Abstract
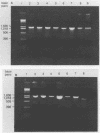
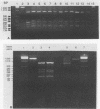
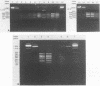
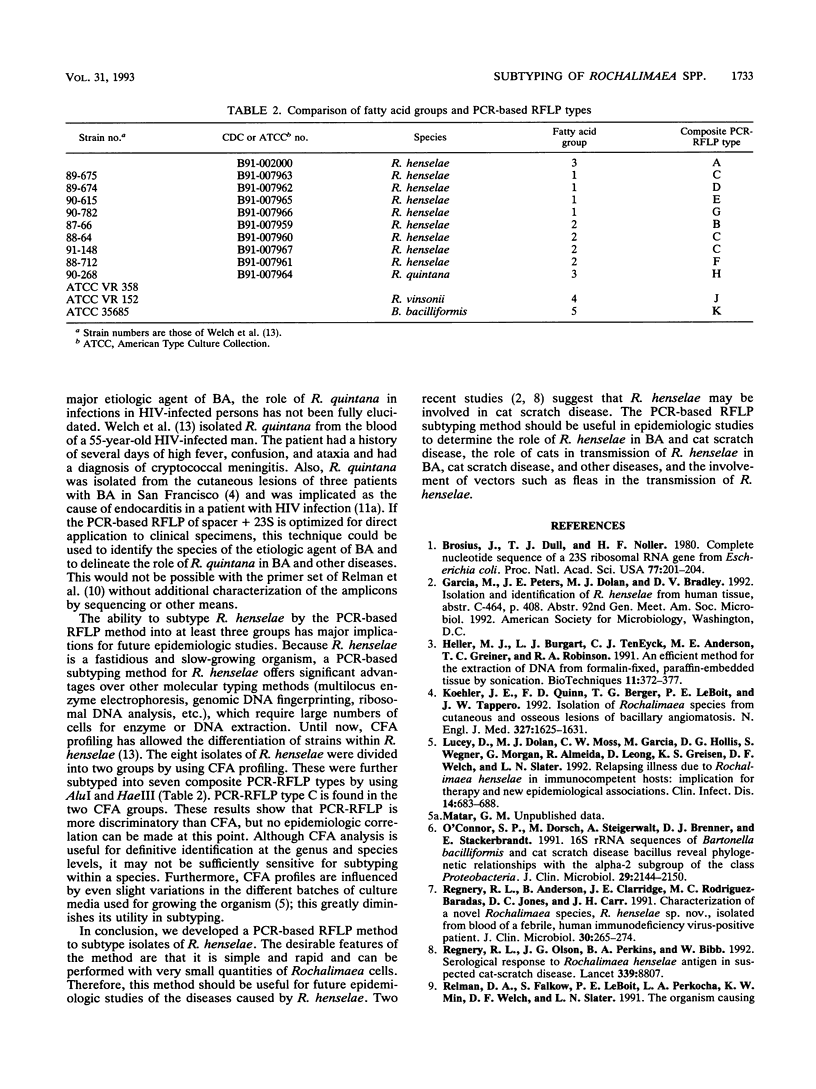
Restriction endonuclease analysis of a polymerase chain reaction-amplified DNA fragment which included the spacer region between the genes coding for 16S and 23S rRNAs and a portion of the gene coding for 23S rRNA (spacer + 23S) was done on 10 previously characterized clinical isolates of Rochalimaea henselae, one clinical isolate of Rochalimaea quintana, and the type strains of R. henselae, R. quintana, Rochalimaea vinsonii, and Bartonella bacilliformis. Brucella abortus DNA was not amplified by the primer set used. The clinical isolates of Rochalimaea were obtained from blood or tissue from patients with and without preexisting disease. The amplicon from each strain was digested with five endonucleases (AluI, HaeIII, TaqI, HinfI, and MseI). AluI and HaeIII were useful in species differentiation and subtyping of R. henselae. R. henselae isolates showed six different restriction patterns with AluI and four patterns with HaeIII. TaqI, HinfI, and MseI were useful only in species differentiation. These observations indicate that PCR amplification of the spacer + 23S region of the ribosomal DNA of Rochalimaea spp., along with restriction endonuclease analysis, allows differentiation of Rochalimaea spp. from closely related genera, differentiation among the species within Rochalimaea, and differentiation of strains within R. henselae. The subtyping potential of this method may be useful for further clinical and epidemiologic studies of the spectrum of diseases caused by R. henselae.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brosius J., Dull T. J., Noller H. F. Complete nucleotide sequence of a 23S ribosomal RNA gene from Escherichia coli. Proc Natl Acad Sci U S A. 1980 Jan;77(1):201–204. doi: 10.1073/pnas.77.1.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller M. J., Burgart L. J., TenEyck C. J., Anderson M. E., Greiner T. C., Robinson R. A. An efficient method for the extraction of DNA from formalin-fixed, paraffin-embedded tissue by sonication. Biotechniques. 1991 Sep;11(3):372-4, 376-7. [PubMed] [Google Scholar]
- Koehler J. E., Quinn F. D., Berger T. G., LeBoit P. E., Tappero J. W. Isolation of Rochalimaea species from cutaneous and osseous lesions of bacillary angiomatosis. N Engl J Med. 1992 Dec 3;327(23):1625–1631. doi: 10.1056/NEJM199212033272303. [DOI] [PubMed] [Google Scholar]
- Lucey D., Dolan M. J., Moss C. W., Garcia M., Hollis D. G., Wegner S., Morgan G., Almeida R., Leong D., Greisen K. S. Relapsing illness due to Rochalimaea henselae in immunocompetent hosts: implication for therapy and new epidemiological associations. Clin Infect Dis. 1992 Mar;14(3):683–688. doi: 10.1093/clinids/14.3.683. [DOI] [PubMed] [Google Scholar]
- O'Connor S. P., Dorsch M., Steigerwalt A. G., Brenner D. J., Stackebrandt E. 16S rRNA sequences of Bartonella bacilliformis and cat scratch disease bacillus reveal phylogenetic relationships with the alpha-2 subgroup of the class Proteobacteria. J Clin Microbiol. 1991 Oct;29(10):2144–2150. doi: 10.1128/jcm.29.10.2144-2150.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regnery R. L., Anderson B. E., Clarridge J. E., 3rd, Rodriguez-Barradas M. C., Jones D. C., Carr J. H. Characterization of a novel Rochalimaea species, R. henselae sp. nov., isolated from blood of a febrile, human immunodeficiency virus-positive patient. J Clin Microbiol. 1992 Feb;30(2):265–274. doi: 10.1128/jcm.30.2.265-274.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slater L. N., Welch D. F., Min K. W. Rochalimaea henselae causes bacillary angiomatosis and peliosis hepatis. Arch Intern Med. 1992 Mar;152(3):602–606. [PubMed] [Google Scholar]
- Spach D. H., Callis K. P., Paauw D. S., Houze Y. B., Schoenknecht F. D., Welch D. F., Rosen H., Brenner D. J. Endocarditis caused by Rochalimaea quintana in a patient infected with human immunodeficiency virus. J Clin Microbiol. 1993 Mar;31(3):692–694. doi: 10.1128/jcm.31.3.692-694.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisburg W. G., Barns S. M., Pelletier D. A., Lane D. J. 16S ribosomal DNA amplification for phylogenetic study. J Bacteriol. 1991 Jan;173(2):697–703. doi: 10.1128/jb.173.2.697-703.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch D. F., Pickett D. A., Slater L. N., Steigerwalt A. G., Brenner D. J. Rochalimaea henselae sp. nov., a cause of septicemia, bacillary angiomatosis, and parenchymal bacillary peliosis. J Clin Microbiol. 1992 Feb;30(2):275–280. doi: 10.1128/jcm.30.2.275-280.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson K. H., Blitchington R. B., Greene R. C. Amplification of bacterial 16S ribosomal DNA with polymerase chain reaction. J Clin Microbiol. 1990 Sep;28(9):1942–1946. doi: 10.1128/jcm.28.9.1942-1946.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]