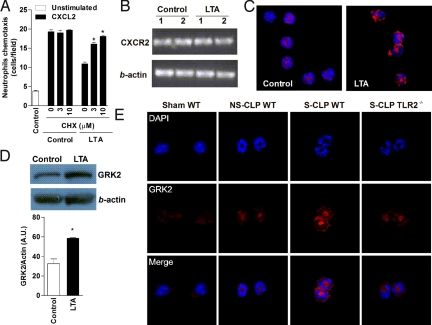
To investigate the mechanisms through which TLR2 activation exerts its regulatory effect on CXCR2, we first used PCR to explore whether changes in the surface expression of CXCR2 reflect changes in the gene expression of this receptor. As illustrated in Fig. 5B, constitutive expression of mRNA to CXCR2 was detected in control neutrophils and was unaffected by incubation with LTA for 1 h. This finding suggests that the reduction of CXCR2 in the neutrophil surface is not due to regulation of its gene transcription. However, the inhibitory effect of LTA on chemotaxis to CXCL2 was abrogated by cycloheximide (CHX) (Fig. 5A), indicating a protein synthesis–dependent mechanism of the cell surface CXCR2 loss after TLR2 activation. Because neutrophils can produce CXCR2 ligands in response to TLR agonists, a potential mechanism for LTA-induced reduced chemotaxis may be involve receptor desensitization by autocrine CXCR2 ligand signaling. After the l-h LTA challenge, we found increased mRNA expression of CXCL1 and CXCL2; however, we did not detect any of these proteins in cell supernatants (data not shown), providing evidence against the aforementioned autocrine mechanism.
Fig. 5.
TLR2 signaling up-regulates GRK2 expression in neutrophils. (A) CXCR2 and β-actin gene expression in LTA-treated neutrophils by RT-PCR. (B) Neutrophils chemotaxis to CXCL2 after treatment with CHX, a protein synthesis inhibitor, for 30 min and later with LTA (10 μg/mL) for 1 h or more. Data are mean ± SEM. *P < .001 relative to the WT control group plus CXCL2. (C) Representative fluorescence microscopy for GRK2 (red) in BM neutrophils after treatment with LTA (10 μg/mL) for 1 h. Nuclei were stained by DAPI (blue). (D) Representative immunoblot analysis of GRK2 and β-actin in neutrophil lysates after treatment with LTA (10 μg/mL) for 1 h. The graph shows data in arbitrary units of the density of GRK2 per β-actin band. *P < .01. (E) Representative fluorescence microscopy for GRK2 (red) in blood neutrophils isolated from septic WT and TLR2−/− mice 2 h after CLP. Nuclei were stained by DAPI (blue).
Several studies have demonstrated a prominent role of GRK2 in the phosphorylation and internalization of chemokine receptors in leukocytes (15, 16). The ability of GRK2 to modulate a chemokine receptor is consistent with the high expression of this kinase in cells (17). We used fluorescence microscopy and immunoblot analysis to explore whether LTA (10 μg/mL) stimulation increased the expression of GRK2 in neutrophils. We found significant up-regulation of GRK2 protein expression in LTA-treated neutrophils compared with control cells (Fig. 5 C and D). To extend the in vitro data to in vivo, we examined whether circulating neutrophils from septic mice expressed GRK2. Neutrophils from WT mice subjected to S-CLP demonstrated a profound increase in GRK2 protein expression compared with sham and NS-CLP mice (Fig. 5E). Notably, the GRK2 protein expression was substantially reduced in neutrophils from TLR2−/− S-CLP mice compared with those from WT mice (Fig. 5E).
Discussion
This in vitro study examined the role of TLR2 on the regulation of neutrophil CXCR2 expression and the outcome of this regulation in experimental sepsis. We found that TLR2 agonist negatively regulated CXCR2 expression on the surface of isolated neutrophils by up-regulating the transcription of GRK2. Moreover, we found that peripheral blood neutrophils from severely septic mice expressed high levels of GRK2 and low levels of CXCR2. Notably, reduced expression of CXCR2 in circulating neutrophils was correlated with failure of migration to the site of infection, development of systemic inflammation, and high mortality. Thus, the present study provides a comprehensive set of data demonstrating for the first time the harmful role of TLR2 signaling in the pathophysiology of polymicrobial sepsis.
The recognition of bacteria by TLRs is important for eliminating invading pathogens. In particular, TLR2 plays a crucial role in the host defense against Gram-positive bacterial infection (18). However, the results reported here indicate that TLR2−/− mice display improved bacterial clearance and reduced mortality during severe polymicrobial sepsis as a consequence of the increased neutrophil recruitment to the infectious site. One potential explanation for this apparent discrepancy may be the diversity and magnitude of TLR stimulation between infection with a single pathogen and polymicrobial peritonitis. Therefore, we hypothesize that during an infection in which different bacterial ligands are present, the absence of signaling of one TLR does not impair the genesis of a local inflammatory response, but that the onset of the systemic inflammatory response resulting in impaired neutrophil migration depends on the activation of all TLRs by their respective agonists.
Chemokines coordinate the migration of leukocytes during inflammation via binding to specific chemokine receptors (19). CXCR2 plays a central role in the recruitment of neutrophils from the circulation into the site of inflammation (20). Impaired neutrophil migration during sepsis is correlated with down-regulation of CXCR2 expression on circulating neutrophils (14). We have identified TLR2 signaling as a crucial pathway in this phenomenon. We found that TLR2 deficiency prevented the down-regulation of CXCR2 on circulating neutrophils from mice with severe polymicrobial sepsis. Moreover, acute administration of the TLR2 agonist LTA in a single i.v. injection triggered a drastic reduction in the surface expression of CXCR2 on circulating neutrophils. Interestingly, this decreased CXCR2 expression was accompanied by reduced chemotaxis to CXCL2, similar to that observed on blood neutrophils in WT mice with severe sepsis. Furthermore, thioglycollate-induced peritoneal neutrophil migration, which is mediated mainly by CXCR2, was significantly inhibited by the i.v. injection of LTA. These findings indicate that the presence of bacterial products in the circulation promotes down-regulation of CXCR2 expression on blood neutrophils and impairs neutrophil migration. Indeed, LTA was able to down-regulate the expression of CXCR2 on the surface of isolated neutrophils in vitro, which was associated with reduction of chemotaxis and F-actin polymerization induced by CXCL2. Moreover, in adoptive transfer experiments, we found that in vitro LTA-stimulated neutrophils transferred into naïve WT mice exhibited a significant reduced capacity to migrate into the peritoneal cavity in response to thioglycollate. Thus, direct activation of TLR2 on neutrophils is able to down-regulate chemokine receptor expression and migration.
How important is the modulation of chemokine receptor expression by TLRs? Once at the infectious focus, neutrophils no longer need to continue migrating, but they must respond by producing antimicrobial mediators to eliminate the invading pathogens. Thus, it is logical to suppose that TLRs might down-modulate neutrophil trafficking to keep these cells at the site of infection. Indeed, it has been shown that bacteria phagocytosis results in decreased surface expression of CXCR1 and CXCR2 in human neutrophils (21, 22). Extrapolating to a systemic infection context with high blood levels of TLR agonists, the down-regulation of chemokine receptors in peripheral circulating neutrophils (“wrong compartment”) may explain the drastic reduction of these cells in the infection focus.
A fundamental question that remains is how TLR activation down-regulates CXCR2 expression on neutrophils. Chemokine receptors belong to the G protein-coupled receptor family (GPCR), in which the major regulatory mechanisms are desensitization and internalization (23). GRKs are serine/threonine protein kinases that regulate receptor internalization of almost all GPCR families, including chemokine receptors (24). GRK2 has been implicated in the regulation of chemokine receptors (e.g., CCRs and CXCRs) and of innate chemoattractant receptors (e.g., fMLP and C5a receptors) (25, 26). We previously reported markedly increased GRK2 levels in neutrophils from septic patients (27), associated with decreased chemotactic responses to IL-8, LTB4 and fMLP (7, 27). Recently, it has been shown that GRK2 protein levels are markedly increased by activation of TLR2 in macrophages (28). In the present study, we found that TLR2 activation substantially increased the protein expression of GRK2 in isolated neutrophils. Notably, peripheral blood neutrophils isolated from TLR2−/− mice with severe sepsis displayed reduced levels of GRK2 compared with those from WT mice. In conclusion, we have identified TLR2 as a regulator of CXCR2 expression in circulating neutrophils during severe sepsis by up-regulation of GRK2 expression.
Materials and Methods
Mice.
TLR2−/−, TLR6−/−, and MyD88−/− mice on the C57BL/6 background were obtained from the School of Medicine of Ribeirão Preto. All experiments were performed in accordance with the ethical guidelines of the School of Medicine of Ribeirão Preto, University of Sao Paulo.
Sepsis Induction.
Sepsis was induced by CLP model as described previously (11). In brief, after the mouse was anesthetized, an incision was made on the abdomen. The cecum was exposed and ligated below the ileocecal junction, after which a single puncture was made through the cecum. A 30- or 18-gauge needle was used to induce NS-CLP or S-CLP, respectively. The survival rate was determined daily for 7 days after CLP induction.
Neutrophil Migration.
Neutrophil migration was performed as described previously (10). In brief, peritoneal lavage fluids were harvested and total cell counts were performed using a Coulter ACT series cell counter. Differential cell counts were carried out on cytocentrifuge slides (Cytospin 3; Shandon Southern Products) stained by the May-Grünwald-Giemsa method.
Bacterial Counts.
Bacterial counts were determined as described previously (29). In brief, samples of peritoneal lavage fluid and blood were harvested, plated on Muller-Hinton agar dishes (Difco Laboratories), and incubated for 24 h at 37 °C.
Cytokine and Chemokine Determination.
Cytokine and chemokine concentrations were measured by ELISA using antibodies from R&D Systems.
Neutrophil Isolation.
For BM neutrophils, femurs and tibias were removed and flushed with HBSS. The BM cells were suspended in HBSS and laid on top of a 2-layer Percoll (Sigma) gradient (72% and 65% in HBSS), and then centrifuged at 1200 × g for 30 min at 25 °C. Mature neutrophils recovered at the interface of the 65%–72% fractions were > 95% pure as determined by May-Grünwald-Giemsa staining and by Gr-1high expression by flow cytometry. For blood neutrophils, mice were anesthetized and blood was collected via cardiac puncture. The blood was suspended in HBSS, laid on top of a 3-layer Percoll gradient (78%, 69%, and 52%), and then centrifuged at 1200 × g for 30 min at 25 °C. The neutrophils were collected from the 69%–78% interface fractions.
Chemotaxis Assay.
Chemotaxis was performed in a 48-well microchamber (Neuro Probe) using a 5-μm-pore polycarbonate membrane. Neutrophils (1 × 106 cells/mL) were allowed to migrate toward CXCL2 (30 ng/mL) or medium alone at 37 °C with 5% CO2. After 1 h, the membrane was removed, fixed, and stained. Neutrophils that migrated through the membrane were counted under a light microscope on at least 5 randomly selected fields.
F-Actin Assembly.
Neutrophils were incubated with CXCL2 (30 ng/mL) at 37 °C for 5 min. Cells were fixed, permeabilized, and stained with rhodamine-phalloidin (Molecular Probes). Microscopic analysis of fluorescent images was performed using an Olympus BX40-F4 epifluorescence microscope. The mean fluorescence intensity was determined from a linear measurement of individual cells' fluorescence. All cells of at least 5 randomly chosen fields of each slide were analyzed.
Flow Cytometry Analysis.
Blood was collected via cardiac puncture. Aliquots (100 μL) of whole blood were incubated with anti-CD16/CD32 mAbs (BD PharMingen), followed by incubation with phycoerythrin-conjugated anti-CXCR2 mAb (R&D Systems) and peridinin-chlorophyll-protein complex–conjugated anti–Gr-1 mAb (BD Biosciences). The cells were washed, depleted of red blood cells by hypotonic lysis, fixed, and analyzed by flow cytometry using a BD Biosciences FACSort flow cytometer.
Apoptosis Assay.
Apoptosis was determined by Annexin V apoptosis detection kit (Bender MedSystems). The cells were analyzed by flow cytometry using a BD Biosciences FACSort flow cytometer.
Fluorescence Microscopy.
Neutrophils were affixed on glass slides by cytospin centrifugation and then incubated with rabbit anti-mouse GRK2 Ab or isotype control (Santa Cruz Biotechnology). Then Alexa-Fluor 594–conjugated goat anti-rabbit IgG Ab (Invitrogen) was added. The cells were incubated with DAPI (Invitrogen) to stain the cell nucleus. Microscopic analysis of fluorescent images was performed using an Olympus BX-50 epifluorescence microscope.
Adoptive Cell Transfer.
BM neutrophils were purified and incubated with LTA (10 μg/mL) or control medium (unstimulated) for 1 h. Then LTA and unstimulated neutrophils (107/mL in PBS) were incubated with 5.0 μM or 0.5 μM carboxyfluorescein diacetate succinimidyl ester (CFDA-SE; Molecular Probes), respectively, at 37 °C for 10 min. The cells were washed, and then a 1:1 ratio of LTA/unstimulated neutrophils (107 total cells) was injected i.v. into WT recipient mice. To induce neutrophil migration, 3% thioglycollate was instilled into the peritoneum at the time of cell transfer. At 4 h postinjection, peritoneal lavage fluids were harvested, and the cells were analyzed for CFSE expression by flow cytometry using a BD Biosciences FACSort flow cytometer.
Statistical Analysis.
Data are expressed as mean ± SEM. Student's unpaired t test was used to evaluate the differences between the WT and TLR2−/− groups. Survival rates were analyzed with the log-rank test, and bacterial counts were analyzed using the Mann-Whitney U test. A P value < .05 was considered significant.
Supplementary Material
Acknowledgments.
This work was supported by grants from Fundação de Amparo à Pesquisa do Estado de São Paulo and Conselho Nacional de Pesquisa e Desenvolvimento Tecnológico.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0900196106/DCSupplemental.
References
- 1.Nathan C. Neutrophils and immunity: Challenges and opportunities. Nat Rev Immunol. 2006;6:173–182. doi: 10.1038/nri1785. [DOI] [PubMed] [Google Scholar]
- 2.Benjamim CF, Ferreira SH, Cunha FdQ. Role of nitric oxide in the failure of neutrophil migration in sepsis. J Infect Dis. 2000;182:214–223. doi: 10.1086/315682. [DOI] [PubMed] [Google Scholar]
- 3.Crosara-Alberto DP, et al. Involvement of NO in the failure of neutrophil migration in sepsis induced by Staphylococcus aureus. Br J Pharmacol. 2002;136:645–658. doi: 10.1038/sj.bjp.0704734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Orinska Z, et al. IL-15 constrains mast cell–dependent antibacterial defenses by suppressing chymase activities. Nat Med. 2007;13:927–934. doi: 10.1038/nm1615. [DOI] [PubMed] [Google Scholar]
- 5.Alves-Filho JC, et al. The role of neutrophils in severe sepsis. Shock. 2008;30(Suppl 1):3–9. doi: 10.1097/SHK.0b013e3181818466. [DOI] [PubMed] [Google Scholar]
- 6.Chishti AD, Shenton BK, Kirby JA, Baudouin SV. Neutrophil chemotaxis and receptor expression in clinical septic shock. Intensive Care Med. 2004;30:605–611. doi: 10.1007/s00134-004-2175-y. [DOI] [PubMed] [Google Scholar]
- 7.Tavares-Murta BM, et al. Failure of neutrophil chemotactic function in septic patients. Crit Care Med. 2002;30:1056–1061. doi: 10.1097/00003246-200205000-00017. [DOI] [PubMed] [Google Scholar]
- 8.Akira S, Takeda K. Toll-like receptor signaling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 9.Plitas G, et al. Toll-like receptor 9 inhibition reduces mortality in polymicrobial sepsis. J Exp Med. 2008;205:1277–1283. doi: 10.1084/jem.20080162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alves-Filho JC, de Freitas A, Russo M, Cunha FQ. Toll-like receptor 4 signaling leads to neutrophil migration impairment in polymicrobial sepsis. Crit Care Med. 2006;34:461–470. doi: 10.1097/01.ccm.0000198527.71819.e1. [DOI] [PubMed] [Google Scholar]
- 11.Wichterman KA, Baue AE, Chaudry IH. Sepsis and septic shock: A review of laboratory models and a proposal. J Surg Res. 1980;29:189–201. doi: 10.1016/0022-4804(80)90037-2. [DOI] [PubMed] [Google Scholar]
- 12.Cavaillon JM, et al. Cytokine cascade in sepsis. Scand J Infect Dis. 2003;35:535–544. doi: 10.1080/00365540310015935. [DOI] [PubMed] [Google Scholar]
- 13.Brown KA, et al. Neutrophils in development of multiple organ failure in sepsis. Lancet. 2006;368:157–169. doi: 10.1016/S0140-6736(06)69005-3. [DOI] [PubMed] [Google Scholar]
- 14.Rios-Santos F, et al. Down-regulation of CXCR2 on neutrophils in severe sepsis is mediated by inducible nitric oxide synthase–derived nitric oxide. Am J Respir Crit Care Med. 2007;175:490–497. doi: 10.1164/rccm.200601-103OC. [DOI] [PubMed] [Google Scholar]
- 15.Aragay AM, et al. Monocyte chemoattractant protein-1–induced CCR2B receptor desensitization mediated by the G protein–coupled receptor kinase 2. Proc Natl Acad Sci U S A. 1998;95:2985–2990. doi: 10.1073/pnas.95.6.2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oppermann M, Mack M, Proudfoot AEI, Olbrich H. Differential effects of CC chemokines on CC chemokine receptor 5 (CCR5) phosphorylation and identification of phosphorylation sites on the CCR5 carboxyl terminus. J Biol Chem. 1999;274:8875–8885. doi: 10.1074/jbc.274.13.8875. [DOI] [PubMed] [Google Scholar]
- 17.Fan J, Malik AB. Toll-like receptor 4 (TLR4) signaling augments chemokine-induced neutrophil migration by modulating cell surface expression of chemokine receptors. Nat Med. 2003;9:315–321. doi: 10.1038/nm832. [DOI] [PubMed] [Google Scholar]
- 18.Takeuchi O, Hoshino K, Akira S. Cutting edge: TLR2-deficient and MyD88-deficient mice are highly susceptible to Staphylococcus aureus infection. J Immunol. 2000;165:5392–5396. doi: 10.4049/jimmunol.165.10.5392. [DOI] [PubMed] [Google Scholar]
- 19.Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: The leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7:678–689. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- 20.Olson TS, Ley K. Chemokines and chemokine receptors in leukocyte trafficking. Am J Physiol Regul Integr Comp Physiol. 2002;283:R7–R28. doi: 10.1152/ajpregu.00738.2001. [DOI] [PubMed] [Google Scholar]
- 21.Kobayashi SD, et al. From the cover: Bacterial pathogens modulate an apoptosis differentiation program in human neutrophils. Proc Natl Acad Sci U S A. 2003;100:10948–10953. doi: 10.1073/pnas.1833375100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Doroshenko T, et al. Phagocytosing neutrophils down-regulate the expression of chemokine receptors CXCR1 and CXCR2. Blood. 2002;100:2668–2671. doi: 10.1182/blood.100.7.2668. [DOI] [PubMed] [Google Scholar]
- 23.Métayé T, Gibelin H, Perdrisot R, Kraimps J-L. Pathophysiological roles of G-protein–coupled receptor kinases. Cell Signal. 2005;17:917–928. doi: 10.1016/j.cellsig.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 24.Pitcher JA, Freedman NJ, Lefkowitz RJ. G protein–coupled receptor kinases. Annu Rev Biochem. 1998;67:653–692. doi: 10.1146/annurev.biochem.67.1.653. [DOI] [PubMed] [Google Scholar]
- 25.Petra Langkabel JZMO. Ligand-induced phosphorylation of anaphylatoxin receptors C3aR and C5aR is mediated by G protein–coupled receptor kinases. Eur J Immunol. 1999;29:3035–3046. doi: 10.1002/(SICI)1521-4141(199909)29:09<3035::AID-IMMU3035>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 26.Prossnitz ER, Kim CM, Benovic JL, Ye RD. Phosphorylation of the N-formyl peptide receptor carboxyl terminus by the G protein–coupled receptor kinase, GRK2. J Biol Chem. 1995;270:1130–1137. doi: 10.1074/jbc.270.3.1130. [DOI] [PubMed] [Google Scholar]
- 27.Arraes SMA, et al. Impaired neutrophil chemotaxis in sepsis associates with GRK expression and inhibition of actin assembly and tyrosine phosphorylation. Blood. 2006;108:2906–2913. doi: 10.1182/blood-2006-05-024638. [DOI] [PubMed] [Google Scholar]
- 28.Loniewski K, Shi Y, Pestka J, Parameswaran N. Toll-like receptors differentially regulate GPCR kinases and arrestins in primary macrophages. Mol Immunol. 2008;45:2312–2322. doi: 10.1016/j.molimm.2007.11.012. [DOI] [PubMed] [Google Scholar]
- 29.Godshall CJ, et al. Genetic background determines susceptibility during murine septic peritonitis. J Surg Res. 2002;102:45–49. doi: 10.1006/jsre.2001.6319. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.