Abstract
Objective:
Smoking and family history of aneurysmal subarachnoid hemorrhage (aSAH) are independent risk factors for aSAH. Using a population-based case-control study of hemorrhagic stroke, we hypothesized that having both a first-degree relative with a brain aneurysm or SAH (+FH) and current smoking interact to increase the risk of aSAH.
Methods:
Cases of aneurysmal SAH were prospectively recruited from all 17 hospitals in the five-county region around the University of Cincinnati. Controls were identified by random digit dialing. Controls were matched to cases of aSAH by age (±5 years), race, and sex. Conditional multiple logistic regression was used to identify independent risk factors. For deviation from the additive model, the interaction constant ratio test was used.
Results:
A total of 339 cases of aSAH were matched to 1,016 controls. Compared to current nonsmokers with no first-degree relatives with aSAH (−FH), the odds ratio (OR) for aSAH for current nonsmokers with +FH was 2.5 (95% confidence interval [CI] 0.9–6.9); for current smokers with −FH, OR = 3.1 (95% CI 2.2–4.4); and for current smokers with +FH, OR = 6.4 (95% CI 3.1–13. 2). The interaction constant ratio, which measured the deviation from the additive model, was significant: 2.19 (95% CI 0.80–5.99). The lower bound of the 95% CI >0.5 signifies a departure from the additive model.
Conclusion:
Evidence of a gene–environment interaction with smoking exists for aneurysmal subarachnoid hemorrhage. This finding is important to counseling family members and for screening of intracranial aneurysm (IA) as well as the design and interpretation of genetic epidemiology of IA studies.
GLOSSARY
- aSAH
= aneurysmal subarachnoid hemorrhage;
- CI
= confidence interval;
- GERFHS
= Genetic and Environmental Risk Factors of Hemorrhagic Stroke;
- IA
= intracranial aneurysm;
- ICH
= intracerebral hemorrhage;
- ICR
= interaction contrast ratio;
- OR
= odds ratio.
One of the challenges in genetic epidemiology of complex traits is identification of gene–environment interactions. Environmental and behavioral factors may be necessary for triggering the manifestation of risk or benefit of some genetic variations, or enhancing the effect. Current and past smoking has been consistently found to be associated with aneurysmal subarachnoid hemorrhage (aSAH) and is considered to be the most significant modifiable risk factor for aSAH.1,2 In population-based or cohort studies, 70–75% of persons with SAH have a history of smoking, and 50–60% are current smokers.1,2 Smoking behavior is known to aggregate within families, and similarly, a family history of brain aneurysm or aSAH have been found to aggregate within families, independent of smoking.1,3,4
However, few studies have explored the possibility that current smoking and a positive family history may interact to further increase the risk of aSAH than their individual risks. Such evidence for a gene–environment interaction may have significant implications for screening family members of patients with an aneurysmal SAH, analysis of genetic association studies, and counseling of family members regarding smoking behavior.
An additive or “no interaction” relationship between risk factors is one in which the presence of both factors yields a risk that is equivalent to adding the individual risks together. An interaction occurs when the presence of both factors leads to a greater (or lesser) risk of the outcome than simply adding the individual risks. For most interactions, however, a minority of cases will have both risk factors (without bias, roughly equivalent to the prevalence of each factor multiplied together). Thus, the power to identify interactions using traditional logistic regression methods is limited in power as a function of the analytical technique. Newer methods to identify interactions seek to identify a significant departure from the additive model. We used the interaction constant ratio as described in Methods and estimated the 95% confidence interval (CI) of the result to determine significance.
We hypothesized that current smoking and having a first-degree relative were independent risk factors and that the coexistence of these risk factors was greater than additive in risk.
METHODS
The methods of the Genetic and Environmental Risk Factors of Hemorrhagic Stroke (GERFHS) Study (National Institute of Neurological Disorders and Stroke: NS36695) have been published previously.5,6 Institutional Review Board approval was obtained from all participating hospitals and informed consent was obtained from all subjects. HIPAA authorization was obtained from all subjects after April 2003. All patients with a potential intracerebral hemorrhage (ICH) or SAH who reside within 50 miles of the University of Cincinnati are identified by surveillance of hospital emergency and radiology departments and hospital discharge diagnoses.
Aneurysmal SAH was identified in patients with SAH by review of neuroimaging, including CT, MRI, and angiography scanning. Intracranial aneurysms (IA) were identified by CTA or MRA if >4 mm, by cerebral angiography, or at autopsy. Fusiform aneurysms, aneurysms related to arteriovenous malformations, and subarachnoid hemorrhage related to trauma or brain tumor were excluded. Patients were eligible if ≥18 years of age.
A subset of cases consented to direct interview and genetic sampling (50% of all cases). This subset was previously reported to be similar to non-interviewed cases with respect to major risk factors such as smoking history and family history of aSAH by chart abstraction.1 If a patient was unable to be interviewed, a proxy was interviewed (99 cases). If the case agreed to be interviewed, two matched controls (age ± 5 years, race, and gender), identified by random digit dialing (50% response rate of potential participants meeting demographic criteria), underwent an identical interview that included smoking history, average pack-years, and family history of brain aneurysm or subarachnoid hemorrhage. As part of the overall GERFHS study, ICH cases also had been interviewed and matched to controls. To maximize power for the current analysis by using three controls for each case, an extra control from the pool of ICH controls, if available, was matched to each aSAH case.
Statistical analysis was done using SAS, version 9.1 (SAS Institute, Cary, NC). A mixed general linear model and conditional logistic regression were used, as appropriate, to compare cases to controls for the continuous and categorical variables. As the study design involved matching, all analyses involved the use of statistical methods for matched data. In order to investigate the effect of family history of a first-degree relative with SAH or IA and current smoking status on aSAH, conditional multiple logistic regression was used. The initial model included a multiplicative interaction term; then further modeling incorporated dummy variables that defined an additive interaction. The reference condition for the additive interaction was no family history and no current smoking. The methodology described by Botto and Khoury7 was used for examination of the multiplicative and additive interaction effects and deviations from these assumptions. We estimated the deviation from the additive model using the interaction contrast ratio (ICR).8 An ICR of 0 denotes an additive interaction, and 0.5 or greater would be considered to be greater than additive. With a lower 95% CI >0.5, we would conclude that the interaction is more than additive.
RESULTS
Between July 1998 and July 2006, we recruited 339 cases of angiogram-confirmed aSAH. All but one case was matched to three controls; the remaining case had two control matches. Thus, there were a total of 1,016 controls. Table 1 presents the demographic data for the cases and controls. Because controls were matched to cases on a case-by-case basis, there were no significant differences in the average age, percent female, or percent black between cases and controls.
Table 1 Subject characteristics
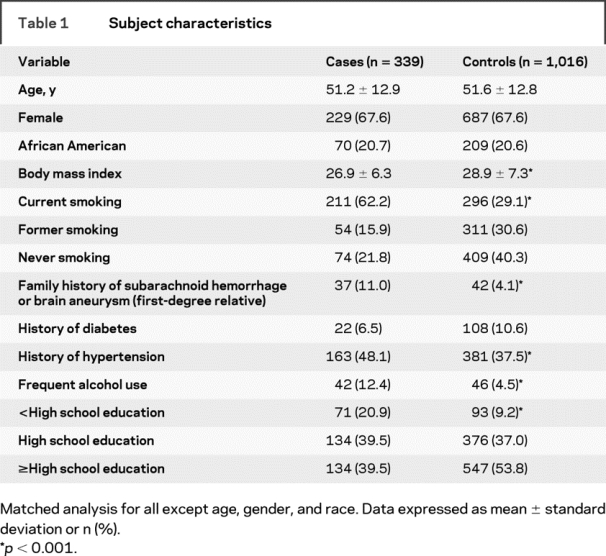
Table 2 presents the results for the analysis of factors representing the family history by smoking additive interaction. Both current smoking (p < 0.0001) and a family history of brain aneurysm/SAH (p = 0.03) were independently associated with aSAH. However, having both aSAH and a family history of SAH was associated with a markedly increased risk of aSAH (odds ratio = 6.4, p < 0.0001) after adjustment for risk factors. These latter comparisons were made using the absence of current smoking and a family history of SAH as the referent group. We examined the models of both multiplicative and additive interactions. A formal test for a multiplicative interaction was not significant (p = 0.80).
Table 2 Conditional logistic regression results for additive interaction model of family history of intracranial aneurysm and smoking history
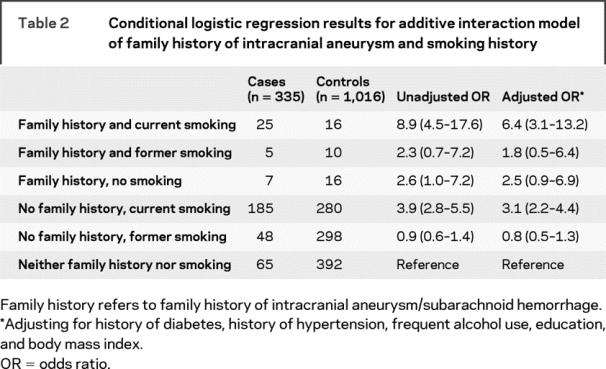
We then examined for deviation from the additive model using the ICR. The ICR was 2.19 with a 95% CI of 0.80 to 5.99. The lower bound of 0.80, being >0.50, suggests departure from the additive model. These models were adjusted for hypertension, diabetes, frequent alcohol use, body mass index, and education level.
DISCUSSION
We report evidence of an interaction between current smoking and the familial aggregation of IA/SAH. We were unable to find a similar association with former smoking and family history of IA/SAH, which suggests that the risk conferred by an interaction may be lowered by quitting smoking. This finding has relevance for both clinical management as well as investigation of the genetic epidemiology of aSAH. Clinicians should assess family history and smoking exposure to quantify and decrease risk to family members of a patient with aSAH. An understanding of the interaction between smoking exposure and family history of aSAH is necessary to enable future genetic research that will allow us to best understand the biology of aneurysm formation and rupture.
Clinically, family members of persons with aSAH should be advised of the markedly increased risk for aSAH with the combination of family history and smoking exposure. While all smokers should be advised to quit smoking, recent evidence suggests that a life-altering event, specifically aSAH, has a significant impact on the behavior of smoking9 and may also be used in counseling family members about the importance of not initiating smoking behavior. Information from the current study may help to increase the potential for quitting prior to life-threatening aSAH, which is fatal in 35–40% of patients.10
Our data also emphasize the importance of screening family members with a family history of aSAH who smoke. Our results are congruent with the recent findings from the Familial Intracranial Aneurysm Study, which reported identifying intracranial aneurysms in 20% of family members without a known history of IA when screened only if they had a history of smoking or hypertension.11 This high rate of detection suggests that the use of risk factors may increase the yield of identifying unruptured intracranial aneurysms in relatives of cases.
Almost all linkage studies for IA reported thus far did not include smoking behavior.12–16 If a gene–environment interaction exists, it is possible that without analyzing this critical trait a potential factor may have been missed. The Familial Intracranial Aneurysm Study recently reported that three of four chromosomal regions with possible linkage to IA appeared to have greater effect in those families with the heaviest smoking.11
A multiplicative interaction (the risk for a disease in those with both risk factors is equal to or greater than the multiplied risk ratios of each risk factor alone) is the most parsimonious determination of interaction. Restriction to accepting only this type of interaction would eliminate the possibility of identifying non-multiplicative interactions (e.g., the observed risk for a disease in those with both risk factors is greater than adding the individual risk ratios of each risk factor separately).17,18 Our findings suggest that we cannot reject a multiplicative interaction (one may still exist). The use of the ICR test is inappropriate for diseases that are not rare (>5%). However, the test is appropriate for this phenotype given the low prevalence (1% prevalence of IA).
One explanation for a non-multiplicative interaction is that the same risk factor does not exist consistently across all families and thus, a purely multiplicative interaction is unlikely to be identified. Intracranial aneurysm is likely a complex polygenic trait, and thus, different genetic factors may have different degrees of interaction with risk factors (smoking and otherwise) that influence the result.
Familial aggregation of a particular phenotype does not necessarily indicate a genetic factor has led to that familial aggregation. Many nongenetic factors such as behavioral or cultural aggregation of risk factors may explain a familial aggregation of a phenotype. However, we have included the major known risk factors for aSAH and have found that the association of a positive family history was independent of their presence, which suggests this would have a limited impact on the finding. A reporting bias may also occur among patients with IA/SAH compared to controls wherein cases are more likely to report a positive family history. However, we would anticipate that the reporting rate would be similar between subjects with and without smoking. Therefore the possibilities are that the finding is spurious (requires confirmation), is associated with some nongenetic familial factor that has not been controlled for, or that provides evidence of a gene–environment interaction.
Address correspondence and reprint requests to Dr. Daniel Woo, Department of Neurology, University of Cincinnati College of Medicine, 260 Stetson Street ML 0525, Cincinnati, OH 45267-0525 Daniel.woo@uc.edu.
Funded by a grant from the NIH (National Institute of Neurological Disorders and Stroke NS36695).
Disclosure: The authors report no disclosures.
Received April 7, 2008. Accepted in final form September 23, 2008.
REFERENCES
- 1.Kissela BM, Sauerbeck L, Woo D, et al. Subarachnoid hemorrhage: a preventable disease with a heritable component. Stroke 2002;33:1321–1326. [DOI] [PubMed] [Google Scholar]
- 2.Broderick JP, Viscoli CM, Brott T, et al. Major risk factors for aneurysmal subarachnoid hemorrhage in the young are modifiable. Stroke 2003;34:1375–1381. [DOI] [PubMed] [Google Scholar]
- 3.Nakagawa T, Hashi K, Kurokawa Y, Yamamura A. Family history of subarachnoid hemorrhage and the incidence of asymptomatic, unruptured cerebral aneurysms. J Neurosurg 1999;91:391–395. [DOI] [PubMed] [Google Scholar]
- 4.Kubota M, Yamaura A, Ono J. Prevalence of risk factors for aneurysmal subarachnoid haemorrhage: results of a Japanese multicentre case control study for stroke. Br J Neurosurg 2001;15:474–478. [DOI] [PubMed] [Google Scholar]
- 5.Woo D, Sauerbeck LR, Kissela BM, et al. Genetic and environmental risk factors for intracerebral hemorrhage: preliminary results of a population-based study. Stroke 2002;33:1190–1195. [DOI] [PubMed] [Google Scholar]
- 6.Woo D, Sekar P, Chakraborty R, et al. Genetic epidemiology of intracerebral hemorrhage. J Stroke Cerebrovasc Dis 2005;14:239–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Botto LD, Khoury MJ. Commentary: facing the challenge of gene-environment interaction: the two-by-four table and beyond. Am J Epidemiol 2001;153:1016–1020. [DOI] [PubMed] [Google Scholar]
- 8.Rothman KJ, Grenland S. Modern Epidemiology. Philadelphia, PA: Lippincott Williams & Wilkins; 1998. [Google Scholar]
- 9.Sauerbeck LR, Khoury JC, Woo D, Kissela BM, Moomaw CJ, Broderick JP. Smoking cessation after stroke: education and its effect on behavior. J Neurosci Nurs 2005;37:316–319, 325. [PubMed] [Google Scholar]
- 10.Hop JW, Rinkel GJ, Algra A, van Gijn J. Case-fatality rates and functional outcome after subarachnoid hemorrhage: a systematic review. Stroke 1997;28:660–664. [DOI] [PubMed] [Google Scholar]
- 11.Foroud T, Koller D, Lai D, et al. Genome-wide SNP linkage screen for intracranial aneurysm susceptibility genes. Stroke 2007;38:455. Abstract.
- 12.Nahed BV, Seker A, Guclu B, et al. Mapping a Mendelian form of intracranial aneurysm to 1p34.3-p36.13. Am J Hum Genet 2005;76:172–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mineharu Y, Inoue K, Inoue S, et al. Model-based linkage analyses confirm chromosome 19q13.3 as a susceptibility locus for intracranial aneurysm. Stroke 2007;38:1174–1178. [DOI] [PubMed] [Google Scholar]
- 14.Ozturk AK, Nahed BV, Bydon M, et al. Molecular genetic analysis of two large kindreds with intracranial aneurysms demonstrates linkage to 11q24-25 and 14q23-31. Stroke 2006;37:1021–1027. [DOI] [PubMed] [Google Scholar]
- 15.Roos YB, Pals G, Struycken PM, et al. Genome-wide linkage in a large Dutch consanguineous family maps a locus for intracranial aneurysms to chromosome 2p13. Stroke 2004;35:2276–2281. [DOI] [PubMed] [Google Scholar]
- 16.Verlaan DJ, Dube MP, St-Onge J, et al. A new locus for autosomal dominant intracranial aneurysm, ANIB4, maps to chromosome 5p152-143. J Med Genet 2006;43:e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khoury MJ, Adams Jr. MJ, Flanders WD. An epidemiologic approach to ecogenetics. Am J Hum Genet 1988;42:89–95. [PMC free article] [PubMed] [Google Scholar]
- 18.Moore JH, Williams SM. Traversing the conceptual divide between biological and statistical epistasis: systems biology and a more modern synthesis. Bioessays 2005;27:637–646. [DOI] [PubMed] [Google Scholar]


