Abstract
Escherichia coli is the dominant facultative bacterium in the normal intestinal flora. E. coli is, however, also responsible for the majority of serious extraintestinal infections. There are distinct serotypical differences between facultative and invasive E. coli strains. Invasive strains frequently produce virulence factors such as α-hemolysin (HlyA), which causes hemolysis by forming pores in the erythrocyte membrane. The present study reveals that this pore formation triggers purinergic receptor activation to mediate the full hemolytic action. Non-selective ATP-receptor (P2) antagonists (PPADS, suramin) and ATP scavengers (apyrase, hexokinase) concentration dependently inhibited HlyA-induced lysis of equine, murine, and human erythrocytes. The pattern of responsiveness to more selective P2-antagonists implies that both P2X1 and P2X7 receptors are involved in HlyA-induced hemolysis in all three species. In addition, our results also propose a role for the pore protein pannexin1 in HlyA-induced hemolysis, as non-selective inhibitors of this channel significantly reduced hemolysis in the three species. In conclusion, activation of P2X receptors and possibly also pannexins augment hemolysis induced by the bacterial toxin, HlyA. These findings potentially have clinical perspectives as P2 antagonists may ameliorate symptoms during sepsis with hemolytic bacteria.
Keywords: alpha-hemolysin, E. coli, erythrocytes, hemolysis, P2X
The dominant facultative intestinal bacterium Escherichia coli (E. coli) frequently induces serious extraintestinal infections as neonatal meningitis, peritonitis, Gram-negative bacteriemia, and urinary infections including pyelonephritis (1, 2). There are, however, distinct serotypical differences between the facultative E. coli and the ones that invade the tissue and cause infection. The invasive E. coli strains frequently produce virulence factors such as the exotoxin α-hemolysin (HlyA) (1, 3). The frequency by which hemolytic E. coli strains can be isolated from patient samples increases with the severity of disease (1).
HlyA is a 107 kDa (4) protein that induces hemolysis by creating ≈2-nm-wide pores in the erythrocyte membrane. These pores are thought to increase the permeability and thereby produce cell swelling, which finally ruptures the erythrocyte. Thus, increasing the osmolality of the extracellular solution with cell-impermeate sugars inhibits the HlyA-induced hemolysis completely (5). If HlyA-induced hemolysis is merely a consequence of inserting non-selective pores into the plasma membrane of red blood cells, it is puzzling that the sensitivity to HlyA varies among species (6). This feature is not unique to HlyA, as the sensitivity to other pore-formers such as α-toxin from Staphylococcus aureus also shows great interspecies variability (7). Regarding S. aureus, the interspecies variation was explained through differences in expression levels of a specific receptor for α-toxin (8). This option has also been suggested for HlyA-induced hemolysis (9) but is not yet generally accepted (10).
In the present study, we investigate the possibility that HlyA requires P2-receptor activation to produce hemolysis. P2X receptors are ligand-gated cation channels activated by extracellular ATP. To date, seven subtypes of P2X receptors have been identified and are referred to as P2X1–7. All P2X receptors are permeable to small monovalent cations and some have significant calcium permeability (11). Here we show that human, murine, and equine erythrocytes use a combination of P2X1 and P2X7 receptor activation for full HlyA-induced hemolysis to occur. This is particularly interesting, as prolonged stimulation of P2X7 receptors are known to increase the plasma membrane permeability to an extent that eventually leads to lysis of certain cells (12). In macrophages it has been shown that pannexin1, a recently discovered pore-forming protein, is required for this increment in permeability (12, 13). Our data also support a role for pannexin channels in addition to P2X channels in HlyA-induced hemolysis.
Results
HlyA-Induced Hemolysis Requires Activation of Purinergic Receptors.
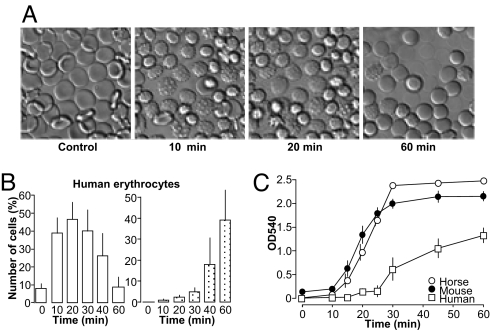
Supernatant from the α-hemolysin (HlyA)–producing E. coli–strain ARD6 lyses equine, human, and murine erythrocytes (Fig. 1). Figure 1 shows the HlyA-induced hemolysis as a function of time. Time-lapse experiments with murine and human erythrocytes attached to coverslips revealed that HlyA-induced hemolysis is a sequential process. Within the first 20 minutes, HlyA induced crenation of the red blood cells as a result of cell shrinkage, followed by a gradual volume increase and finally lysis of the cells (Figs. 1A and 1B, Movie S1). This sequential shrinkage and swelling also applies at the single-cell level. Thus, it is not different populations of red blood cells that either shrink or swell but, rather, that a single erythrocyte first shrinks and then swells as a consequence of HlyA application. The erythrocyte suspension (1.25%) was incubated with dilute E. coli supernatant (50 μl · ml−1). Erythrocytes from the three tested species showed marked difference in the responsiveness to HlyA (Fig. 1C) with the lowest sensitivity to HlyA in human erythrocytes. In all of the following experiments, the amount of added E. coli supernatant was adjusted to produce ≈50% hemolysis after 60 minutes' incubation.
Fig. 1.
α-Hemolysin–induced hemolysis in equine, murine and human erythrocytes. (A) Effect of α-hemolysin containing E. coli (ARD6, serotype OK:K13:H1) supernatant on human erythrocytes attached to a coverslip after 10, 20, and 60 minutes' incubation at 37 °C (see also Movie S1). (B) Summarized data. The total amount of crenated erythrocytes (open columns) and lysed erythrocytes (dotted columns) over time analyzed in image sequences collected over 60 minutes, at 0.1 Hz (n = 8 human). (C) The overall hemolysis is shown as an increase in optical density at 540 nm (OD540) reflecting the hemoglobin concentration in the solution. The erythrocytes were incubated with E. coli supernatant (50 μl·ml−1) from 0 to 60 minutes. n = 5, 7, and 6 for equine, murine, and human, respectively.
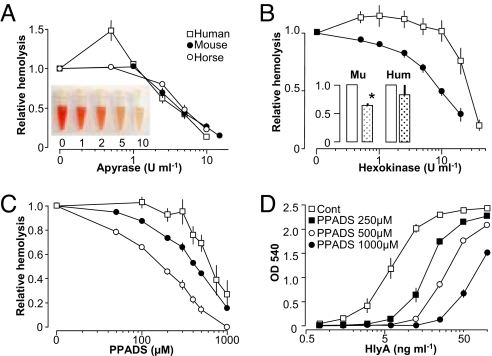
We generally use filtered E. coli (ARD6) supernatant to induce hemolysis unless otherwise stated. This approach was chosen to ensure that our results would also apply in vivo where HlyA is released from E. coli together with various other components. When choosing this approach, we did, however, have to verify that the hemolysis induced by HlyA-producing E. coli could in fact be ascribed to HlyA. Therefore, we purified HlyA from our ARD6-culture. After purification, a suspension of the purified HlyA was separated on a 5–15% sodium dodecyl sulfate (SDS) gel. A single 100-kDa band appeared after Coomassie R staining, and mass spectroscopy identified the band as HlyA (Fig. S1 A and B). As an additional control we used the supernatant from the E. coli strain D2103, a non-pathological laboratory strain of E. coli that does not produce HlyA. The supernatant from these bacteria did not induce hemolysis in human, murine, or equine erythrocytes (Fig. S1D). Furthermore, we compared our findings to HlyA kindly supplied by Prof. Sucharit Bhakdi, University of Mainz, Germany (with the activity of 10 ng·ml−1 for ≈50% hemolysis, Fig. S2). In the following, when purified HlyA is mentioned, it is with reference to this preparation. During our initial tests of the biological activity of HlyA, we discovered that the ATP-scavenger apyrase completely inhibited HlyA-induced hemolysis of equine erythrocytes. This finding was truly surprising, as it implied extracellular ATP necessary for the hemolysis inflicted by HlyA-producing E. coli. As extracellular ATP is a signaling molecule that activates P2 receptors, our findings could suggest that the prevailing pore model for HlyA-induced hemolysis might be a simplification. Therefore, we tested the effect of ATP scavenging more thoroughly. We found that apyrase completely inhibited hemolysis of not only equine but also murine and human erythrocytes (Fig. 2A). In addition, hexokinase, which rapidly degrades adenosine triphosphate (ATP) to adenosine diphosphate (ADP), similarly reduced the HlyA-induced hemolysis in red blood cells of murine and human origins in a concentration-dependent manner (Fig. 2B). This finding was verified by purified HlyA (Fig. 2B, inset). It is worth noticing that, in human erythrocytes, both apyrase and hexokinase potentiated the HlyA-induced hemolysis at lower concentrations. The distinction might suggest a difference in P2 receptor expression pattern on the red blood cells between the species.
Fig. 2.
HlyA-induced hemolysis of erythrocytes is inhibited by ectoATPases and purinergic antagonist. E. coli supernatant (60 minutes) induces hemolysis of human (square), murine (filled circles), and equine (open circles) erythrocytes. (A) Concentration–response curves for the ATP scavenger apyrase. Inset shows a representative picture of supernatant from murine erythrocytes subjected to HlyA in the presence of 0, 1, 2, 5 or 10 U ml−1 apyrase. (B) Effect of hexokinase on the HlyA-induced lysis of human, murine, and equine erythrocytes; inset shows the effect of hexokinase (10 U ml−1) on hemolysis induced by purified HlyA in murine and human erythrocytes). (C) Effect of the non-selective P2 receptor antagonist PPADS on HlyA-induced lysis of erythrocytes from all three species. (D) Concentration–response relationship of PPADS at various concentration of purified HlyA in human erythrocytes. Hemolysis was measured as OD540. Values are mean ± SEM; n = 5–13.
To validate the relevance of this finding, it was important to learn whether P2 receptor antagonists influenced the HlyA-induced hemolysis. The non-selective P2 receptor antagonist PPADS concentration-dependently decreased hemolysis induced by HlyA-producing E. coli in equine, murine, and human erythrocytes (Fig. 2C). The EC50 value for PPADS was 520 μM, 400 μM, and 180 μM for human, murine, and equine erythrocytes, respectively. This finding was substantiated for the whole range of HlyA concentrations (Fig. 2D) tested in human erythrocytes exposed to purified HlyA. The concentration–response relationship was compatible with competitive antagonism, and it should be noted that the effect of even maximal toxin concentrations was reduced by the P2 receptor blocker. Thus, P2 receptor activation seems to be involved in HlyA-induced hemolysis. The non-selective P2 receptor antagonist suramin also concentration-dependently decreased HlyA-induced hemolysis in all three species (data not shown). In higher concentrations suramin does, however, cause dramatic erythrocyte shrinkage, and thus may not be suitable for evaluating P2 receptor implication in erythrocytes.
To evaluate whether the effect of the purinergic antagonist on hemolysis was merely a result of increased osmolality, we tested the effect of extracellular sucrose on the HlyA-induced hemolysis (data not shown). Sucrose (1 mM) only slightly decreased hemolysis (5.1% ± 1.7%), whereas 10 mM and 75 mM sucrose markedly decreased hemolysis (28.5% ± 5.0%, 82.8% ± 5.2%). Given that the concentrations of the antagonists and ATPases used in this study never exceeded 1 mM, the effect cannot be the result of increased osmolarity. Neither did our results reflect unselective binding between the antagonists and the toxin. This was tested in equine erythrocytes, which were preincubated with HlyA for 10–15 minutes at 37 °C or for 30 minutes at 4 °C, thoroughly washed, and re-suspended with or without the antagonists. Because HlyA is incorporated into the membrane during the preincubation, the erythrocytes proceeded to lysis in the absence of free HlyA. Fig. S2 shows that various pharmacological interventions reduced hemolysis after HlyA was prebound to the erythrocytes. The antagonists were, however, less efficient when added to washed erythrocytes in which the lytic process was already initiated.
Which P2 Receptor(s) Is Involved in HlyA-Induced Hemolysis?
Erythrocytes express various types of P2 receptors. The P2 receptors that have been reported to be expressed in mature human erythrocytes include P2Y1 (14), P2Y2 (15), P2Y13 (15), P2X1 (15), and P2X7 (16), whereas P2Y1, P2X1, P2X4, and P2X7 appear to be present in erythroid progenitor cells (17). To test which of these purinergic receptors participates in the HlyA-induced hemolysis, we addressed the receptors in question individually. As the P2Y1 receptor is implicated in sorbitol-induced hemolysis of plasmodium-infected human and murine erythrocytes (14), we tested whether this receptor was responsible for the HlyA-induced hemolysis. The P2Y1 receptor antagonist MRS2179 did not affect the HlyA-induced hemolysis (Fig. S3A) at concentrations (up to 500 μM) beyond what was needed to inhibit hemolysis in Plasmodium berghei– infected erythrocytes (14). As there are no specific antagonists for P2Y2 receptors, we examined the effect of HlyA in transgenic mice. The HlyA-induced hemolysis was similar in erythrocytes from P2Y2−/− and P2Y2+/+ mice (Fig. S3B). In the case of the P2Y13 we tested the antagonist MRS2211, which has been reported to display some selectivity toward the P2Y13 receptor (18). MRS2211 decreased HlyA-induced hemolysis significantly in human and murine erythrocytes (Fig. S3C). This finding contradicts our results with hexokinase (degrading ATP to ADP), which should stimulate rather than inhibit the ADP-sensitive P2Y13 receptor. Therefore, hexokinase and MRS2211 should give opposing results if the P2Y13 receptor is involved. As this is not the case, the P2Y13 receptor is an unlikely candidate for the P2 receptor involved in HlyA-induced hemolysis. We cannot exclude the possibility that the inhibition produced by MRS2211 is mediated through another P2 receptor.
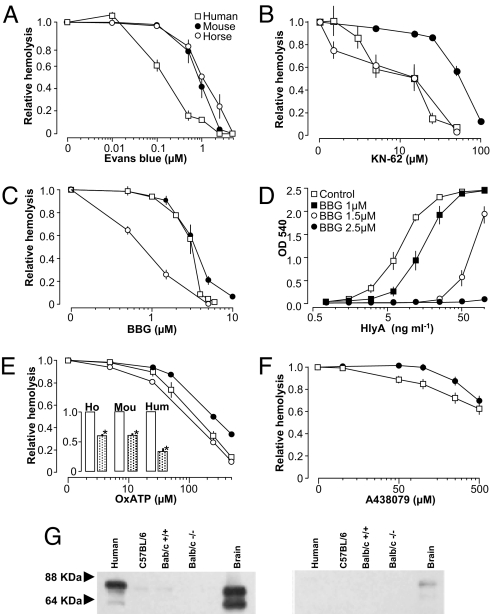
In principle, this leaves only the P2X receptors to be considered. Fig. 3A shows that the non-selective blocker of P2X receptors Evans blue potently reduced the HlyA-induced hemolysis, suggesting that a P2X-receptor is involved in this hemolysis. Of the P2X-receptors expressed in erythrocytes, we regarded the P2X7 as the most likely mediator of HlyA-induced hemolysis for the following reasons. The P2X7 receptors are known to undergo a transition to a greater permeability state, which eventually leads to lysis in certain cells (12). The P2X7 receptor has been reported to interact with the channel protein pannexin1 (12), and the complex creates a sizeable pore permeable to larger molecules such as ethidium bromide (13). Pannexin1 is expressed in human red blood cells (19) and has recently been suggested as the ATP release channel in erythrocytes (20). To test whether P2X7 receptors participate in HlyA-induced hemolysis, we used antagonists with relative selectivity for P2X7: Brilliant Blue G (BBG), ATP-2′,3′-dialdehyde (OxATP), and KN-62 (21). All antagonists concentration-dependently decreased hemolysis in equine, murine, and human erythrocytes (Fig. 3). Equine and human erythrocytes were more sensitive to all of the tested substances compared with murine erythrocytes. In this context, it should be mentioned that the murine P2X7 receptor is known to be less sensitive to KN-62 compared with the human receptor (22). The protection against hemolysis by P2X receptor antagonism was again substantiated for the whole concentration range of purified HlyA in human erythrocytes using BBG as an example of a P2X7 antagonist (Fig. 3D). Again the antagonist shows a substantial effect on HlyA-induced hemolysis even under HlyA concentrations that produced maximal hemolysis. The inhibition of hemolysis by OxATP was verified using purified HlyA in murine and human erythrocytes (Fig. 3E, inset). The novel selective, competitive P2X7 receptor antagonist A438079 reduced the hemolysis in human erythrocytes, but was less efficient in murine erythrocytes (Fig. 3F). Immunoblots of plasma membrane-fractions for the P2X7 receptor confirm that human and murine erythrocytes express a protein of relevant size (66 kDa, Fig. 3G, and in full in Fig. S4B). It will require further investigation to fully establish the relative contribution of P2X receptors in the HlyA-induced hemolysis. With our current tools, we cannot exclude the possibility of contributions from other P2X receptors in the HlyA-induced hemolysis in any of the species studied.
Fig. 3.
HlyA-induced hemolysis is inhibited by P2X7 receptor antagonists. HlyA-induced hemolysis in human (squares), mouse (filled circles), and horse (open circles). Hemolysis induced by HlyA-producing E. coli was reduced by increasing concentrations of (A) Evans Blue, (B) KN-62, and (C) Brilliant Blue G (BBG). (D) Concentration-dependent effect of BBG at various concentrations of purified HlyA. ATP-2′,3′-dialdehyde (OxATP) (E) likewise reduced the hemolysis induced by HlyA-producing E. coli and by the purified toxin (inset, OxATP, 500 μM). (F) The selective P2X7 antagonist A438079 showed an effect mainly on human erythrocytes. Values are mean ± SEM, n = 5–13. (G) Immunoblots with a C-terminal antibody directed against P2X7 receptor (dilution 1:200). Left panel show a similar blot with peptide preadsorption.
Fig. S4A shows the HlyA-induced hemolysis in murine (P2X7+/+ and P2X7−/−) erythrocytes. The murine erythrocytes show a similar degree of hemolysis in response to HlyA irrespective of their genotype. The P2X7−/− mice and P2X7+/+ mice were originally generated by Pfizer and were backcrossed into BALB/c background. We did not detect any discrepancies between the sensitivity to HlyA-induced hemolysis in erythrocytes isolated from BALB/c and C57BL/6 mice (data not shown), even though the C57BL/6-strain is known to have a genetic variation in the C terminus of the P2X7 receptor (23). These data are consistent with the miniscule effect of A438079 on murine erythrocytes and the low protein expression of the P2X7 receptor in murine erythrocytes (Figs. 3F and 3G).
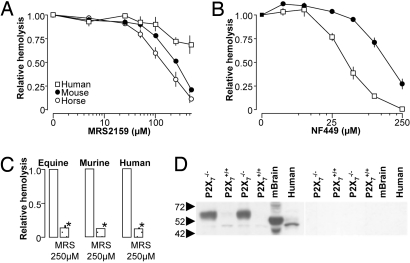
These results imply that there is at least one additional P2 receptor involved in the HlyA-induced hemolysis in murine erythrocytes. As the P2X1 and P2X7 share similar inhibitor profiles for BBG, KN-62 and OxATP (24), we tested the P2X1 antagonists MRS2159 and NF449. MRS2159 concentration-dependently inhibited hemolysis in erythrocytes from horse (EC50:150 μM) and mouse (EC50: ≈250 μM). Human erythrocytes were relatively insensitive to the antagonist, but at a concentration above 250 μM, we did see a small and statistically significant reduction (Fig. 4A). This effect was much more pronounced if purified HlyA was used (Fig. 4C). This implies that there might be differences in the cellular response in respect to whether they are subjected HlyA in a pure form or in combination with other E. coli constituents. NF449 concentration-dependently inhibits the HlyA-induced hemolysis in human (Fig. 4B). NF449 was much less efficient in murine erythrocytes in agreement with the murine P2X1 receptor being relative resistant to this inhibitor (25). It should be emphasized that even though NF449 is a suramin derivative it did not provoke the same volume changes in erythrocytes as suramin. Immunoblots of the P2X1 receptor are known to show up to 4 bands in various tissues; a 45 kDa non-glycosylated, a 60 kDa glycosylated and a 95/120 kDa band that might be the polymerized form of the receptor (26, 27). In our hands the P2X1 receptor antibody consistently recognized a 45 KD band and a very weak 60 kDa band in blots of plasma membranes from murine and human erythrocytes (Fig. 4D). Interestingly, we found that the expression level 60 kDa band was much higher in the P2X7−/− mice as compared to controls (similar in three preparations, Fig. 4D). In this immunoblot the protein levels are adjusted to avoid overloading of the bands from the P2X7−/− mice, which leaves the 60 kDa band almost undetectable in the P2X7+/+ mice. This apparent up-regulation of the P2X1 receptor might potentially conceal a hemolytic phenotype in the P2X7 receptor–deficient mice. Taken together, these data support the hypothesis that both the P2X1 and P2X7 receptor are relevant for the HlyA-induced hemolysis. Our results point to significant interspecies variations, in which the P2X7 receptor is more important for hemolysis in human erythrocytes.
Fig. 4.
Effect of the P2X1 antagonists (MRS2159 and NF449) on HlyA-induced hemolysis in equine, murine, and human erythrocytes. (A) Erythrocytes were incubated with HlyA-containing E. coli supernatant and increasing concentrations of MRS2159 (mean ± SEM, n = 7–8). (B) Erythrocytes were incubated with purified HlyA and increasing concentrations of NF449 (mean ± SEM, n = 5–6). (C) Effect of 250 μM MRS2159 in hemolysis induced by purified HlyA. (D) Immunoblotting with an antibody directed against P2X1 receptor (diluted 1:200); right panel shows a parallel blot with peptide preadsorption. Protein isolation and immunoblotting were repeated three times, with similar results.
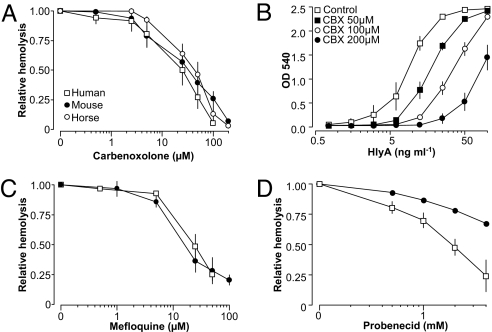
HlyA-Induced Hemolysis Is Prevented by Pannexin1 Antagonists.
Carbenoxolone (28), mefloquine and probenecid (30) have been used as antagonists with relative selectivity for pannexin1. Carbenoxolone significantly decreased the level of hemolysis in all three species with similar sensitivity (Fig. 5A). The effect of carbenoxolone was again tested for the whole range of HlyA concentrations (purified toxin, Fig. 5B), also showing sizeable effects under maximal HlyA concentrations. Mefloquine and probenecide were tested only in murine and humane erythrocytes. The EC50 for mefloquine was 25 μM in human and 18 μM in murine erythrocytes. Probenecid inhibited hemolysis in human erythrocytes, with an EC50 of 2 mM, but was less effective in murine erythocytes. Recently, the known Cl−-channel antagonists NPPB and niflumic acid have been shown to inhibit pannexin channels as well (31). Both substances reduced the HlyA-induced hemolysis, with a substantially more pronounced effect on human erythrocytes (Fig. S5).
Fig. 5.
HlyA-induced hemolysis of human, murine, and equine erythrocytes is inhibited by pannexin1 antagonists. Hemolysis was induced by HlyA-containing supernatant from E. coli. The hemolysis was concentration-dependently decreased by carbenoxolone (A), which also reduced the hemolysis induced by purified HlyA over a wide range of concentrations (B). Hemolysis induced by HlyA-producing E. coli was also reduced by mefloquine (C) and probenecid (D). Values are given as mean ± SEM; n = 5–13.
Discussion
α-Hemolysin from E. coli (HlyA) is known to lyse cells by forming pores in the plasma membrane (1, 5, 6). HlyA is able to permebilize both biological membranes and artificial lipid bilayers by inserting itself in an apparently receptor-independent manner (32). To our surprise, we discovered that this pore-formation triggers P2X receptor and pannexin channel activation in red blood cells of equine, murine, and human origins. This means that the HlyA-pores takes advantage of a specific cellular amplification system to inflict the full hemolytic response.
Purinergic Antagonists and EctoATPases.
This conclusion is based on the striking blocking effect on HlyA-induced hemolysis imposed by various inhibitors of purinergic signaling. The ATP scavenging enzymes apyrase and hexokinase continuously degrade extracellular ATP, which might otherwise stimulate P2 receptors. These enzymes almost abolished the HlyA-induced hemolysis in all three species that we tested, suggesting that ATP is being released in sufficient amounts to amplify the hemolytic process. We substantiated this finding by testing P2 receptor antagonists that were structurally different from apyrase and hexokinase. PPADS, a non-selective purinergic antagonist, like apyrase, entirely eliminates the HlyA-induced hemolysis in all three species. Taken together, these data imply a central role for P2 receptors in HlyA-induced hemolysis. In this context it is important to underscore that suramin (another non-selective P2 antagonist) has previously been shown to reduce both the lethal (33) and hemolytic actions (34) of α-toxin from S. aureus in mice and to inhibit hemolysis induced by complement activation in red blood cells from sheep (35). We found that suramin did inhibit HlyA-induced hemolysis. Unfortunately, suramin inflicted fairly significant shape changes (crenation) in the erythrocytes, and therefore we are not confident that the effect of suramin on hemolysis results from P2 receptor inhibition.
This new idea of P2 receptor–dependent amplification of HlyA-induced hemolysis is supported by the obvious sequential events the process. Within the first 20 minutes, HlyA produced intense crenation of the red blood cells, which was likely a result of volume reduction. This was followed by a gradual swelling that eventually ruptured the cells. The crenation and swelling did not occur in different population of red blood cells but could be observed within a single red blood cell.
Which P2 Receptors Mediate the HlyA-Induced Hemolysis?
HlyA-induced hemolysis is caused by swelling and rupture of the erythrocytes, which very likely result from formation of pores in the plasma membrane. Our results imply that HlyA triggers P2 receptor activation, which eventually leads to membrane rupture. The P2X7 receptor was the most likely P2-receptor candidate in HlyA-induced hemolysis. The rationale is that the P2X7 has been shown to dilate during continual ATP stimulation, creating a pore with very high conductivity (36, 37), a feature ascribed either to intrinsic characteristics of P2X receptors (38, 39) or to interaction with pannexin1 (12, 13).
This finding led us to test a series of antagonists with known selectivity toward the P2X7 receptor. We were able to show that BBG, KN-62, and OxATP all drastically reduced the HlyA-induced hemolysis in all species. P2X7 receptors have previously been shown to be expressed in human (11), rat (40), and canine erythrocytes (16, 41), and we confirmed P2X7 receptor expression by immunoblotting in both human and murine erythrocytes.
In human erythrocytes, the P2X7 receptor seems to be the main receptor involved in HlyA-induced hemolysis. However, as the new selective P2X7 receptor antagonist A438079 only partially reduced the HlyA-induced hemolysis, and as P2X1 receptor antagonists inhibited lysis of human erythrocytes in higher concentrations, we cannot exclude a contribution of P2X1 receptor subtype in the response. Similarly, we are not able to exclude heteromers of P2X receptors, including P2X1/P2X4 (42) and P2X4/P2X7 (43).
The HlyA-induced hemolysis in murine erythrocytes was found to be less sensitive to P2X7 receptor antagonists. This means that P2X7 receptors play a lesser role in HlyA-induced hemolysis in murine erythrocytes. The HlyA-induced lysis of murine erythrocytes was, however, very sensitive to the P2X1 receptor antagonist MRS2159. The other P2X1 inhibitor, NF449, reduced hemolysis of murine erythrocytes only in high concentrations, consistent with the murine P2X1 receptor being resistant to this suramin analogue (25). These data implicate the P2X1 and P2X7 receptors as the functionally relevant P2 receptors for HlyA-induced hemolysis in mice. This finding might very well be relevant for other pore-forming toxins belonging to the RTX-family (44).
The Role of ATP in Hemolysis.
Regardless of the P2 receptor involvement in HlyA-induced hemolysis, ATP alone, even in high concentrations (1 mM, 24 hours), does not by itself induce hemolysis in any of the three species tested here (data not shown). Sluyter et al. reported a similar resistance to ATP in human erythrocytes (1 mM, 24 hours), whereas ATP exposure in canine erythrocytes led to a significant degree of hemolysis (41). This resistance to ATP in murine, equine, and human red blood cells is quite surprising in light of our current data. This means that ATP is required, but not sufficient, to induce hemolysis in most species. One possibility is that the hemolysis is a combination of at least two events, in which HlyA primes the red blood cells to become sensitive to ATP. Resent data imply that ATP itself inhibits pannexins (31, 45). Thus an alternative explanation is that ATP in high concentration prevents itself from being pro-hemolytic. Further studies are obviously needed to pinpoint the exact signal transduction pathway for the HlyA-induced hemolysis.
HlyA Sensitivity.
A consequence of the above-mentioned finding is that the action of HlyA is likely to be determined by the P2 receptor expression pattern of the given tissue. This could explain the interspecies variation in the sensitivity to HlyA. As one example, our study confirms the previous finding that human erythrocytes are more resistant to HlyA than murine (5). One possible explanation is that the sensitivity to HlyA relates to the size of the erythrocytes. It has previously been shown that the osmotic resistance of erythrocytes is a function of their size, with smaller cells being more susceptible to hemolysis (46). As equine and murine erythrocytes are about half the size of human erythrocytes, we cannot exclude the possibility that the difference in HlyA-sensitivity is related to cell size. It should be emphasized that our results show slight variation with regard to whether hemolysis was induced by E. coli supernatant or purified toxin. There is, however, no specific pattern; some antagonist are more effective in supernatant-induced hemolysis (OxATP) and some when the purified toxin is used (BBG and MRS2159). The main point is that purinergic receptor inhibition is likely to protect against the cell damage inflicted by hemolytic E. coli.
Interaction Between P2X and Pannexins.
Our data suggest that pannexin channels are involved in HlyA-induced hemolysis. Prolonged stimulation of P2X7 primes the receptor to undergo a transition to a second permeability state (11–13). It has been suggested that interaction with the pore-protein pannexin1 is responsible for the observed pore enlargement (12, 13), which in turn can lead to cell lysis. In the present study three nonselective antagonists of pannexin channels, carbenoxolone (13), mefloquine (47), and probenecid (30), markedly decreased hemolysis in both murine and human red cells. Carbenoxolone and mefloquine also inhibit connexins; but this cross-reactivity has little significance, as they are unlikely to be expressed in red blood cells. In this regard it is worth mentioning that Brilliant Blue G and A438079 recently has been shown to reduced the conductance of Xenopus oocytes injected with pannexin 1 alone (45). The two Cl− channel blockers, NPPB and niflumic acid, were recently shown to reduce pannexin1 currents (niflumic acid to a lesser degree). Both substances also inhibited lysis of human erythrocytes but had little effect on murine erythrocytes. As various P2X receptor subtypes are involved in the HlyA-induced hemolysis, it is unlikely that P2X7-pannexin1 complex is an absolute requirement for the hemolysis. Our results favor the conclusion that any P2X receptor would be able to trigger the suggested pannexin activation, possibly via a rise of intracellular Ca2+ concentration. One can speculate that the toxin lyses only cells expressing P2X receptors and pannexins. In this context, it is noteworthy that α-toxin (48) and HlyA (49) induce interleukin β1 (IL-1β) release from human monocytes, a process that, in human macrophages, is known to result from P2X7 and pannexin activation (50). This could suggest that cells that express P2X7 and pannexins respond to HlyA through activation of these proteins.
Clinical Perspectives.
The question remains as to whether these findings are relevant in a clinical setting. Gram-negative sepsis is usually not associated with massive intravascular hemolysis. Sepsis is, however, in general followed by a reduced amount of red blood cells, partly as a result of hemolysis and partly because of eryptosis (“apoptosis” in erythrocytes) (51). It is known that pore-forming toxins activate human platelets (52), and that platelet ADP receptors contribute to the initiation of intravascular coagulation (29). Pore-forming bacterial toxins in the blood are likely to release ATP, which might in the end trigger thrombocyte aggregation. Thus, the cellular effects of hemolysins might add to the total clinical picture of sepsis. As both the purified toxin and the bacterial supernatant are antagonized with P2X and pannexin blockers, it is possible that inhibition of P2X receptors and pannexin channels also ameliorate cytotoxic effects of α-hemolytic E. coli in vivo. Further studies are, however, needed to determine whether these speculations have any validity.
Methods
Preparations of Erythrocytes.
Equine blood was purchased from Statens Serum Institut (Copenhagen, Denmark). Human blood was collected from seven healthy volunteers. The experiments were approved by the Danish National Committee on Biomedical Research Ethics. Murine blood was obtained from C57BL/6 and BALB/c mice after cervical dislocation. The P2X7 knock out mice (P2X7−/−) mice were bred in house according to the Danish law on research animal use (see SI Methods).
Preparation of Bacteria and Purification of E. coli HlyA.
HlyA was purified according to the method described by Bhakdi et al. (5). Hemolysis was measured spectrophotometrically in erythrocyte supernatant (Ultraspec III, LKB Biochrom, Cambridge, UK) as optical density at 540 nm (see SI Text).
Immunoblotting.
Isolated plasma membrane proteins from human and murine erythrocytes were separated and blotted by standard procedures (primary antibodies, Alomone, Jerusalem, Israel and secondary peroxidase-conjugated antibody; DAKO, Glostrup, Denmark). Preadsoption controls were included for all antibodies with 1:1 peptide-antibody ratio (see also SI Methods).
Solutions, Materials, Data Analysis, and Statistics.
Data are presented as mean ± SEM. The n value indicates number of trials for each drug. For the experiments on P2X7−/− vs. P2X7+/+ mice erythrocytes, n equals number of animals. (For statistics, please see SI Text).
Supplementary Material
Acknowledgments.
We thank for the skilled technical assistance from Christian Westberg, Edith Bjoern Moeller, Helle Hoeyer, Anne Strandsby, and Inger-Merete Paulsen. Furthermore, we thank Jeppe Praetorius and Robert A. Fenton for valued discussion, Bent Honoré for performing mass spectroscopy, and Friedrich Koch-Nolte for supplying the Pfizer-P2X7 knock out mice. The project is financially supported by: The Danish Medical Research Council, Danish National Research Foundation. Nyreforeningens forskningsfond, The Aarhus University Research Foundation, Eva og Henry Frænkels Mindefond, The A.P. Møller Foundation for the Advancement of Medical Science and R.I.E. König-Petersen Forskningsfond for Nyresygdomme. The Water and Salt Research Center at the University of Aarhus is established and supported by the Danish National Research Foundation (Danmarks Grundforskningsfond).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. S.B. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/cgi/content/full/0807044106/DCSupplemental.
References
- 1.Cavalieri SJ, Bohach GA, Snyder IS. Escherichia coli alpha-hemolysin: Characteristics and probable role in pathogenicity. Microbiol Rev. 1984;48:326–343. doi: 10.1128/mr.48.4.326-343.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johnson JR, Stell AL. Extended virulence genotypes of Escherichia coli strains from patients with urosepsis in relation to phylogeny and host compromise. J Infect Dis. 2000;181:261–272. doi: 10.1086/315217. [DOI] [PubMed] [Google Scholar]
- 3.Bhakdi S, Mackman N, Menestrina G, Gray L, Hugo F, Seeger W, Holland IB. The hemolysin of Escherichia coli. Eur J Epidemiol. 1988;4:135–143. doi: 10.1007/BF00144740. [DOI] [PubMed] [Google Scholar]
- 4.Felmlee T, Pellett S, Welch RA. Nucleotide sequence of an Escherichia coli chromosomal hemolysin. J Bacteriol. 1985;163:94–105. doi: 10.1128/jb.163.1.94-105.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhakdi S, Mackman N, Nicaud JM, Holland IB. Escherichia coli hemolysin may damage target cell membranes by generating transmembrane pores. Infect Immun. 1986;52:63–69. doi: 10.1128/iai.52.1.63-69.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhakdi S, Bayley H, Valeva A, Walev I, Walker B, et al. Staphylococcal alpha-toxin, streptolysin-O, and Escherichia coli hemolysin: Prototypes of pore-forming bacterial cytolysins. Arch Microbiol. 1996;165:73–79. doi: 10.1007/s002030050300. [DOI] [PubMed] [Google Scholar]
- 7.Bhakdi S, Tranum-Jensen J. Alpha-toxin of Staphylococcus aureus. Microbiol Rev. 1991;55:733–751. doi: 10.1128/mr.55.4.733-751.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Valeva A, Hellmann N, Walev I, Strand D, Plate M, et al. Evidence that clustered phosphocholine head groups serve as sites for binding and assembly of an oligomeric protein pore. J Biol Chem. 2006;281:26014–26021. doi: 10.1074/jbc.M601960200. [DOI] [PubMed] [Google Scholar]
- 9.Cortajarena AL, Goni FM, Ostolaza H. A receptor-binding region in Escherichia coli alpha-haemolysin. J Biol Chem. 2003;278:19159–19163. doi: 10.1074/jbc.M208552200. [DOI] [PubMed] [Google Scholar]
- 10.Valeva A, Walev I, Kemmer H, Weis S, Siegel I, et al. Binding of Escherichia coli hemolysin and activation of the target cells is not receptor-dependent. J Biol Chem. 2005;280:36657–36663. doi: 10.1074/jbc.M507690200. [DOI] [PubMed] [Google Scholar]
- 11.North RA. Molecular physiology of P2X receptors. Physiol Rev. 2002;82:1013–1067. doi: 10.1152/physrev.00015.2002. [DOI] [PubMed] [Google Scholar]
- 12.Locovei S, Scemes E, Qiu F, Spray DC, Dahl G. Pannexin1 is part of the pore forming unit of the P2X7 receptor death complex. FEBS Lett. 2007;581:483–488. doi: 10.1016/j.febslet.2006.12.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pelegrin P, Surprenant A. Pannexin-1 mediates large pore formation and interleukin-1β release by the ATP-gated P2X7 receptor. EMBO J. 2006;25:5071–5082. doi: 10.1038/sj.emboj.7601378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tanneur V, Duranton C, Brand VB, Sandu CD, Akkaya C, et al. Purinoceptors are involved in the induction of an osmolyte permeability in malaria-infected and oxidized human erythrocytes. FASEB J. 2006;20:133–135. doi: 10.1096/fj.04-3371fje. [DOI] [PubMed] [Google Scholar]
- 15.Wang L, Olivecrona G, Gotberg M, Olsson ML, Winzell MS, Erlinge D. ADP acting on P2Y13 receptors is a negative feedback pathway for ATP release from human red blood cells. Circ Res. 2005;96:189–196. doi: 10.1161/01.RES.0000153670.07559.E4. [DOI] [PubMed] [Google Scholar]
- 16.Sluyter R, Shemon AN, Barden JA, Wiley JS. Extracellular ATP increases cation fluxes in human erythrocytes by activation of the P2X7 receptor. J Biol Chem. 2004;279:44749–44755. doi: 10.1074/jbc.M405631200. [DOI] [PubMed] [Google Scholar]
- 17.Hoffman JF, Dodson A, Wickrema A, Dib-Hajj SD. Tetrodotoxin-sensitive Na+ channels and muscarinic and purinergic receptors identified in human erythroid progenitor cells and red blood cell ghosts. Proc Natl Acad Sci USA. 2004;101:12370–12374. doi: 10.1073/pnas.0404228101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim YC, Lee JS, Sak K, Marteau F, Mamedova L, et al. Synthesis of pyridoxal phosphate derivatives with antagonist activity at the P2Y13 receptor. Biochem Pharmacol. 2005;70:266–274. doi: 10.1016/j.bcp.2005.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Locovei S, Bao L, Dahl G. Pannexin 1 in erythrocytes: Function without a gap. Proc Natl Acad Sci USA. 2006;103:7655–7659. doi: 10.1073/pnas.0601037103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bao L, Locovei S, Dahl G. Pannexin membrane channels are mechanosensitive conduits for ATP. FEBS Lett. 2004;572:65–68. doi: 10.1016/j.febslet.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 21.Di VF. Novel data point to a broader mechanism of action of oxidized ATP: The P2X7 receptor is not the only target. Br J Pharmacol. 2003;140:441–443. doi: 10.1038/sj.bjp.0705469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hibell AD, Thompson KM, Xing M, Humphrey PP, Michel AD. Complexities of measuring antagonist potency at P2X7 receptor orthologs. J Pharmacol Exp Ther. 2001;296:947–957. [PubMed] [Google Scholar]
- 23.Adriouch S, Dox C, Welge V, Seman M, Koch-Nolte F, Haag F. Cutting edge: A natural P451L mutation in the cytoplasmic domain impairs the function of the mouse P2X7 receptor. J Immunol. 2002;169:4108–4112. doi: 10.4049/jimmunol.169.8.4108. [DOI] [PubMed] [Google Scholar]
- 24.Seyffert C, Schmalzing G, Markwardt F. Dissecting individual current components of co-expressed human P2X1 and P2X7 receptors. Curr Top Med Chem. 2004;4:1719–1730. doi: 10.2174/1568026043387160. [DOI] [PubMed] [Google Scholar]
- 25.Sim JA, Broomhead HE, North RA. Ectodomain lysines and suramin block of P2X1 receptors. J Biol Chem. 2008;283:29841–29846. doi: 10.1074/jbc.M802523200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jiang L, Bardini M, Keogh A, dos Remedios CG, Burnstock G. P2X1 receptors are closely associated with connexin 43 in human ventricular myocardium. Int J Cardiol. 2005;98:291–297. doi: 10.1016/j.ijcard.2003.11.036. [DOI] [PubMed] [Google Scholar]
- 27.Clifford EE, Parker K, Humphreys BD, Kertesy SB, Dubyak GR. The P2X1 receptor, an adenosine triphosphate-gated cation channel, is expressed in human platelets but not in human blood leukocytes. Blood. 1998;91:3172–3181. [PubMed] [Google Scholar]
- 28.Bruzzone R, Barbe MT, Jakob NJ, Monyer H. Pharmacological properties of homomeric and heteromeric pannexin hemichannels expressed in Xenopus oocytes. J Neurochem. 2005;92:1033–1043. doi: 10.1111/j.1471-4159.2004.02947.x. [DOI] [PubMed] [Google Scholar]
- 29.Leon C, Alex M, Klocke A, Morgenstern E, Moosbauer C, et al. Platelet ADP receptors contribute to the initiation of intravascular coagulation. Blood. 2004;103:594–600. doi: 10.1182/blood-2003-05-1385. [DOI] [PubMed] [Google Scholar]
- 30.Silverman W, Locovei S, Dahl G. Probenecid, a gout remedy, inhibits pannexin 1 channels. Am J Physiol Cell Physiol. 2009;295:C761–C767. doi: 10.1152/ajpcell.00227.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ma W, Hui H, Pelegrin P, Surprenant A. Pharmacological characterization of pannexin-1 currents expressed in mammalian cells. J Pharmacol Exp Ther. 2008;328:409–418. doi: 10.1124/jpet.108.146365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koschinski A, Repp H, Unver B, Dreyer F, Brockmeier D, et al. Why Escherichia coli alpha-hemolysin induces calcium oscillations in mammalian cells—the pore is on its own. FASEB J. 2006;20:973–975. doi: 10.1096/fj.05-4561fje. [DOI] [PubMed] [Google Scholar]
- 33.Arbuthnott JP, Lominski IR, Wright MR. Inhibition of staphylococcal alpha-toxin. The effect of aromatic polysulphonic acids on the lethal effect of alpha-toxin in mice. Biochem J. 1968;108:49–55. doi: 10.1042/bj1080049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wright MR, Arbuthnott JP, Lominski IR. Inhibition of staphylococcal alpha-toxin. A kinetic evaluation of aromatic polysulphonic acids as inhibitors of haemolysis. Biochem J. 1968;108:41–48. doi: 10.1042/bj1080041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eisen V, Loveday C. Effects of suramin on complement, blood clotting, fibrinolysis and kinin formation. Br J Pharmacol. 1973;49:678–687. doi: 10.1111/j.1476-5381.1973.tb08544.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Virginio C, MacKenzie A, Rassendren FA, North RA, Surprenant A. Pore dilation of neuronal P2X receptor channels. Nat Neurosci. 1999;2:315–321. doi: 10.1038/7225. [DOI] [PubMed] [Google Scholar]
- 37.Khakh BS, Bao XR, Labarca C, Lester HA. Neuronal P2X transmitter-gated cation channels change their ion selectivity in seconds. Nat Neurosci. 1999;2:322–330. doi: 10.1038/7233. [DOI] [PubMed] [Google Scholar]
- 38.Chaumont S, Khakh BS. Patch-clamp coordinated spectroscopy shows P2X2 receptor permeability dynamics require cytosolic domain rearrangements but not Panx-1 channels. Proc Natl Acad Sci USA. 2008;105:12063–12068. doi: 10.1073/pnas.0803008105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yan Z, Li S, Liang Z, Tomic M, Stojilkovic SS. The P2X7 receptor channel pore dilates under physiological ion conditions. J Gen Physiol. 2008;132:563–573. doi: 10.1085/jgp.200810059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jiang H, Zhu AG, Mamczur M, Falck JR, Lerea KM, McGiff JC. Stimulation of rat erythrocyte P2X7 receptor induces the release of epoxyeicosatrienoic acids. Br J Pharmacol. 2007;151:1033–1040. doi: 10.1038/sj.bjp.0707311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sluyter R, Shemon AN, Hughes WE, Stevenson RO, Georgiou JG, et al. Canine erythrocytes express the P2X7 receptor: Greatly increased function compared to human erythrocytes. Am J Physiol Regul Integr Comp Physiol. 2007 doi: 10.1152/ajpregu.00166.2007. [DOI] [PubMed] [Google Scholar]
- 42.Nicke A, Kerschensteiner D, Soto F. Biochemical and functional evidence for heteromeric assembly of P2X1 and P2X4 subunits. J Neurochem. 2005;92:925–933. doi: 10.1111/j.1471-4159.2004.02939.x. [DOI] [PubMed] [Google Scholar]
- 43.Guo C, Masin M, Qureshi OS, Murrell-Lagnado RD. Evidence for functional P2X4/P2X7 heteromeric receptors. Mol Pharmacol. 2007;72:1447–1456. doi: 10.1124/mol.107.035980. [DOI] [PubMed] [Google Scholar]
- 44.Welch RA. Pore-forming cytolysins of Gram-negative bacteria. Mol Microbiol. 1991;5:521–528. doi: 10.1111/j.1365-2958.1991.tb00723.x. [DOI] [PubMed] [Google Scholar]
- 45.Qiu F, Dahl GP. A permeant regulating its permeation pore: Inhibition of pannexin 1 channels by ATP. Am J Physiol Cell Physiol. 2008 doi: 10.1152/ajpcell.00433.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Aldrich K, Saunders DK, Sievert MS, Sivert G. Comparison of erythrocyte osmotic fragility among ectotherms and endotherms at three temperatures. J Therm Biol. 2001;26:179–182. doi: 10.1016/s0306-4565(00)00040-1. [DOI] [PubMed] [Google Scholar]
- 47.Iglesias R, Locovei S, Roque A, Alberto AP, Dahl G, et al. P2X7 receptor-Pannexin1 complex: Pharmacology and signaling. Am J Physiol Cell Physiol. 2008;295:C752–C760. doi: 10.1152/ajpcell.00228.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bhakdi S, Muhly M, Korom S, Hugo F. Release of interleukin-1 beta associated with potent cytocidal action of staphylococcal alpha-toxin on human monocytes. Infect Immun. 1989;57:3512–3519. doi: 10.1128/iai.57.11.3512-3519.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bhakdi S, Muhly M, Korom S, Schmidt G. Effects of Escherichia coli hemolysin on human monocytes. Cytocidal action and stimulation of interleukin 1 release. J Clin Invest. 1990;85:1746–1753. doi: 10.1172/JCI114631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pelegrin P, Surprenant A. Pannexin-1 couples to maitotoxin- and nigericin-induced interleukin-1beta release through a dye uptake-independent pathway. J Biol Chem. 2007;282:2386–2394. doi: 10.1074/jbc.M610351200. [DOI] [PubMed] [Google Scholar]
- 51.Kempe DS, Akel A, Lang PA, Hermle T, Biswas R, et al. Suicidal erythrocyte death in sepsis. J Mol Med. 2007;85:269–277. doi: 10.1007/s00109-006-0123-8. [DOI] [PubMed] [Google Scholar]
- 52.Arvand M, Bhakdi S, Dahlback B, Preissner KT. Staphylococcus aureus α-toxin attack on human platelets promotes assembly of the prothrombinase complex. J Biol Chem. 1990;265:14377–14381. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.