Abstract
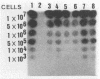
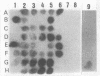
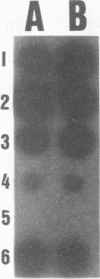
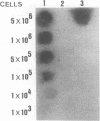
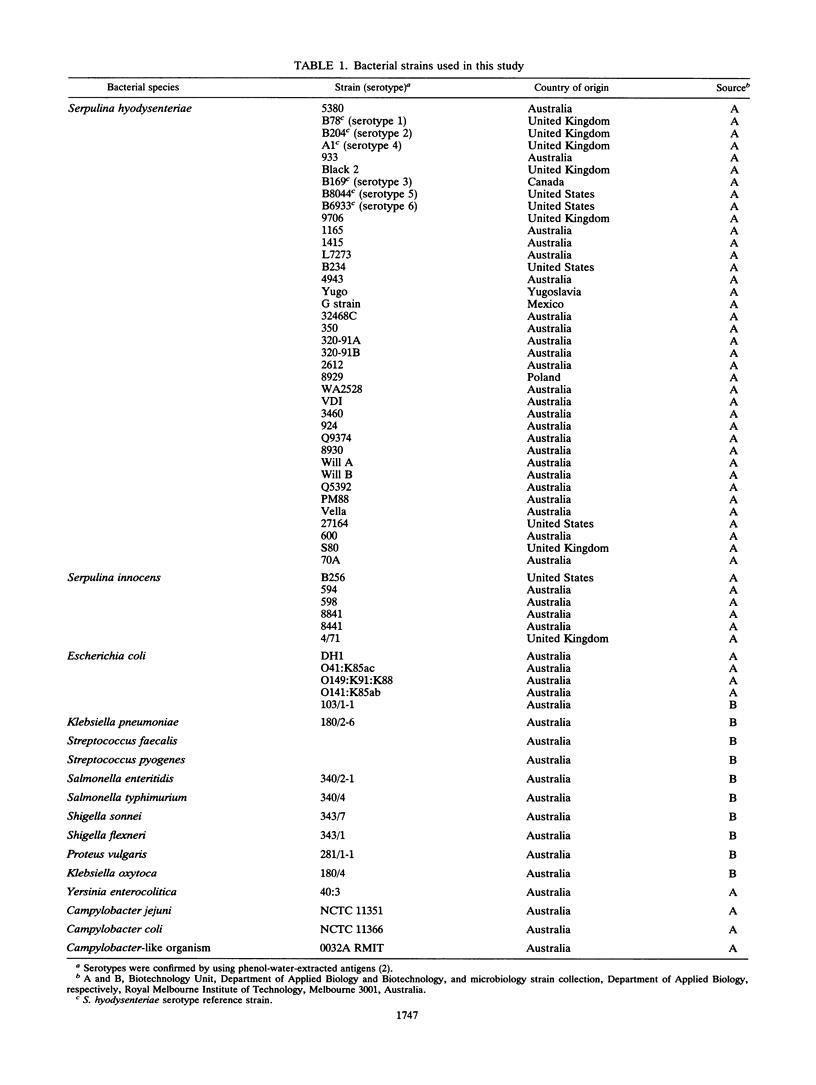
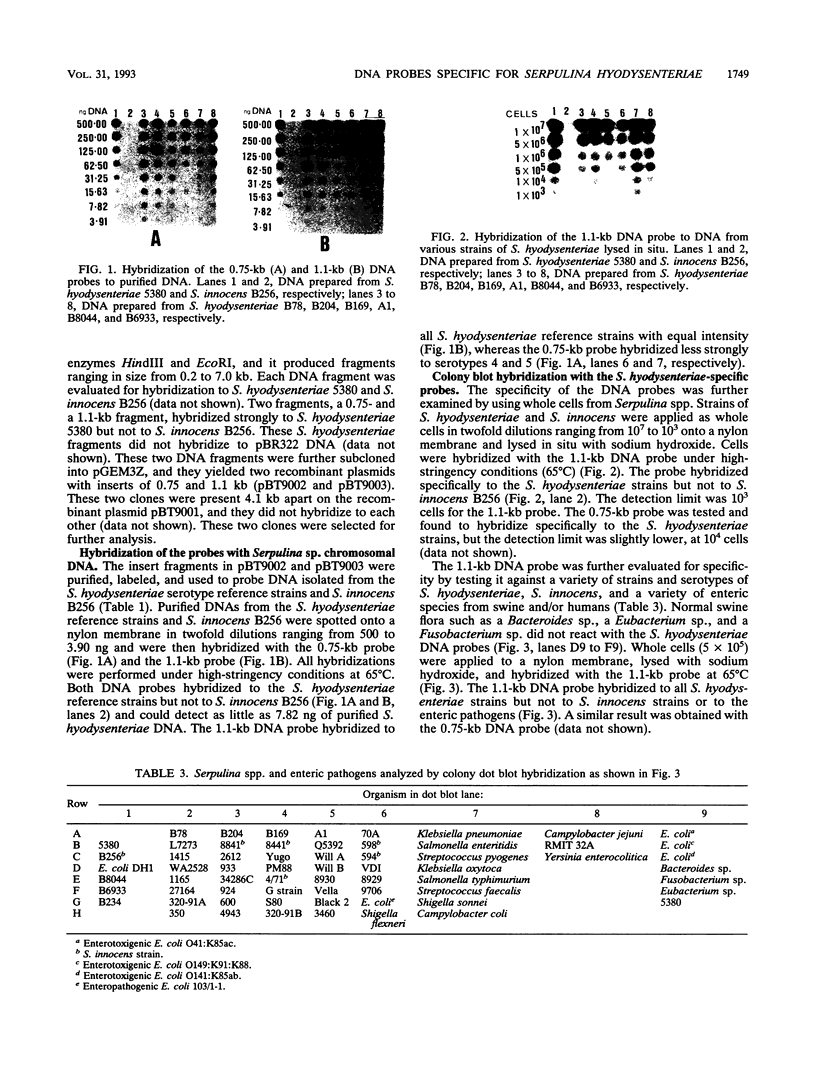
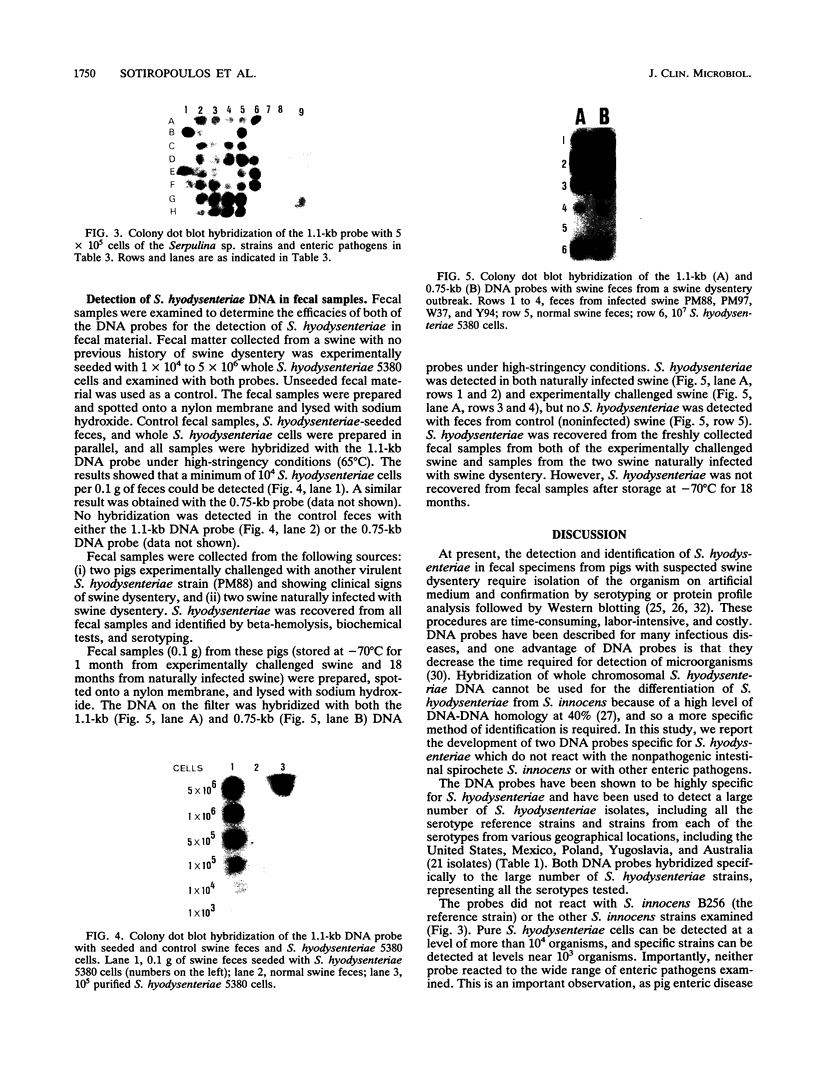
Two DNA probes, one 1.1- and one 0.75-kb probe, specific for Serpulina hyodysenteriae were isolated from a genomic library generated from virulent S. hyodysenteriae 5380. These probes are highly specific and react with all S. hyodysenteriae strains tested. Under stringent conditions, the DNA probes did not react with the nonpathogenic species Serpulina innocens or with other species of enteric bacteria, including Escherichia coli. Both probes are able to detect S. hyodysenteriae in colony blot hybridizations, and when applied to fecal specimens, they can detect 10(4) S. hyodysenteriae cells in 0.1 g of seeded fecal matter. Both probes can detect S. hyodysenteriae in fecal specimens from swine with clinical signs of swine dysentery after experimental challenge and from swine from a herd with an acute outbreak of swine dysentery. These probes have application as a diagnostic tool in veterinary microbiology.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baum D. H., Joens L. A. Serotypes of beta-hemolytic Treponema hyodysenteriae. Infect Immun. 1979 Sep;25(3):792–796. doi: 10.1128/iai.25.3.792-796.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Bélanger M., Jacques M. Evaluation of the An-Ident system and an indole spot test for the rapid differentiation of porcine treponemes. J Clin Microbiol. 1991 Aug;29(8):1727–1729. doi: 10.1128/jcm.29.8.1727-1729.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins D. M., Ross D. E. Restriction endonuclease analysis of Campylobacter strains with particular reference to Campylobacter fetus ss. fetus. J Med Microbiol. 1984 Aug;18(1):117–124. doi: 10.1099/00222615-18-1-117. [DOI] [PubMed] [Google Scholar]
- Combs B., Hampson D. J., Mhoma J. R., Buddle J. R. Typing of Treponema hyodysenteriae by restriction endonuclease analysis. Vet Microbiol. 1989 Apr;19(4):351–359. doi: 10.1016/0378-1135(89)90100-4. [DOI] [PubMed] [Google Scholar]
- Harris D. L., Glock R. D., Christensen C. R., Kinyon J. M. Inoculation of pigs with Treponema hyodysenteriae (new species) and reproduction f the disease. Vet Med Small Anim Clin. 1972 Jan;67(1):61–64. [PubMed] [Google Scholar]
- Holmes D. S., Quigley M. A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem. 1981 Jun;114(1):193–197. doi: 10.1016/0003-2697(81)90473-5. [DOI] [PubMed] [Google Scholar]
- Hudson M. J., Alexander T. J., Lysons R. J. Diagnosis of swine dysentery: spirochaetes which may be confused with Treponema hyodysenteriae. Vet Rec. 1976 Dec 18;99(25-26):498–500. doi: 10.1136/vr.99.25-26.498. [DOI] [PubMed] [Google Scholar]
- Hunter D., Wood T. An evaluation of the API ZYM system as a means of classifying spirochaetes associated with swine dysentery. Vet Rec. 1979 Apr 28;104(17):383–384. doi: 10.1136/vr.104.17.383. [DOI] [PubMed] [Google Scholar]
- Jensen N. S., Casey T. A., Stanton T. B. Detection and identification of Treponema hyodysenteriae by using oligodeoxynucleotide probes complementary to 16S rRNA. J Clin Microbiol. 1990 Dec;28(12):2717–2721. doi: 10.1128/jcm.28.12.2717-2721.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joens L. A., Glock R. D. Experimental infection in mice with Treponema hyodysenteriae. Infect Immun. 1979 Aug;25(2):757–760. doi: 10.1128/iai.25.2.757-760.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joens L. A., Harris D. L., Baum D. H. Immunity to Swine dysentery in recovered pigs. Am J Vet Res. 1979 Oct;40(10):1352–1354. [PubMed] [Google Scholar]
- Joens L. A., Harris D. L., Kinyon J. M., Kaeberle M. L. Microtitration agglutination for detection of Treponema hyodysenteriae antibody. J Clin Microbiol. 1978 Sep;8(3):293–298. doi: 10.1128/jcm.8.3.293-298.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joens L. A., Songer J. G., Harris D. L., Glock R. D. Experimental infection with Treponema hyodysenteriae in guinea pigs. Infect Immun. 1978 Oct;22(1):132–135. doi: 10.1128/iai.22.1.132-135.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinyon J. M., Harris D. L., Glock R. D. Enteropathogenicity of various isolates of Treponema hyodysenteriae. Infect Immun. 1977 Feb;15(2):638–646. doi: 10.1128/iai.15.2.638-646.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkle R. A., Kinyon J. M. Improved selective medium for the isolation of Treponema hyodysenteriae. J Clin Microbiol. 1988 Nov;26(11):2357–2360. doi: 10.1128/jcm.26.11.2357-2360.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mapother M. E., Joens L. A. New serotypes of Treponema hyodysenteriae. J Clin Microbiol. 1985 Aug;22(2):161–164. doi: 10.1128/jcm.22.2.161-164.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nibbelink S. K., Wannemuehler M. J. Susceptibility of inbred mouse strains to infection with Serpula (Treponema) hyodysenteriae. Infect Immun. 1991 Sep;59(9):3111–3118. doi: 10.1128/iai.59.9.3111-3118.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Schwan T. G., Simpson W. J., Schrumpf M. E., Karstens R. H. Identification of Borrelia burgdorferi and B. hermsii using DNA hybridization probes. J Clin Microbiol. 1989 Aug;27(8):1734–1738. doi: 10.1128/jcm.27.8.1734-1738.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S. C., Barrett L. M., Muir T., Christopher W. L., Coloe P. J. Application and evaluation of enzyme-linked immunosorbent assay and immunoblotting for detection of antibodies to Treponema hyodysenteriae in swine. Epidemiol Infect. 1991 Oct;107(2):285–296. doi: 10.1017/s0950268800048937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S. C., Roddick F., Ling S., Gerraty N. L., Coloe P. J. Biochemical and immunochemical characterisation of strains of Treponema hyodysenteriae. Vet Microbiol. 1990 Jul;24(1):29–41. doi: 10.1016/0378-1135(90)90048-z. [DOI] [PubMed] [Google Scholar]
- Stanton T. B., Jensen N. S., Casey T. A., Tordoff L. A., Dewhirst F. E., Paster B. J. Reclassification of Treponema hyodysenteriae and Treponema innocens in a new genus, Serpula gen. nov., as Serpula hyodysenteriae comb. nov. and Serpula innocens comb. nov. Int J Syst Bacteriol. 1991 Jan;41(1):50–58. doi: 10.1099/00207713-41-1-50. [DOI] [PubMed] [Google Scholar]
- Sueyoshi M., Adachi Y., Shoya S. Enteropathogenicity of Treponema hyodysenteriae in young chicks. Zentralbl Bakteriol Mikrobiol Hyg A. 1987 Oct;266(3-4):469–477. doi: 10.1016/s0176-6724(87)80229-8. [DOI] [PubMed] [Google Scholar]
- Taylor D. J., Alexander T. J. The production of dysentery in swine by feeding cultures containing a spirochaete. Br Vet J. 1971 Nov;127(11):58–61. doi: 10.1016/s0007-1935(17)37282-2. [DOI] [PubMed] [Google Scholar]
- Tenover F. C. Diagnostic deoxyribonucleic acid probes for infectious diseases. Clin Microbiol Rev. 1988 Jan;1(1):82–101. doi: 10.1128/cmr.1.1.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walfield A. M., Hanff P. A., Lovett M. A. Expression of Treponema pallidum antigens in Escherichia coli. Science. 1982 Apr 30;216(4545):522–523. doi: 10.1126/science.7041257. [DOI] [PubMed] [Google Scholar]
- Wright J. C., Wilt G. R., Reed R. B., Powe T. A. Use of an enzyme-linked immunosorbent assay for detection of Treponema hyodysenteriae infection in swine. J Clin Microbiol. 1989 Mar;27(3):411–416. doi: 10.1128/jcm.27.3.411-416.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuerner R. L., Bolin C. A. Repetitive sequence element cloned from Leptospira interrogans serovar hardjo type hardjo-bovis provides a sensitive diagnostic probe for bovine leptospirosis. J Clin Microbiol. 1988 Dec;26(12):2495–2500. doi: 10.1128/jcm.26.12.2495-2500.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]