Abstract
Serum-free mouse embryo (SFME) cells are an epidermal growth factor (EGF)-dependent established line derived from brains of 16-d-old Balb/c mouse embryos. SFME cells grow indefinitely in serum-free medium without replicative senescence, chromosomal abnormalities, or malignant transformation. SFME cells express nestin, a neural stem cell marker, under serum-free conditions. Exposure to serum or transforming growth factor β (TGF-β) leads to a marked increase in differentiation toward the astrocytic lineage with expression of glial fibrillary acidic protein and other astrocyte markers. In this study, we show that treatment of SFME cells with bone morphogenetic protein-4 (BMP-4), another member of the TGF-β family, led to differentiation toward a neuronal lineage under conditions of low mitogenic stimulation (0.5 ng/mL) by EGF and fibroblast growth factor. Maximum mitogenic stimulation with 50 ng/mL EGF blocked the BMP-4 effect on neuronal differentiation, but did not block TGF-β-induced expression of markers of the astrocytic lineage. BMP-4 treatment also enhanced the activity of the neuron-specific enolase (NSE) promoter in SFME-NSE-lacZ cells that carry the gene for bacterial β-galactosidase under the control of the NSE promoter. Extended BMP-4 treatment caused SFME cells to express a neuronal phenotype synthesizing gamma-aminobutyric acid. These results indicate that SFME cells have the capacity to generate both neurons and astrocytes in vitro, which resemble the behavior of EGF-dependent multipotential stem cells in the central nervous system, and establish a relationship between effects of BMP-4 and degree of mitogenic stimulation by other peptide growth factors.
Keywords: Neural stem cells, Bone morphogenetic protein, Epidermal growth factor, Fibroblast growth factor, Neuronal differentiation, Astrocyte differentiation
Serum-free mouse embryo (SFME) cells are a brain-derived line cultured in serum-free basal nutrient medium supplemented with insulin, transferrin, chemically defined lipid concentrate (CDL), selenium, epidermal growth factor (EGF), and/or fibroblast growth factor-2 (FGF-2) with fibronectin as a culture dish-associated attachment protein (Loo et al. 1987; Murayama et al. 2000). SFME cells maintain a stable karyotype with no gross chromosomal aberration or evidence of malignant transformation for more than 100 cell doublings beyond the point at which mouse embryo cells in serum-containing medium undergo growth crisis (Loo et al. 1989; Ernst et al. 1991). SFME cells require EGF or FGF-2 for survival, and growth is inhibited by serum- or platelet-free plasma. In the absence of EGF or FGF, SFME cells undergo rapid apoptosis that is under the influence of BCL-2 and ras or other oncogenes (Rawson et al. 1991; Loo et al. 1998; Slinskey et al. 2000).
Treatment of SFME cells with serum, transforming growth factor β (TGF-β) (Sakai et al. 1990), leukemia inhibitory factor (LIF), ciliary neurotrophic factor (CNTF), or related growth factors (Nishiyama et al. 1993) leads to expression of glial fibrillary acidic protein (GFAP) and other markers for astrocytes (Solem et al. 1990; Weisz et al. 1993). Nestin, a marker of neural progenitor cells, is expressed in SFME cells under serum-free conditions and is downregulated upon induction of astrocytic differentiation by TGF-β or serum exposure (Loo et al. 1994, 1995). In view of these data, previous work by D’Alessandro and Wang (1994a; D’Alessandro et al. 1994b) showing that members of the bone morphogenetic protein (BMP) family influenced the survival and glial differentiation of SFME cells, and extensive work from a variety of laboratories linking BMPs with neuronal and glial differentiation of neural stem cells (Ling and Panchision 2007; Mathieu et al. 2008), we explored the possibility that SFME cells treated with BMP may give rise not only the glial lineage, but also to a neuronal lineage.
In this study, we report that treatment of SFME cells with BMP-4 leads to expression of high-molecular-weight neurofilament protein (NF-H). Neuronal differentiation upon BMP-4 treatment required growth factor-restricted conditions in which concentrations of EGF and FGF were maintained at a level minimally necessary to maintain survival. BMP-4 treatment also activated the promoter for neuron-specific enolase (NSE) in SFME-NSE-lacZ cells that express β-galactosidase (β-gal) under the control of the NSE promoter and led to the development of neurons producing gamma-aminobutyric acid (GABA). The results suggest that SFME cells represent an established cell line representative of EGF-dependent neural stem cells. The data also indicate that mitogen limitation is necessary for BMP-4 induction of a neuronal phenotype.
Materials and Methods
Cell culture
SFME cells were established from brains of 16-d-old Balb/c mouse embryos (Loo et al. 1987, 1989) and grown in Dulbecco-modified Eagle’s medium/Ham’s F12 (DMEM/F12, 1:1) supplemented with 10 µg/mL insulin (Sigma, St. Louis, MO), 10 µg/mL transferrin (Sigma), 1% chemically defined lipid concentrate (CDL, Gibco-BRL, Carlsbad, CA), 10 µM sodium selenite (Sigma), and 50 ng/mL EGF (UBI, Lake Placid, NY) on fibronectin-coated culture dishes. Fibronectin was prepared from bovine serum (Loo et al. 1987). SFME cells used in these experiments had undergone 20 to 40 population doublings. For differentiation experiments, cells were preincubated overnight in DMEM/F12 medium with insulin, transferrin, CDL, selenium, 5 ng/mL EGF, and 5 ng/mL FGF-2 (R&D, Minneapolis, MN) on fibronectin-coated 24-well plates at a density of 5×104 cells per well. The cells were washed with serum-free medium, shifted to medium with insulin, transferrin, CDL, selenium, 0.1% bovine serum albumin (BSA), 0.5 ng/mL EGF, 0.5 ng/mL FGF-2, and 100 ng/mL BMP-4 (R&D), 100 ng/mL CNTF (R&D), 100 ng/mL activin (R&D), or 500 nM all-trans retinoic acid (RA, Sigma), and then cultured for 4 d. For neuronal phenotype experiments, cells were cultured for 4 d in medium with insulin, transferrin, CDL, selenium, 0.1% BSA, 0.5 ng/mL EGF, 0.5 ng/mL FGF-2, and 100 ng/mL BMP-4.
SFME-ras cells (EGF/FGF-independent) were cloned from SFME cells transfected with the human Ha-ras oncogene (Shirahata et al. 1990) and grown in DMEM/F12 medium with insulin, transferrin, CDL, and selenium on fibronectin-coated culture dishes. For differentiation experiments, SFME-ras cells were preincubated overnight in medium with insulin, transferrin, CDL, and selenium on fibronectin-coated 24-well plates (5×104 cells per well). The cells were washed with serum-free medium, shifted to medium with insulin, transferrin, CDL, selenium, 0.1% BSA, and 100 ng/mL BMP-4, and cultured for 4 d.
Immunocytochemistry
Cells were fixed for 15 min at room temperature with 4% paraformaldehyde in 0.1 M sodium phosphate, pH 7.4, gradually permeabilized for 20 min (35% to 95% ethanol), and washed three times with phosphate-buffered saline (PBS), pH 7.4. Nonspecific binding was blocked for 1 h with 1% BSA–PBS. The fixed cells were incubated for 2 h at room temperature with primary antibody diluted in 1% BSA–PBS and washed three times with PBS. Primary antibodies were as follows: anti-NF-high-molecular-weight (NF-H) antibody, 1:400 (Sigma); anti-β-tubulin III antibody, 1:2,000 (Promega, Madison, WI); and anti-GFAP antibody, 1:20 (Boehringer-Mannheim, Mannheim, Germany). For neuronal phenotype immunocytochemistry, cells were fixed for 15 min at room temperature with 4% paraformaldehyde and then incubated for 2 h with anti-GABA antibody, 1:5,000 (Sigma); antiglutamate antibody, 1:100 (Chemicon, Billerica, MA); antityrosine hydroxylase antibody, 1:5,000 (Sigma); anticholine acetyltransferase antibody, 1:100 (Chemicon); or antitryptophan hydroxylase antibody, 1:1,000 (Sigma). The cells were incubated for 1 h at room temperature with secondary antibody conjugated to fluorescein isothiocyanate or rhodamine, 1:750 (Immunotech, Vaudreuil-Dorion, Quebec, Canada) and washed three times with PBS. Labeled cells were visualized with a fluorescence microscope.
Analysis of NSE promoter activity
SFME-NSE-lacZ cells were established by the same method as that used for SFME cells, from the embryonic brains of heterozygous transgenic mice carrying a 1.8-kb rat NSE promoter fragment fused to the Escherichia coli lacZ (β-galactosidase gene) (Forss-Petter et al. 1990) and grown in DMEM/F12 medium with insulin, transferrin, CDL, selenium, and 50 ng/mL EGF on fibronectin-coated culture dishes. For the detection of NSE promoter activity, NSE-lacZ cells (15 to 20 population doublings) were preincubated overnight in medium with insulin, transferrin, CDL, selenium, 5 ng/mL EGF, and 5 ng/mL FGF-2 on fibronectin-coated 24-well plates at a density of 5×104 cells per well. The cells were washed with serum-free medium, shifted to medium with insulin, transferrin, CDL, selenium, 0.1% BSA, 0.5 ng/mL EGF, 0.5 ng/mL FGF-2, and 100 ng/mL BMP-4, and then cultured for 4 d. The cells were fixed for 5 min at 4°C with 2% formaldehyde/0.2% glutaraldehyde in 50 mM HEPES, 150 mM NaCl, pH 7.4 and incubated for 3 h at 37°C in 50 mM HEPES, 150 mM NaCl, pH 7.4 including 5 mM potassium ferricyanide, 5 mM potassium ferrocyanide, 2 mM MgCl2, and 1 mg/mL 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside. Cells stained blue when β-galactosidase was expressed under the control of the NSE promoter.
Results
Neuronal differentiation of SFME cells
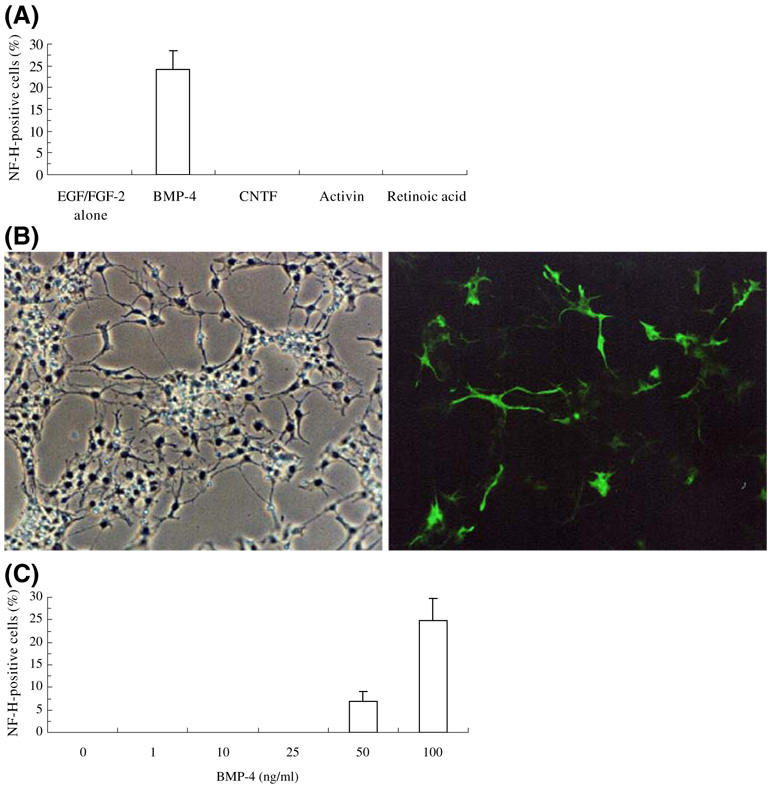
We examined SFME cell cultures maintained under a variety of conditions for evidence of neuronal differentiation, monitored by the expression of high-molecular-weight neurofilament (NF-H). Initially, we found that a low percentage of SFME cells (10.2±2.1%) were immunoreactive for β-tubulin III when incubated for 4 d at low concentrations (0.5 ng/mL) of EGF and FGF-2. We examined neuronal differentiation of SFME cells cultured with 0.5 ng/mL EGF/FGF-2 and 100 ng/mL BMP-4, CNTF, activin, or 0.5 µM RA. In control cultures maintained for 4 d in 0.5 ng/mL EGF/FGF-2, NF-H expression was not detected. However, BMP-4 treatment for 4 d led to the expression of NF-H (24.2±4.4% positive cells) (Fig. 1a and b). To further evaluate the BMP-4 effects on NF-H expression, SFME cells were preincubated overnight in DMEM/F12 medium with 5 ng/mL EGF/FGF-2 and cultured for 4 d at various concentrations of BMP-4 in 0.5 ng/mL EGF/FGF-2. All cultures were fixed after 4 d and the percentage of NF-H-positive cells were determined. NF-H-positive cells were induced at BMP-4 concentrations from 50 to 100 ng/mL with a maximum of 25.0±4.9% NF-H-positive cells. Treatment with less than 25 ng/mL BMP-4 had no effect on NF-H expression (Fig. 1c). Treatment for 4 d with CNTF, activin, or RA had little effect on NF-H expression, but BMP-4 or CNTF treatment did lead to an increase in the percentage of β-tubulin III-positive cells, and CNTF treatment also affected morphology (not shown). β-Tubulin III is indicative of immature neurons.
Figure 1.
Expression of NF-H by SFME cells. Cells were cultured for 4 d in DMEM/F12 medium with 0.5 ng/mL EGF/ FGF-2 alone, 100 ng/mL BMP-4, 100 ng/mL CNTF, 100 ng/mL activin, or 500 nM RA and then subjected to immunofluorescent staining for NF-H. (A) Percentage of cells staining positive for NF-H (mean±SD); (B) NF-H expression (green) in 100 ng/mL BMP-4 (left, phase contrast image; right, fluorescence image); (C) concentration dependence of BMP-4 on expression of NF-H.
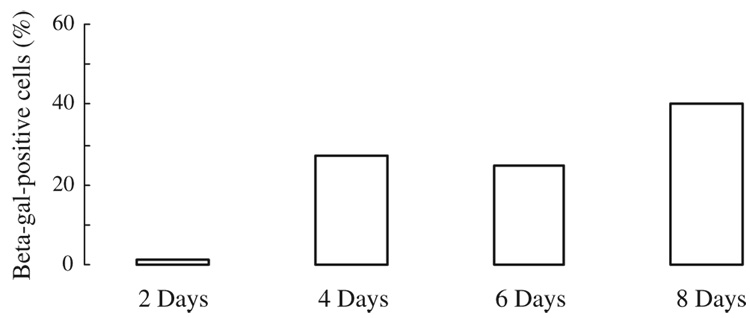
We examined the effect of BMP-4 treatment on the activation of the promoter for NSE. SFME-NSE-lacZ cells were established by the same method as that used for SFME cells, from the embryonic brains of transgenic mice carrying β-galactosidase under the control of NSE promoter (Loo et al. 1989; Forss-Petter et al. 1990). The cells were preincubated overnight in 5 ng/mL EGF/FGF-2 and cultured in 0.5 ng/mL EGF/FGF-2 with or without 100 ng/mL BMP-4. Cultures were fixed after 2 to 8 d of exposure to BMP-4 and the activity of the NSE promoter evaluated as the proportion of β-galactosidase-expressing cells. Staining indicating β-galactosidase activity was observed within 3 h of incubation with substrate. Control staining (cultures in 0.5 ng/mL EGF/FGF-2 alone) was not detectable over this incubation period. BMP-4 treatment resulted in 30% to 40% of the cells staining positive for β-galactosidase (Fig. 2).
Figure 2.
BMP-4 activation of the NSE promoter in SFME-NSE-lacZ cells. The cells were cultured in 0.5 ng/mL EGF/FGF-2 with 100 ng/mL BMP-4. All cultures were fixed after 2 to 8 d of BMP-4 treatment and the activity of the NSE promoter evaluated as the proportion of β-galactosidase-expressing cells.
Maximum mitogenic stimulation blocks BMP-4-induced neuronal differentiation
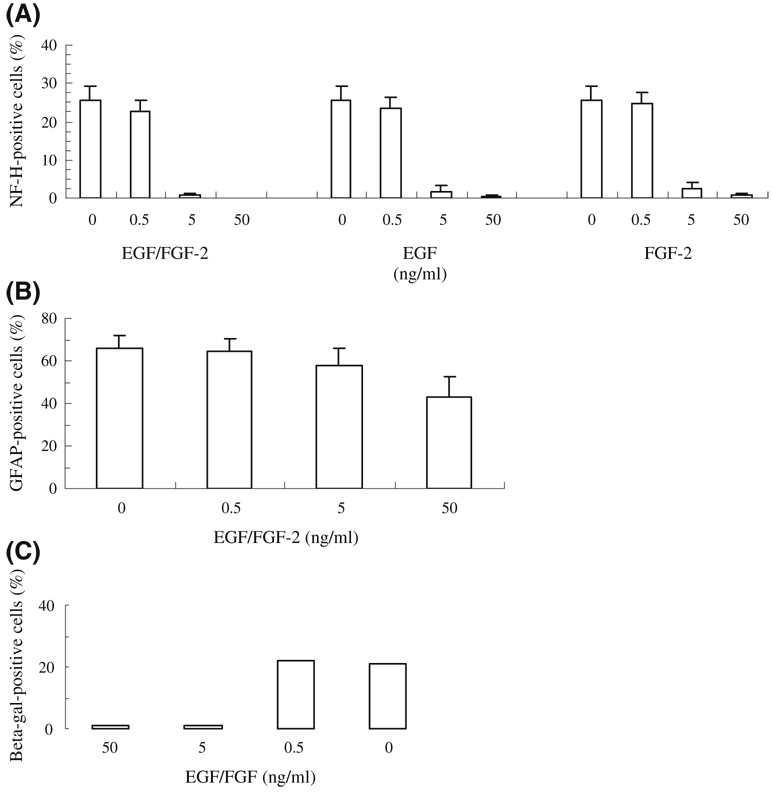
SFME cells are routinely cultured at a high concentration of EGF (50 ng/mL) to protect from apoptotic cell death resulting from the depletion of EGF from the culture medium. Previously, we found that the incubation of SFME cells in lower concentrations of EGF promoted astrocytic differentiation upon treatment with CNTF, leukemia inhibitory factor (LIF), interleukin 6, or oncostatin M, all growth factors related through the mechanism of action (gp130) (Nishiyama et al. 1993). Because of these results, we explored the effect of similar mitogen restriction on BMP-4 effects in SFME cell cultures. The cells were preincubated overnight in 5 ng/mL EGF/FGF-2, then cultured for 4 d at various concentrations of EGF/FGF-2 with 100 ng/mL BMP-4. When cells were cultured in 50 ng/mL EGF/FGF-2, NF-H expression was completely blocked (Fig. 3a). The percentage of NF-H-positive cells upon BMP-4 treatment increased with decreasing concentrations of EGF/FGF-2. The effective neurogenic concentration was less than 0.5 ng/mL EGF/FGF-2, while treatment with more than 5 ng/mL EGF or FGF-2 significantly decreased the number of NF-H-positive neurons. BMP-4 treatment also allowed the survival of SFME cells in the absence of EGF/FGF, as reported previously by D’Alessandro and Wang (1994a). Because these authors also reported that treatment of SFME cells with BMPs leads to astrocytic differentiation identified by GFAP expression (D’Alessandro and Wang 1994a; D’Alessandro et al. 1994b), we explored the BMP-4 induction of GFAP at various concentrations of EGF/FGF-2. BMP-4 treatment resulted in GFAP expression that, unlike NF-H expression, was not markedly blocked at high concentrations of EGF/FGF-2 (Fig. 3b).
Figure 3.
EGF/FGF-2 concentration dependence of BMP-4-induced NF-H and GFAP expression in SFME cells. Cells were cultured for 4 d at various concentrations of EGF and/or FGF-2 with 100 ng/mL BMP-4. (A) NF-H expression, percentage of positive cells (mean±SD); (B) GFAP expression; (C) β-galactosidase expression in SFME-NSE-lacZ cells treated with BMP-4 at various concentrations of EGF/FGF.
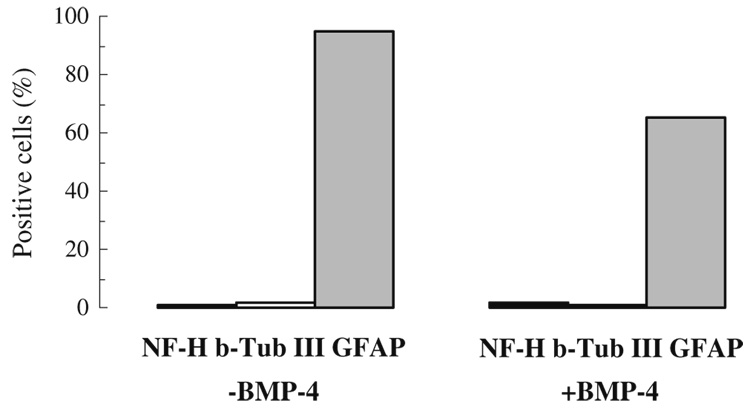
Another means to achieve maximal mitogenic stimulation of SFME cells is through the action of oncogenes. For these experiments, we used SFME-ras cells, cloned from SFME cells transfected with the human Ha-ras oncogene (Shirahata et al. 1990). This oncogene is involved in the downstream signaling of tyrosine kinase receptors for growth factors including EGF and FGF-2 and allows the growth of SFME cells in the absence of EGF or FGF. Most of these cells expressed GFAP, even in the absence of added growth factors. When these cells were cultured for 4 d in 100 ng/mL BMP-4 without EGF/FGF-2, the percentage of β-tubulin III (marker for immature neurons) and NF-H-positive neurons was very low (0.21±0.17% for NF-H), while the percentage of GFAP-positive cells was somewhat reduced, but remained greater than 60% (Fig. 4). No NF-H-positive cells were observed in controls without BMP-4.
Figure 4.
BMP-4 treatment and marker expression of SFME-ras cells. The cells were cultured for 4 d in 100 ng/mL BMP-4 without EGF/FGF-2, and percentage of cells positive for NF-H, β-tubulin III, and GFAP determined by immunofluorescence.
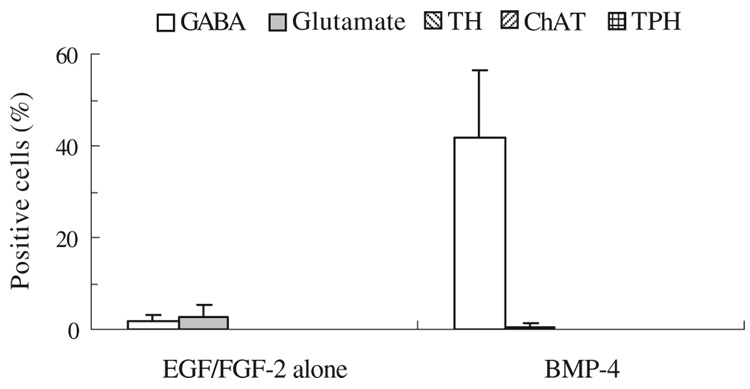
BMP-4 enhances development into GABAergic neurons
Differentiation of SFME to specific neuronal phenotypes was examined by assay for GABA, glutamate, tyrosine hydroxylase (TH), choline acetyltransferase (ChAT), and tryptophan hydroxylase (TPH). SFME cells were preincubated overnight in 5 ng/mL EGF/FGF-2 and cultured for 4 d in 0.5 ng/mL EGF/FGF-2 with or without 100 ng/mL BMP-4. All cultures were fixed after 4 d and examined for evidence of GABAergic, glutaminergic, dopaminergic, and cholinergic neuronal phenotypes. In the control (cells cultured for 4 d in 0.5 ng/mL EGF/FGF-2 alone), few cells were immunoreactive for GABA (1.64±1.49%) or glutamate (2.78±2.36%) with no cells exhibiting TH, ChAT, or TPH (Fig. 5). BMP-4 treatment augmented the percentage of cells expressing GABA about 25-fold over control cultures (41.7±14.6%), whereas glutamate-positive cells were few (0.6±0.65%) and the other phenotypes were negative. When cells were also stained for NF-H, we found that 79.8±10.7% of the NF-H-positive cells were also expressing GABA.
Figure 5.
BMP-4 induction of GABAergic neuronal phenotype in SFME cells. Cells were cultured for 4 d in 0.5 ng/mL EGF/FGF-2 with or without 100 ng/mL BMP-4 and then subjected to immunofluorescent staining for GABA, glutamate, TH, ChAT, and TPH. Percentage of positive cells (mean±SD) is shown.
Discussion
Previously, it has been reported that SFME cells express nestin in the undifferentiated state and that serum or TGF-β induces the expression of astrocytic markers and reduces the expression of nestin (Sakai et al. 1990; Loo et al. 1994, 1995). In this study, we demonstrate that SFME cells can express neuronal markers under specific culture conditions. Under conditions that were suboptimal for cell proliferation, BMP-4 significantly augmented the number of NF-H-positive neurons and activated the NSE promoter.
A variety of findings have supported a role for the BMP family in neuronal and glial differentiation. Pleiotropic effects are observed on prenatal and postnatal neural stem cells; BMP-4 has been implicated in the control of differentiation of neuronal cells and has multiple and complicated effects on various cell types (Angley et al. 2003; Ling and Panchision 2007). BMP antagonists promote neuronal differentiation of progenitors in the adult mouse subventricular zone and inhibit glial cell differentiation (Lim et al. 2000; Lein et al. 2002), and BMPs promote astrocytic differentiation of progenitors in the murine embryonic subventricular zone (Gomes et al. 2003). BMPs trigger neuronal differentiation of neocortical precursors in the mouse ventricular zone (Li et al. 1998). These and other results indicate that the effects are both cell type- and BMP subtype-specific (Lopez-Coviella et al. 2000; Yabe et al. 2002). Similarly, EGF or EGF-like molecules and FGF-2 play essential roles in neuronal development (Deleyrolle et al. 2006) and BMP-4 and FGF-2 may act in an antagonistic manner (Ota and Ito 2006).
Interestingly, we found that, under conditions of maximal mitogenic stimulation of SFME cells (EGF/FGF-2 more than 5 ng/mL), BMP-4 treatment failed to generate cells displaying NF-H, but did induce expression of GFAP. These results point out that BMP-4 or other growth factor effects on neuronal differentiation may be mitigated in highly mitogenic environments and suggest the potential for factors that inhibit EGF or FGF mitogenicity to play a role in neuronal differentiation.
Growth factor-stimulated tyrosine kinase receptors for EGF or FGF-2 activate a number of signaling pathways to promote either growth or differentiation. Ras is one mediator that is activated by various influences and is involved in pathways of tyrosine kinase growth factor receptors. In this study, we found that BMP-4 had little effect on the generation of NF-H-positive neurons by SFME cells transfected with Ha-ras oncogene (SFME-ras cells). Thus, the constant expression of constitutively active ras acted in a manner similar to maximal mitogenic stimulation by EGF/FGF-2.
In our studies, BMP-4-treated SFME cells exhibited the capacity to develop into GABAergic neurons under growth factor-reduced conditions. These neurons in vivo function as the main neurotransmitter of inhibitory postsynaptic potential in the central nervous system. Low levels of SFME cell differentiation to glutaminergic neurons were observed, and these were not increased by BMP-4 treatment. FGF-2-responsive murine subventricular zone progenitors are reported to have the capacity to generate neurons immunoreactive for GABA, glutamate, or ChAT (Gritti et al. 1996). In parallel studies, EGF-responsive progenitors are unable to generate TH, glutamate, or 5-TH-positive neurons (Reynolds and Weiss 1992; Reynolds et al. 1992). It has been shown previously that brain-derived neurotrophic factor (BDNF) enhances the differentiation of GABAergic neurons in mouse striatum neurons or in rat hippocampus neurons (Arsenijevic and Weiss 1998; Vicario-Abejon et al. 1998). Thus far, we have seen no effect of BDNF in our SFME cell culture system.
SFME cells represent an established, chromosomally stable cell line that is self-renewing under culture conditions, which favors the multipotential stem cell state, and the cell line is capable of generating neurons and astrocytes under appropriate conditions in vitro. These results support the proposal that comparable EGF-dependent multipotential neural cells in the mouse embryonic central nervous system may have an unlimited proliferative capability. These results also point out the usefulness of the SFME cell line as a stable system for the introduction or inhibition of genes of interest in understanding growth factor-influenced pathways in neural progenitor proliferation and differentiation.
Acknowledgments
We thank Drs. D. Sato and. A. Toumadjie for the advice, P. Mericko and L. Chen for the technical assistance, and N. Young and A. Miller for the inspiration. NSE transgenic mice were a gift of Dr. J. G. Sutcliffe. This study was supported by NIH Grants P40RR15452, P20RR16463 and ES03828 and a grant from the Hansen Foundation.
Contributor Information
Ken-ichi Kusumoto, Department of Biological Chemistry, Biotechnology and Food Research Institute, Fukuoka Industrial Technology Center, Kurume, Fukuoka 8390861, Japan.
Angela Parton, Mount Desert Island Biological Laboratories, P.O. Box 35, Salisbury Cove, ME 04672, USA.
David Barnes, Email: dbarnes@mdibl.org, Mount Desert Island Biological Laboratories, P.O. Box 35, Salisbury Cove, ME 04672, USA.
References
- Angley C, Kumar M, Dinsio KJ, Hall AD, Siegel RE. Signaling by bone morphogenetic proteins and Smad1 modulates the postnatal differentiation of cerebellar cells. J. Neurosci. 2003;23:260–268. doi: 10.1523/JNEUROSCI.23-01-00260.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arsenijevic Y, Weiss S. Insulin-like growth factor-I is a differentiation factor for postmitotic CNS stem cell-derived neuronal precursors: distinct actions from those of brain-derived neurotrophic factor. J. Neurosci. 1998;18:2118–2128. doi: 10.1523/JNEUROSCI.18-06-02118.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Alessandro JS, Wang EA. Bone morphogenetic proteins inhibit proliferation, induce reversible differentiation and prevent cell death in astrocyte lineage cells. Growth Factors. 1994a;11:45–52. doi: 10.3109/08977199409015050. [DOI] [PubMed] [Google Scholar]
- D’Alessandro JS, Yetz-Aldape J, Wang EA. Bone morphogenetic proteins induce differentiation in astrocyte lineage cells. Growth Factors. 1994b;11:53–69. doi: 10.3109/08977199409015051. [DOI] [PubMed] [Google Scholar]
- Deleyrolle L, Marchal-Victorion S, Dromard C, Fritz V, Saunier M, Sabourn J-C, Ba C, Privat A, Hugnot J-P. Exogenous and fibroblast growth factor 2/epidermal growth factor-regulated endogenous cytokines regulate neural precursor cell growth and differentiation. Stem Cells. 2006;24:748–762. doi: 10.1634/stemcells.2005-0138. [DOI] [PubMed] [Google Scholar]
- Ernst T, Jackson C, Barnes D. Karyotypic stability of serum-free mouse embryo cells. Cytotechnology. 1991;5:211–222. doi: 10.1007/BF00556291. [DOI] [PubMed] [Google Scholar]
- Forss-Petter S, Danielson PE, Catsicas S, Battenberg E, Price J, Nerenberg M, Sutcliffe JG. Transgenic mice expressing beta-galactosidase in mature neurons under neuron-specific enolase promoter control. Neuron. 1990;5:187–197. doi: 10.1016/0896-6273(90)90308-3. [DOI] [PubMed] [Google Scholar]
- Gomes WA, Mehler MF, Kessler JA. Transgenic overexpression of BMP4 increases astroglial and decreases oligodendrioglial lineage commitment. Dev. Biol. 2003;255:164–177. doi: 10.1016/s0012-1606(02)00037-4. [DOI] [PubMed] [Google Scholar]
- Gritti A, Parati EA, Cova L, Frolichsthal P, Galli R, Wanke E, Faravelli L, Morassutti DJ, Roisen F, Nickel DD, Vescovi AL. Multipotential stem cells from the adult mouse brain proliferate and self-renew in response to basic fibroblast growth factor. J. Neurosci. 1996;16:1091–1100. doi: 10.1523/JNEUROSCI.16-03-01091.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lein PH, Beck HN, Chandrasekaran V, Gallagher PH, Chen HL, Lin Y, Guo X, Kaplan PL, Tiedge H, Higgins D. Glia induce dendritic growth in cultured sympathetic neurons by modulating the balance between bone morphogenetic proteins (BMPs) and BMP antagonists. J. Neurosci. 2002;22:10377–10387. doi: 10.1523/JNEUROSCI.22-23-10377.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Cogswell CA, LoTurco JJ. Neuronal differentiation of precursors in the neocortical ventricular zone is triggered by BMP. J. Neurosci. 1998;18:8853–8862. doi: 10.1523/JNEUROSCI.18-21-08853.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim DA, Tramontin AD, Trevejo JM, Herrera DG, Garcia-Verdugo JM, Alvarez-Buylla A. Noggin antagonizes BMP signaling to create a niche for adult neurogenesis. Neuron. 2000;28:713–726. doi: 10.1016/s0896-6273(00)00148-3. [DOI] [PubMed] [Google Scholar]
- Ling H-L, Panchision DM. Concise review: bone morphogenetic protein pleitrophism in neural stem cells and their derivatives-alternative pathways, convergent signals. Stem Cells. 2007;25:63–68. doi: 10.1634/stemcells.2006-0339. [DOI] [PubMed] [Google Scholar]
- Loo DT, Althoen MC, Cotman CW. Down regulation of nestin by TGF-beta or serum in SFME cells accompanies differentiation into astrocytes. NeuroReport. 1994;5:1585–1588. doi: 10.1097/00001756-199408150-00011. [DOI] [PubMed] [Google Scholar]
- Loo DT, Althoen MC, Cotman CW. Differentiation of serum-free mouse embryo cells into astrocytes is accompanied by induction of glutamine synthetase activity. J. Neurosci. Res. 1995;42:184–191. doi: 10.1002/jnr.490420205. [DOI] [PubMed] [Google Scholar]
- Loo DT, Bradford S, Sharps A, Barnes D. BCL-2 inhibits cell death of serum-free mouse embryo cells caused by EGF deprivation. Cell Biol. Toxicol. 1998;14:375–382. doi: 10.1023/a:1007518909429. [DOI] [PubMed] [Google Scholar]
- Loo DT, Fuquay JI, Rawson CL, Barnes DW. Extended culture of mouse embryo cells without senescence: inhibition by serum. Science. 1987;236:200–202. doi: 10.1126/science.3494308. [DOI] [PubMed] [Google Scholar]
- Loo DT, Rawson C, Helmrich A, Barnes D. Serum-free mouse embryo (SFME) cells: growth responses in vitro. J. Cell. Physiol. 1989;139:484–491. doi: 10.1002/jcp.1041390306. [DOI] [PubMed] [Google Scholar]
- Lopez-Coviella I, Berse B, Krauss R, Thies RS, Blusztajn JK. Induction and maintenance of the neuronal cholinergic phenotype in the central nervous system by BMP-9. Science. 2000;289:313–316. doi: 10.1126/science.289.5477.313. [DOI] [PubMed] [Google Scholar]
- Mathieu C, Sii-Felice K, Fouchet P, Etienne O, Haton C, Mabondzo A, Boussin FD, Mouthon M-A. Endothelial cell-derived bone morphogenetic proteins control proliferation of neural stem/progenitor cells. Mol. Cell. Neurosci. 2008;38:569–577. doi: 10.1016/j.mcn.2008.05.005. [DOI] [PubMed] [Google Scholar]
- Murayama K, Singh NN, Helmrich A, Barnes DW. Neural cell lines. In: Federhoff S, Richards A, editors. Protocols for neural cell cultures. Totowa, New Jersey, USA: Humana Press; 2000. pp. 219–228. [Google Scholar]
- Nishiyama K, Collodi P, Barnes D. Regulation of glial fibrillary acidic protein in serum-free mouse embryo (SFME) cells by leukemia inhibitory factor and related peptides. Neurosci. Lett. 1993;163:114–116. doi: 10.1016/0304-3940(93)90242-d. [DOI] [PubMed] [Google Scholar]
- Ota M, Ito K. BMP and FGF-2 regulate neurogenin-2 expression and the differentiation of sensory neurons and glia. Dev. Dyn. 2006;235:646–655. doi: 10.1002/dvdy.20673. [DOI] [PubMed] [Google Scholar]
- Rawson C, Loo D, Hedstrom O, Schmidt E, Barnes D. Death of serum-free mouse embryo (SFME) cells caused by EGF deprivation. J. Cell. Biol. 1991;113:671–680. doi: 10.1083/jcb.113.3.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds BA, Tetzlaff W, Weiss S. A multipotent EGF-responsive striatal embryonic progenitor cell produces neurons and astrocytes. J. Neurosci. 1992;12:4565–4574. doi: 10.1523/JNEUROSCI.12-11-04565.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255:1707–1710. doi: 10.1126/science.1553558. [DOI] [PubMed] [Google Scholar]
- Sakai Y, Rawson C, Lindburg K, Barnes D. Serum and transforming growth factor beta regulate glial fibrillary acidic protein in serum free-derived mouse embryo cells. Proc. Natl. Acad. Sci. U. S. A. 1990;87:8378–8382. doi: 10.1073/pnas.87.21.8378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirahata S, Rawson C, Loo D, Chang Y, Barnes D. Ras and neu oncogenes reverse serum inhibition and epidermal growth factor dependence of serum-free mouse embryo (SFME) cells. J. Cell. Physiol. 1990;144:69–76. doi: 10.1002/jcp.1041440110. [DOI] [PubMed] [Google Scholar]
- Slinskey A, Barnes D, Pipas JM. SV40 large T antigen J domain and RB-binding motif are sufficient to block apoptosis induced by growth factor withdrawal in a neuronal stem cell line. J. Virol. 2000;73:6791–6799. doi: 10.1128/jvi.73.8.6791-6799.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solem M, Rawson C, Lindburg K, Barnes D. Transforming growth factor beta regulates cystatin C in serum-free mouse embryo (SFME) cells. Biochem. Biophys. Res. Commun. 1990;172:945–951. doi: 10.1016/0006-291x(90)90767-h. [DOI] [PubMed] [Google Scholar]
- Vicario-Abejon C, Collin C, McKay RD, Segal M. Neurotrophins induce formation of functional excitatory and inhibitory synapses between cultured hippocampal neurons. J. Neurosci. 1998;18:7256–7271. doi: 10.1523/JNEUROSCI.18-18-07256.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisz PV, Solem M, Barnes D. Expression of a TGFβ regulated, brain-specific mRNA in serum-free mouse embryo (SFME) cells. Neurosci. Lett. 1993;154:153–156. doi: 10.1016/0304-3940(93)90194-p. [DOI] [PubMed] [Google Scholar]
- Yabe T, Samuels I, Schwartz JP. Bone morphogenetic proteins BMP-6 and BMP-7 have differential effects on survival and neurite outgrowth of cerebellar granule cell neurons. J. Neurosci. Res. 2002;68:161–168. doi: 10.1002/jnr.10210. [DOI] [PubMed] [Google Scholar]