Abstract
The prenatal diet can program an individual’s cardiovascular system towards later higher resting blood pressure (BP) and kidney dysfunction but the extent to which these programmed responses are directly determined by the timing of maternal nutritional manipulation is unknown. In this study we examined whether maternal nutrient restriction targeted over the period of maximal placental growth i.e. 28-80 d gestation resulted in altered BP or kidney development in the juvenile offspring. This was undertaken in 6-month-old sheep born to mothers fed control (100-150% recommended metabolisable energy (ME) intake for that stage of gestation) or nutrient-restricted (NR; 50%; n 6) diets between 28-80 d gestation. Controls were additionally grouped according to normal (C; ≥3, n 7) or low body condition score (LBCS; ≤2, n 6) thereby enabling us to examine the effect of maternal body composition on later cardiovascular function. From day 80 to term (∼147 d) all sheep were fed to 100% ME. Offspring were weaned at 12 weeks and pasture-reared until 6 months of age when cardiovascular function was determined. Both LBCS and NR sheep tended to have lower resting systolic (C, 85 (SEM 2); LBCS, 77 (SEM 3); NR, 77 (SEM 3) mmHg) and diastolic BP relative to controls. Total nephron count was markedly lower in both LBCS and NR relative to controls (LBCS, 59 (SEM 6); NR, 56 (SEM 12) %). Our data suggest maternal body composition around conception is as important as the level of nutrient intake during early pregnancy in programming later cardiovascular health.
Keywords: Programming, Nutrition, Nephron, Blood pressure
Introduction
The developmental origins of adult disease hypothesis (Barker 2001) has stimulated a worldwide research effort, precipitating major advances in our knowledge of fetal and neonatal biology per se, but also potentially accounting for a proportion of the variation in disease status of adult individuals. A role for the nutritional status of the mother as she enters pregnancy and throughout the periods of gestation and lactation is clearly implicated in the programming of later disease risk (Barker et al. 1993). However, the precise interactions between maternal body composition and macronutrient intake during pregnancy, birth weight, postnatal growth rate and postnatal dietary exposure are less well defined in terms of programmable endpoints such as blood pressure. Clearly each has a role, but their relative importance has yet to be described.
Through the use of animal models cardiovascular programming in the offspring has been shown to be associated with either gross maternal nutritional imbalance in terms of macronutrients (Langley & Jackson 1994) and energy (Ozaki et al. 2001; Gardner et al. 2004b; Gopalakrishnan et al. 2004a), as well as with the deficiency of single amino acids such as glycine (Jackson et al. 2002). In large animals such as sheep global caloric restriction targeted over the first 30 or 95 d gestation has limited effect on resting blood pressure per se, but does result in a leftward shift of their baroreceptor curve coupled with a reduced bradycardia during hypertensive challenges (Gardner et al. 2004b; Gopalakrishnan et al. 2004a). Taken together these findings suggest that these individuals may be at an increased risk of developing later hypertension and coronary heart disease as is recognised in humans (Eckberg 1979; Ookuwa et al. 1987). The extent to which cardiovascular function may be reset in earlier life remains to be established but may be potentially significant given the strong influence of placental to fetal weight ratio on later hypertension (Barker et al. 1990). In this regard it has been established that maternal nutrient restriction targeted over the period of maximal placental growth initially restricts placental mass (Clarke et al. 1998) but subsequently results in a disproportionately larger placenta at term (Heasman et al. 1998), adaptations that may be predicted to contribute later cardiovascular dysfunction.
Maternal anthropometrics account for ∼50% variation in intrauterine growth which are mediated in part through the mothers ability to maintain placental growth which is in turn related to maternal body mass and fat stores prior to conception (Robinson et al. 1994). For example, sheep which are light at conception and then go on to lose body condition through gestation produce growth retarded offspring with reduced fat stores (Clarke et al. 1997). These adverse fetal adaptations occur even when maternal food intake is maintained through late gestation. In addition, while a low plane of nutrition throughout gestation results in reduced birth weight; so does a high plane given to young growing sheep (Wallace et al. 1996). Therefore it would appear that regardless of the maternal intake, it is the actual fetal nutritional exposure that is of utmost importance. This may be modulated by the condition of the mother prior to, and food intake during, pregnancy. For this reason the present study has established two control groups, well-fed ewes in normal or low body condition as assessed by palpation of their lumbar region (Russel et al. 1969) in order to establish whether the long term cardiovascular outcomes were comparable to those observed following a targeted period of maternal nutrient restriction between early to mid gestation.
In search of a mechanistic basis for the programming of later cardiovascular dysfunction emphasis has been placed on the kidney as it may underpin a majority of the programmable adaptations within the cardiovascular system. To this extent total nephron number which is determined prior to term in most species, except the litter-bearing rat and pig, is one factor mediating early life programming of cardiovascular dysfunction (Mackenzie & Brenner 1995). Indeed, 48 h exposure to dexamethasone in sheep only programs adult hypertension when given during early differentiation of the metanephros (days 28-30; (Dodic et al. 2002)). A maternal low protein diet programs increased apoptosis of the metanephros in rats (Welham et al. 2002) and hypertension after this dietary regimen tends to be associated with the nephron complement in the offspring rather than glucocorticoid status of the mother (McMullen & Langley-Evans 2004). Thus in the present study we have focussed our attention upon cardiovascular and renal function and the extent to which these control mechanisms may be programmed by either a defined period of maternal nutrient restriction between 28-80 d gestation or a low maternal body weight and condition score at conception. As an index of cardiovascular function we have measured resting blood pressure, heart rate and pressor responses to exogenous infusion of the potent vasopressor hormones angiotensin II and noradrenaline (Gardner et al. 2004b). In addition, during the short-term pressor challenges, we have also assessed baroreflex function through the parallel changes in blood pressure and heart rate. For an index of renal function we have measured nephron number and the relative abundance of cytochrome C and voltage dependent anion channel (VDAC) - as measures of overall renal metabolic activity. The current study also assesses the resting plasma concentration of leptin - as an indicator of body fat mass, together with glucose and cortisol in order to provide indices of resting metabolic and stress status, respectively.
Materials and methods
Animals
All procedures were performed under the UK Animals (Scientific Procedures) Act, 1986. Nineteen mature Welsh Mountain ewes of similar age (2-3 years), parity (2nd - 3rd pregnancy), live weight (42.8 (SEM 0.9) kg) and body condition score (2.3 (SEM 0.1) arbitrary units) were kept on grass at the University of Nottingham’s animal facility at Sutton Bonington for a year prior to mating. At mating a single ram was used for all ewes, which were then allocated to receive either a control (C: n 13; 7 bearing singletons, 6 bearing twins) or nutrient restricted (NR: n 6; all singleton bearing) diet from day 28-80 of gestation (term ∼147 d gestation). Allocation of ewes into control or NR groups was influenced by their body condition score (BCS) - a manual assessment of fat depth in the lumbar region (Russel et al. 1969). Ewes with a BCS of ≤2 (scale of 0, very thin to 5, obese) were allocated to the control diet to comply with Home Office legislation. Thus the ewes fed control diet formed two groups; those with 1) a BCS ≥3 (C, n 7; 3 singleton bearing and 4 twin bearing ewes) and 2) a BCS ≤2 (LBCS, low BCS; n 6, 4 singleton bearing and 2 twin bearing ewes). NR ewes had a BCS of ≥3 units. The period of NR was specifically chosen to target the duration of maximal placental growth and has previously been shown to programme many aspects of feto-placenta;, neonatal and adult development/function (Heasman et al. 1998; Whorwood et al. 2001; Bispham et al. 2003; Gopalakrishnan et al. 2004a). From 28-80 d gestation ewes were singly housed under the prevailing day length conditions with unlimited access to water and controls fed to appetite; equating to 150% metabolisable energy (ME) requirements for live weight maintenance as well as meeting the additional requirements for growth of the conceptus (10-12 MJ.d-1) as defined by the Agricultural and Food Research Council (Agricultural and Food Research Council 1993), while NR sheep were fed to 50% estimated requirement (4-5 MJ.d-1). After 80 d, all ewes received diet calculated to meet 100% AFRC requirements (8-10 MJ.d-1). The diet comprised chopped hay with an ME content of 7.91 MJ.kg dry matter-1 and crude protein content (nitrogen × 6.25) of 69 g/kg dry matter and a barley-based concentrate that had an ME content of 11.6 MJ.kg dry matter-1 and a crude protein content of 162 g.kg dry matter-1 (Mostyn et al. 2003). The proportion of hay to concentrate fed was approximately 3:1 with respect to dry weight. All diets contained adequate minerals and vitamins.
For all ewes the level of feed offered during gestation was based upon fetal number i.e. those bearing twins received a higher allowance, and the changing demands associated with increasing conceptus weight as gestation progresses (Agricultural Research Council 1980). All ewes were weighed and body condition scored at 14-day intervals. At term, lambs (males/females: C, 2/5; LBCS, 3/3; NR, 2/4) were delivered naturally with no intervention and birth weights recorded. All offspring were ewe reared as singletons (one twin lamb from any twin litter was randomly selected for humane euthanasia at term) until weaning at 10 weeks of age and thereafter grass-fed at Sutton Bonington until 6 months of age. Not all data were available for all animals in each group, and therefore a corresponding n has been ascribed to each data set.
Experimental protocols
Surgery
At 6 months of age and at approximately one week prior to surgery all sheep were group housed indoors. For 24 h prior to surgery all food, but not water, was withdrawn from the animals. Anaesthesia was induced with sodium thiopentone (20 mg.kg-1 I.V. Intraval Sodium; Rhone Mérieux, Dublin, Ireland) and maintained with 1-2% halothane in 50:50 O2/N2O. Left carotid and jugular catheters (Fecalon universal polyvinyl tubing; 1.2mm ID 1.8mm OD) were inserted into each sheep, secured and the neck incision closed. Catheters emerging from the neck were coiled and protected within a 10” bandage. All sheep received a dose of long-acting antibiotic (15 mg.kg-1 I.M. amoxycillin, ‘Duphamox’; Fort Dodge Animal Health Ltd, Southampton, UK) and analgesia (1 mg.kg-1 flunixin meglumine; ‘Finadyne’; Shering-Plough, Kenilworth, UK) post-operatively. Catheter patency was maintained by daily flushing with heparinized saline (50 I.U. heparin.ml-1). Catheterised sheep were housed individually, but within sight and touch of other sheep in a highly-ventilated air-conditioned building with controlled lighting (12 h on 12 h off; 8.00 - 20.00 h). Sheep were feeding 1 hr after surgery and showed no visible signs of discomfort for the duration of the experimental period. A period of 2-3 d post-operative recovery was allowed prior to any experiment being performed and the investigator was blinded to the dietary origin of the sheep.
In vivo experiments
In total 4 experiments were performed over a 5-7 day period, the order of which was randomised. All experiments were begun between 9.00-10.00 h and, at the end of all experiments, the animals were humanely euthanased with a lethal overdose of I.V. sodium pentobarbitone (170mg.kg-1; “Dolethal”, Vétoquinol, Bicester, UK). For cardiovascular recording the carotid catheter was connected to a pre-calibrated pressure transducer (SensorNor 840; S 4925), attached at heart level, and linked to a data acquisition system (Po-Ne-Mah; Version 3, Gould Instrument Systems Inc). Hay and water was available at all times. Analogue signals for real-time systolic, diastolic, mean arterial pressure and heart rate were recorded sec-by-sec for a one-hour baseline period and subsequently during the experimental challenge. All data were immediately digitized and downloaded to an Excel spreadsheet for further analysis. From these data, pulse pressure (systolic-diastolic) was derived. For experiments 1-3, a 2 ml blood sample was taken before (30 min) and then immediately after (5 min) infusion of the highest dose of pressor agent.
Experiment 1: Cardiovascular response to angiotensin II infusion
After a baseline period of 10 min, step-wise I.V. increases in angiotensin II (0, 1, 2, 4, 8, 16 & 32 ng.kg-1.min-1) were administered every 10 min, followed by a 30 min recovery period in which cardiovascular variables returned to baseline.
Experiment 2: Cardiovascular response to noradrenaline infusion
After a baseline period of 10 min, step-wise I.V. increases in noradrenaline (0, 2, 4, 8, 16, 32 & 48 ng.kg-1.min-1) were administered every 10 min, followed by a 10 min recovery period in which cardiovascular variables returned to baseline.
Experiment 3: Cardiovascular responses to captopril infusion
After a baseline period of 30 min captopril was infused for 30 min at a dose of 0.12 mg.kg-1.hr-1. This dose has been previously validated to be the lowest effective dose to completely block the pressor effect of 0.5 μg angiotensin I (Smith et al. 1997). After infusion, cardiovascular variables were recorded for a further 20 min recovery period or until blood pressure had returned to baseline.
Experiment 4: Basal endocrine status
Blood samples (2 ml) were taken every 30 min for a total period of 6 hrs. The blood was drawn into heparinised (lithium heparin) syringes, placed in chilled blood tubes and centrifuged at 3500 rpm (800 g), 4° for 5 min and the resultant plasma stored at -20° for later analysis of glucose, cortisol and leptin concentration.
Hormone analysis
Plasma concentrations of glucose were measured enzymatically (Trinder; glucose oxidase) as described by Symonds et al. (1986). Plasma concentrations of leptin were assayed using a double antibody RIA, validated for use with ovine plasma as previously described in detail (Delavaud et al. 2000). Samples were assayed in duplicate (200 μl) using a rabbit anti-ovine leptin primary antibody, iodinated ovine leptin and sheep antirabbit secondary antibody. The leptin assay has a sensitivity of 0.10 ng.ml-1 with intra- and interassay coefficients of variation of 4 and 11% (n 5), respectively. Total cortisol was measured using a commercially available coated-tube RIA kit (Coat-a-Count cortisol, Diagnostic Products Corp, Ltd, Caernarfon, UK) validated for use with ovine plasma (Bispham et al. 2003). The minimum detection limit for the assay was 0.5 ng.ml-1 and the intra- and interassay (n 5) coefficients of variation were 6 and 9%, respectively.
Nephron counts
Determination of the total renal nephron complement was conducted in all C (n 7) and LBCS (n 6) and NR (n 6) sheep using an adaptation of a mild acid-hydrolysis method (Welham et al. 2002) as recently described for use in sheep (Brennan et al. 2005). In brief, whole frozen kidneys were horizontally cut through the hilum and from one section two 1g sliced sections of renal tissue were derived. These sections were covered in 1M hydrochloric acid and incubated for 30 minutes at 37°C. Acid was then removed and replaced with a known volume (20ml) of 50 mM phosphate buffered saline (PBS; pH 7.4). The tissue was homogenised using a bench top homogeniser (Yellowline disperser; IKA Works Inc, USA) and a 20 μl sample subsequently taken and placed on a slide and overlaid with a coverslip. Using an x10 objective lens, the number of glomeruli in the aliquot was counted in triplicate for each of the 2 kidney sections. The six results were averaged and used to determine the total number of glomeruli in the sample and therefore the whole kidney. The intra- and inter-assay variations were 11% and 16%, respectively.
This method for analysis of nephron number in large adult kidneys was validated to give a representative value for whole kidney nephron number as follows: in a whole kidney from 10 sheep, 2 × 1g hilar sections were removed as above. The remaining kidney tissue (18-31 g, comprising 94-97 % of the total organ weight) was acid digested as described above for 30 mins and then homogenised in 200ml PBS, using a Waring blender. Glomeruli and thus nephrons were then counted in quadruplicate 20 μl aliquots from each preparation. There was very close agreement in the estimated total nephron number between these two methods that were strongly correlated i.e. R = 0.967, (P<0.001) indicating there is 93.5% agreement between the two methods. Values are appropriate for the species (Wintour et al. 2003) indicating that in large adult sheep kidneys a representative 1g portion from the hilar region appears to be a valid approach to determining nephron number. This procedure has the further benefit of preserving the rest of the organ for additional histological and/or molecular analyses.
Tissue cytochrome C and VDAC abundance
Mitochondria were prepared from frozen renal tissue in C (n 6) and NR (n 6) as described by Symonds et al. (1992). No data were available in LBCS for tissue cytochrome C and VDAC. Abundance of cytochrome C was determined on 10 μg mitochondrial protein using an antibody (‘Santa Cruz’, Santa Cruz, CA, USA) at a dilution of 1 in a 1000. VDAC abundance was determined using an ovine-specific antibody prepared ‘in house’ as previously described by Mostyn et al. (2003) and used at a dilution of 1 in 2000.
11β-hydroxysteroid dehydrogenase type 2 (11ß-HSD2) activity
Renal 11ß-HSD2 enzyme activity was determined by measuring the rate of conversion of cortisol to cortisone according to Yang et al. (1994). In brief, the homogenate was diluted and protein estimated by the Lowry method before measurement of 11ß-HSD2 activity, using NAD as the cofactor. Each assay contained tritiated cortisol (45000 d.p.m., specific activity 58 Ci.mmol-1; Amersham Pharmacia Biotech, Amersham, Bucks, UK), unlabelled cortisol (0·1 μM; Sigma, Poole, Dorset, UK) and NAD (400 μM; Sigma) in 0·4 ml Krebs-Henseleit bu.er (pH 7·4). The mixture of substrate and cofactor was warmed at 37° for 10 min before 100 μl tissue homogenate containing 200 μg protein was added. After incubation for 10 min (reaction rate was linear from 5-40 min), the reaction was stopped by addition of ice-cold ethyl acetate (5 ml) containing 20 μg cold cortisol and 20 μg cold cortisone (Sigma) as internal carriers for the chromatography. After extraction of the steroids, the extracts were dried under an air stream at 37°, redissolved in 120 μl ethanol and spotted onto thin-layer chromatography plates (Silica gel 150Å; Whatman, Clifton, New Jersey, USA). The plates were developed using a mixture of chloroform and methanol (9:1, v/v). The bands containing cortisol and cortisone were visualized under UV light and excised into scintillation vials. Liquid scintillation cocktail (Optiphase II, Hisafe; Wallac Oy, Turku, Finland) was added and the resulting counts corrected for quenching. The conversion of cortisol to cortisone was expressed as the percentage conversion of recovered tritium counts in the cortisone band. Using the specific activity of the cortisol and the percentage conversion to cortisone, the conversion rate of cortisol to cortisone was calculated and expressed as the amount of cortisone (pmol) synthesized per min. The assay was performed in duplicate and included blanks that contained no tissue homogenate to allow correction for non-enzymatic oxidation. The results are expressed as pmol.min-1.mg protein-1.
Statistical analyses
All data are expressed as Means ± S.E.M. unless otherwise stated. Cardiovascular variables (blood pressures, heart rate, rate pressure product) were first grouped into summary measures i.e. before, during and after a pressor challenge (Matthews et al. 1990) and mean values analysed by two-way ANOVA with repeated measures for effects of group e.g. control vs. LBCS and NR, time e.g. prior to, during and after pressor challenges and any interaction between group*time using SPSS 11.5.2 (SPSS Inc; Chicago, IL). Sex of the offspring and fetal number were included as covariates in the analysis. Where indicated post hoc statistics were run with Bonferronis correction. For glucose and hormone data, values were analysed by either two-way repeated measures ANOVA (Experiment 4) or paired t-test (before and after noradrenaline infusion). Total nephron counts and 11ß-HSD2 activity were not normally distributed and were analysed by Mann Whitney U-test. For all statistical comparisons significance was accepted when P<0.05.
Results
Maternal data and lamb characteristics
At the start of the study, despite all ewes sharing similar grazing and nutritional regimes during a one-year acclimatization after arriving at the University of Nottingham’s farm at Sutton Bonington, a subgroup of ewes did not gain weight or body condition score (group, LBCS). Consequently, these ewes were significantly (P<0.05) lighter and in poorer condition at conception when compared to their contemporaneous controls and NR group (Table 1), despite being indistinguishable in all other respects e.g. daily behaviour, appetite and food intake throughout pregnancy and conception.
Table 1.
Maternal body weight and condition score (BCS) at start of study and at conception (1 year later) plus energy intake during gestation (Values are means with their standard errors)
| At start of study | At conception (1 year later) | Energy Intake (MJ.d-1) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Weight (kg) | BCS (units) | Weight (kg) | BCS (units) | Early-Mid (28-80) | Late (81-140) | |||||||
| Mean | SE | Mean | SE | Mean | SE | Mean | SE | Mean | SE | Mean | SE | |
| Control (n 7) |
42.0 | 0.4 | 2.3 | 2.3 | 48.5 | 1.5 | 3.0 | 0.2 | 12.2 | 0.6 | 10.2 | 0.6 |
| LBCS (n 6) |
42.7 | 2.0 | 2.3 | 2.3 | 42.5 | 1.2* | 1.5 | 0.2* | 10.4 | 0.9 | 9.7 | 1.0 |
| NR (n 6) |
43.8 | 2.3 | 2.2 | 2.2 | 49.3 | 1.3 | 3.5 | 0.2 | 4.6 | 0.2*** | 8.4 | 0.4 |
LBCS, low body condition score; NR, nutrient restricted
Statistically significant differences between groups are indicated by P<0.05, Control vs. LBCS and NR
Statistically significant differences between groups are indicated by P<0.001 NR vs. Control and LBCS
The energy intake of ewes in all groups was similar, except during the period of feed restriction NR ate significantly less metabolisable energy (Table 1). During early-mid gestation all ewes gained weight when measured at birth (minus the products of conception), although the magnitude of gain was greater in adequately fed relative to nutrient restricted sheep (LCBS, an increase of 8.83 (SEM 2.22); C, 4.85 (SEM 0.80); NR, 1.83 (SEM 1.13) kg; P=0.04, NR vs. C; P=0.01, NR vs. LBCS). The reduction in BCS usually observed over pregnancy (reflecting fat mobilization to sustain fetal growth) was apparent in C and NR ewes (C, from 3.0 (SEM 0.2) to 1.5 (SEM 0.2); NR, 3.5 (SEM 0.2) to 1.5 (SEM 0.1) units; P=0.005 both cases, paired t-test) but remained unchanged from 1.5 (SEM 0.2) units in LCBS, despite being fed well above (28-80 d) and to (day 80-term) requirements during their pregnancy. Lamb birth weight was similar in adequately fed control ewes (C, 3.48 (SEM 0.32) and LBCS, 3.76 (SEM 0.78) kg, respectively) and was similar between singletons and twins within these groups, but was increased in NR relative to controls (4.68 (SEM 0.14) kg). Over the first two 3-month periods after birth, the rates of growth were similar in all groups of sheep (0-3 months: C, 265 (SEM 13); LBCS, 240 (SEM 20); NR, 257 (SEM 21) g.d-1; 3-6months: C, 65 (SEM 13); LBCS, 41 (SEM 8); NR, 36 (SEM 5) g.d-1). Growth in NR tended (P=0.06) to be slower from 3-6 months as compared to C. However, at 6 months of age, there was no difference in body weight between the three groups of sheep (C, 33.5 (SEM 1.2) LBCS, 29.4 (SEM 0.1) NR, 31.4 (SEM 1.8) kg).
Cardiophysiology of offspring
Basal status
Resting blood pressure was similar in LBCS and NR, but both groups exhibited a trend toward lower (P=0.057) pressures relative to C (Table 2). Values for heart rate were similar between all groups.
Table 2.
Resting cardiovascular status of 6-month-old offspring (Values are means with their standard errors for the average value obtained from second by second recorded data over a 1 h period measured on 3 separate d)
| Controls (n 7) | LBCS (n 6) | NR (n 6) | ||||
|---|---|---|---|---|---|---|
| Mean | SE | Mean | SE | Mean | SE | |
| Systolic blood pressure (mmHg) | 85 | 2 | 77 | 3 | 77 | 3 |
| Diastolic blood pressure (mmHg) | 63 | 2 | 59 | 2 | 56 | 3 |
| Pulse pressure (mmHg) | 23 | 3 | 18 | 2 | 21 | 3 |
| Mean blood pressure (mmHg) | 74 | 4 | 67 | 2 | 66 | 3 |
| Heart rate (bpm) | 96 | 3 | 101 | 7 | 93 | 9 |
LBCS, low body condition score; NR, nutrient restricted
Pulse pressure was calculated as systolic minus diastolic pressure (mmHg)
Pressor responses to angiotensin II infusion
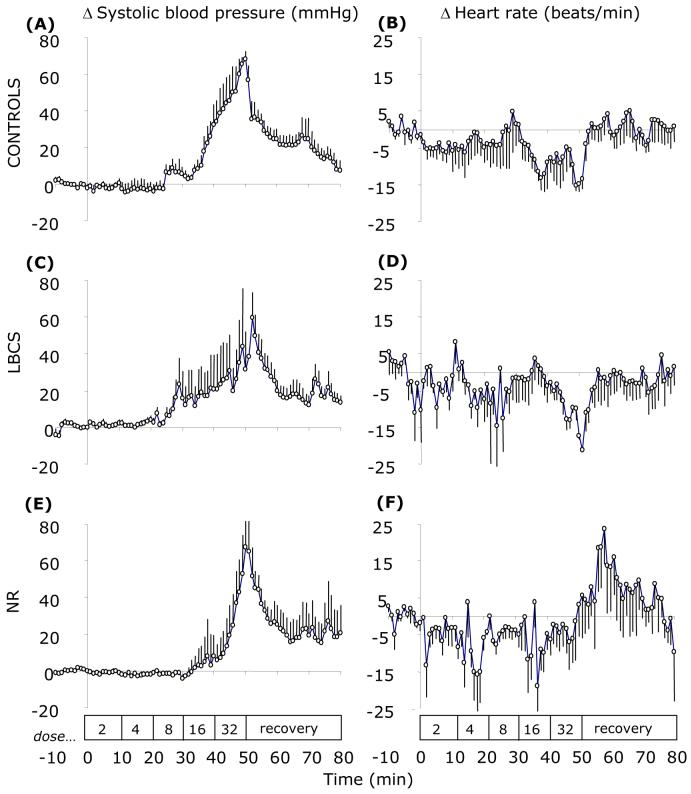
In all groups of sheep, angiotensin II infusion resulted in a dose-dependent increase in systolic blood pressure; the magnitudes of which were similar between groups (Figure 1). At the peak increase in arterial blood pressure (∼50 min), heart rate had decreased by −15 (SEM 5), −17 (SEM 3) beats.min-1 in C and LBCS but remained unaltered in NR (+3 (SEM 8) beats.min-1).
Figure 1.
Mean arterial blood pressure and heart rate response to incremental step-wise infusion of angiotensin II in control (A & B), low body condition score (C & D) and nutrient restricted (E & F) sheep. Values are 5-min means with their standard errors for control (C; n 5), low body condition score controls (LBCS; n 6) and nutrient restricted (NR; n 4) sheep for a baseline period (10 min) and 50 min of angiotensin II infusion (stepwise dose increments of 2-32 ng.kg-1.min-1 every 10 min) followed by 30 min of recovery. Box indicates the period of infusion.
Pressor responses to noradrenaline infusion
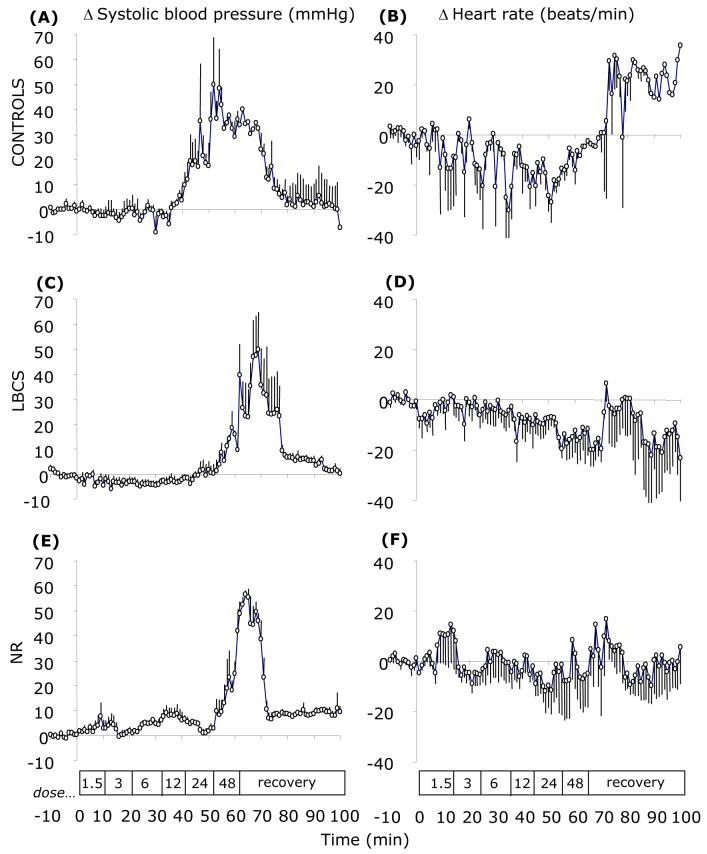
In all groups of sheep, noradrenaline infusion resulted in a dose-dependent increase in systolic blood pressure; the magnitudes of which were similar between groups (Figure 2). At the peak increase in arterial blood pressure (∼65 min), heart rate had decreased by −16 (SEM 8), −15 (SEM 7) and −6 (SEM 11) beats.min-1 in C, LBCS and NR groups, respectively.
Figure 2.
Mean delta arterial blood pressure and heart rate responses to incremental step-wise infusion of noradrenaline in control (A & B), low body condition score (C & D) and nutrient restricted (E & F) sheep. Values are 5-min means with their standard errors for control (C; n 5), low body condition score controls (LBCS; n 6) and nutrient restricted (NR; n 4) sheep for a baseline period (10 min) and 1 h of noradrenaline infusion (stepwise dose increments of 1.5-48 ng.kg-1.min-1 every 10 min) followed by 40 min of recovery. Box indicates the period of infusion.
Pressor responses to captopril infusion
In all offspring 30 minutes of captopril infusion resulted in a decrease in systolic and diastolic arterial blood pressure that was similar in all groups e.g. decrease in systolic blood pressure – C, 8.0 (SEM 4.0); NR, 5.0 (SEM 5.5) mmHg and was not accompanied by any change in heart rate which remained at baseline values (Table 2).
Basal glucose, cortisol and leptin status
Resting plasma concentrations of glucose did not differ between study days or over the 6-hr study period and therefore an average value was calculated for each individual and group. For all resting humoral data, values for C & LBCS were not different and were therefore combined and compared vs. NR. Resting plasma glucose concentration was lower in NR offspring relative to controls (3.46 (SEM 0.27) vs. 4.20 (SEM 0.21) mmol.L-1; P=0.04, NR vs. C & LBCS combined). In addition, plasma glucose concentration was measured after infusion of the highest dose of noradrenaline in order to assess the sympathetically mediated glycaemic response. Plasma glucose increased in response to noradrenaline infusion in all groups (C & LBCS combined: from 3.78 (SEM 0.26) to 5.31 (SEM 0.34) mmol.L-1; NR, from 3.82 (SEM 0.40) to 6.55 (SEM 1.00) mmol.L-1). Values for cortisol (nmoles.L-1) and leptin (ng.ml-1) were similar in all study groups (C, 15.4 (SEM 2.3) and 3.72 (SEM 0.85); LBCS, 16.0 (SEM 1.8) and 4.25 (SEM 1.25); NR, 18.2 (SEM 1.2) and 3.20 (SEM 0.98) for cortisol & leptin, respectively).
Renal histology and molecular biology in offspring at six months of age
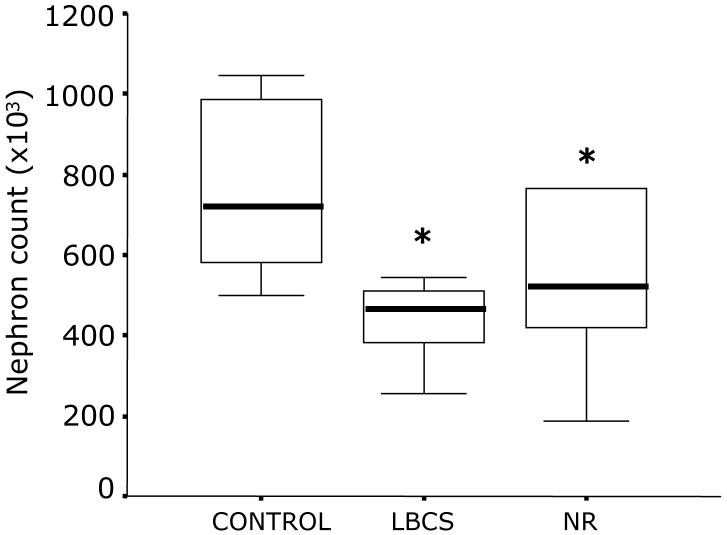
The total nephron count was similar in kidneys from LBCS and NR, but each group had a lower complement of nephrons relative to C sheep (LBCS, 59 (SEM 6); NR, 56 (SEM 12) % relative to C; P=0.01, both cases; Figure 3). The relative abundance of renal cytochrome C was significantly higher in NR relative to C (118 (SEM 14) vs. 86 (SEM 5) arbitrary units; P<0.01). This effect was specific to cytochrome C as there was no difference in relative VDAC abundance between groups (83 (SEM 6) vs. 81 (SEM 6) arbitrary units). In addition, the activity of renal 11ß-HSD2 was similar between groups (C, 1.20 [0.26-1.29]; LBCS, 1.11 [0.31-1.28]; NR, 1.11 [0.86-1.16] pmol.min-1mg protein-1, median with [25th-75th interquartile range]).
Figure 3.
Total kidney nephron number in control and nutrient restricted sheep. Data are represented as a box and whisker plot showing median (bold line within box), 25th and 75th interquartile ranges (bottom and top of box, respectively) and min and max value (lower and upper whiskers, respectively) for control (C; n 7), low body condition score controls (LBCS; n 6) and nutrient restricted (NR; n 6) sheep. Statistical difference between groups are indicated by: *P<0.01, C vs. LBCS and NR.
Sheep biometry at six months of age
When expressed relative to body weight, the spleen was significantly (P<0.01) lighter in NR and perirenal fat significantly (P=0.04) reduced in LBCS (Table 3), relative to all other groups. Weights for all other organs were similar between all groups.
Table 3.
Body composition of 6-month-old offspring (Values are means with their standard errors)
| Controls (n 7) | LBCS (n 6) | NR (n 6) | ||||
|---|---|---|---|---|---|---|
| Mean | SE | Mean | SE | Mean | SE | |
| Body weight (kg) | 33.5 | 1.2 | 29.4 | 2.6 | 31.4 | 1.8 |
| Organ Weights (g.kg-1) | ||||||
| Kidney | 2.99 | 0.16 | 2.86 | 0.21 | 2.91 | 0.12 |
| Liver | 15.3 | 0.5 | 16.3 | 0.9 | 15.1 | 0.45 |
| Spleen | 4.10 | 0.40 | 3.00 | 0.30 | 2.40 | 0.30* |
| Heart | 4.54 | 0.26 | 4.46 | 0.29 | 4.57 | 0.17 |
| Brain | 2.35 | 0.12 | 2.71 | 0.28 | 2.58 | 0.22 |
| Lungs | 9.57 | 1.10 | 10.01 | 0.74 | 9.32 | 0.99 |
| Fat mass (g.kg-1) | ||||||
| Perirenal | 7.84 | 1.01 | 5.02 | 0.68* | 8.34 | 1.83 |
| Omental | 11.83 | 2.08 | 11.99 | 3.10 | 11.81 | 4.15 |
| g total fat.kg body weight-1 | 21.1 | 2.6 | 19.0 | 3.2 | 21.6 | 6.1 |
LBCS, low body condition score; NR, nutrient restricted
Statistically significant differences between groups are indicated by P<0.05 - LBCS and/or NR vs. Controls
Discussion
We have shown that resting blood pressure in offspring born to mothers nutrient restricted between 28-80 d gestation is not raised but tends to be lower compared with offspring born to contemporaneous controls. These findings are therefore in contrast to previous studies that have generally used the rat as an animal model of the developmental programming of adult hypertension, together with tail-cuff plethysmography, in which the expression of the hypertensive state in previously nutrient-restricted offspring often, but not always (Crowe et al. 1995; Gambling et al. 2003), appears demonstrable from a very early age (Langley-Evans 2001). Indeed, initially lower then later higher blood pressure have been noted in rodent models of iron restriction in pregnancy (Crowe et al., 1995; Gambling et al., 2003). Whilst one study in a large animal species (sheep) also demonstrated lower fetal blood pressures after early nutrient restriction, but later higher blood pressure as young lambs (Hawkins et al. 2000). Our data is in accord with these results and extend findings from our previous studies conducted in offspring at later ages which have now shown that the offspring of NR sheep tend to have lower resting arterial pressure as juveniles (current study), the same blood pressure as controls by one year of age (Gardner et al. 2004b), and a higher pressure at three years of age (Gopalakrishnan et al. 2004a). It is acknowledged that breed of sheep is different between these studies but we have found no evidence that this has a significant effect on cardiovascular function (D.S. Gardner and M.E. Symonds unpublished results).
The results from the present study potentially contrast with those recently published in juvenile offspring that were exposed to an identical period of maternal nutrient restriction in which raised mean arterial blood pressure was reported (Gilbert et al. 2005). In this other report, however, the interpretation of the effects of nutrient restriction are potentially confounded by the large number of twins in the control compared with NR group. Indeed comparison of mean arterial blood pressure between twin and singleton offspring in that study indicates as large a difference of fetal number (Twins 76 (SEM 3); Singletons 83 (SEM 5) mmHg (P<0.05)) as of that assigned to maternal diet (Control 73 (SEM 2); NR 89 (SEM 7) mmHg). A potential confounding influence of fetal number is present in the current study in which there are a proportion of twins in the nutritional control groups but not in the NR group. Comparison of mean systolic (and diastolic) blood pressure between twin and singleton offspring in our study reveals very similar blood pressure with respect to fetal number (e.g. C Twins 84 (SEM 3);C Singletons 87 (SEM 3) mmHg). One explanation for the substantial difference in the effect of fetal number between these two studies may relate to the experimental protocols, in that the study of Gilbert et al. (2005) did not take into account the additional ME requirements of twin compared with singleton pregnancies. In addition, in our study each mother was only allowed to raise a single offspring whereas in the study of Gilbert et al. (2005) each mother reared both twins.
A number of publications have shown that the prenatal endocrinological development of twins is different to singletons, primarily reflecting a specific adaptation to their intrauterine environment (Schwartz & Rose 1998; Edwards & McMillen 2002; Gardner et al. 2004a). However, both in prenatal and adult life the available evidence would suggest no overt differences in cardiophysiology between twin and singletons when the mother is fed according to fetal number and twins then reared as singletons (Gardner et al. 2004b). Indeed in this regard our findings are in accord with those of Gilbert et al. (2005) in which there was no effect of litter size on total nephron count but this was specifically reduced in NR offspring by a similar magnitude as we report here. Taken together these findings raise the question as to what age an impairment in nephron number may act to contribute to cardiovascular disease. Interestingly we found no effect of the maternal environment on 11ß-HSD2 activity in the kidney indicating the reduction that is seen at birth following maternal nutrient restriction between 28-80 days gestation is only transient (Whorwood et al. 2001).
The potential divergence between programmed cardiovascular and kidney outcomes that are dependent on the in utero environment is further high-lighted by our findings in offspring born to LCBS mothers. These LBCS mothers and their metabolic response to overfeeding may reflect the overnourished adolescent ewe model of Wallace (2000) in which ewes experience competing metabolic demands for the high nutrient intake from the mother in order to support her own growth and that of her developing conceptus. Ultimately this acts to the detriment of the placenta and thus fetus, which is markedly growth retarded as a result at term. In the present study LBCS sheep put on an extra 4 kg of body weight compared to controls of good body condition at the same time as maintaining a LBCS and producing normal sized offspring. These adaptations suggest that LBCS mothers are protecting/establishing their body reserves during gestation in preparation for the greater demands of lactation. In this respect they have succeeded, as postnatal growth was similar between all nutritional groups. However, the juvenile offspring have a trend for lower blood pressure and reduced nephron number, in similarity to NR offspring. In addition their fat deposition was specifically reduced in the perirenal but not omental depots suggesting that nutrient partitioning within these offspring was different. However, as total fat mass was unaffected it was not unexpected that plasma leptin was similar between groups. Overall this raises an important question - are the fetuses from both groups effectively undernourished during early gestation? This seems difficult to reconcile in LBCS ewes that were eating 150% ME requirements up to 80 days gestation. In overnourished adolescent ewes placental glucose transfer is proportionate between experimental and control groups (Wallace et al. 2003). However, perhaps the disturbance to endogenous metabolic cycles and endocrine milieu concerned with assimilating gross energy intake is similar. Preliminary data from our laboratory indicates that a high-energy intake during early gestation is counterproductive to fetal growth in the sheep (D.S. Gardner and M.E. Symonds - unpublished results), and would itself place specific demands on the mother in order to handle the excess energy.
To date, when considering all of our own studies on young/adult NR offspring, we find two defining characteristics in all NR sheep; 1) an altered pressure-heart rate relationship i.e. reduction in the bradycardia observed during elevations in pressure and 2) a reduced nephron complement (current study and at three years of age: C; 998 [807-1088] vs. NR; 350 [271-372] × 103 nephrons/kidney; median with interquartile ranges (Gopalakrishnan et al. 2004b). The former observation is not apparent in LBCS but the latter is. Without allowing a LBCS group to grow to maturity we cannot say conclusively whether one of these observations, or an interaction between the two, is a prerequisite for development of hypertension after maternal nutrient restriction. The models established within the current study now provide a framework under which to examine these potential mechanisms. A further hypothesis is that the initial stimulus for later cardiovascular programming is altered apoptosis, particularly in the kidney. The widely reported association of programmed hypertension with a deficit in nephron number in rats (Langley-Evans et al. 1999; Woods et al. 2001) and sheep (Wintour et al. 2003) is pre-empted by increased apoptosis of mesenchymal metanephroi (in rats at least), therefore reducing the adult mature nephron complement (Welham et al. 2002), and resulting in glomerular hypertrophy. Consequently, renal functional reserve is more limited in NR. Increased cytochrome C abundance in the kidney of NR, which is perhaps indicative of elevated renal metabolic work, would support this contention.
In conclusion, nutrient restriction between early to mid gestation does not increase resting blood pressure but reduces nephron number as juveniles, relative to controls. However, ewes that ate to or above estimated requirement for metabolisable energy throughout gestation, but who were in low body condition as they entered pregnancy, similarly produced offspring that have the same resting blood pressure and nephron counts as NR offspring. In some respects, however, there appears to be a divergence in response that may indicate differences in the mechanism of programming: while NR offspring failed to show a depressor effect on heart rate with elevations in blood pressure, juveniles from LBCS ewes did. Thus, maternal body composition around conception appears to be as important as the level of nutrient intake during early pregnancy in programming later cardiovascular health of the offspring.
Acknowledgements
The authors wish to acknowledge the staff of the Joint Animals Breeding Unit for the routine care of the animals used in this study. This work was supported by the British Heart Foundation and The Nutricia Foundation.
References
- Agricultural and Food Research Council . Technical Committee on Responses to Nutrients. CAB International; Wallingford, UK: 1993. pp. 812–815. Report No. 9. [Google Scholar]
- Agricultural Research Council . The Nutritional Requirements of Ruminant Livestock. Commonwealth Agricultural Bureau; Slough, UK: 1980. Requirements for energy; pp. 115–119. [Google Scholar]
- Barker DJP. The malnourished baby and infant. British Medical Bulletin. 2001;60:69–88. doi: 10.1093/bmb/60.1.69. [DOI] [PubMed] [Google Scholar]
- Barker DJP, Bull AR, Osmond C, Simmonds SJ. Fetal and placental size and risk of hypertension in adult life. British Medical Journal. 1990;301:259–262. doi: 10.1136/bmj.301.6746.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker DJP, Gluckman PD, Godfrey KM, Harding JE, Owens JA, Robinson JS. Fetal nutrition and cardiovascular disease in later life. Lancet. 1993;341:938–941. doi: 10.1016/0140-6736(93)91224-a. [DOI] [PubMed] [Google Scholar]
- Bispham J, Gopalakrishnan GS, Dandrea J, Wilson V, Budge H, Keisler DH, Broughton Pipkin F, Stephenson T, Symonds ME. Maternal endocrine adaptation throughout pregnancy to nutritional manipulation: consequences for maternal plasma leptin and cortisol and the programming of fetal adipose tissue development. Endocrinology. 2003;144:3575–3585. doi: 10.1210/en.2003-0320. [DOI] [PubMed] [Google Scholar]
- Brennan KA, Gopalakrishnan GS, Kurlak L, Rhind SM, Brooks AN, Rae MT, Olson DM, Stephenson T, Symonds ME. Impact of maternal undernutrition and fetal number on glucocorticoid, growth hormone and insulin-like growth factor receptor mRNA abundance in the ovine fetal kidney. Reproduction. 2005;129:151–159. doi: 10.1530/rep.1.00229. [DOI] [PubMed] [Google Scholar]
- Clarke L, Heasman L, Juniper DT, Symonds ME. Maternal nutrition in early-mid gestation and placental size in sheep. British Journal of Nutrition. 1998;79:359–364. doi: 10.1079/bjn19980060. [DOI] [PubMed] [Google Scholar]
- Clarke L, Yakubu DP, Symonds ME. Influence of maternal bodyweight on size, conformation and survival of newborn lambs. Reproduction, Fertility and Development. 1997;9:509–514. doi: 10.1071/r97016. [DOI] [PubMed] [Google Scholar]
- Crowe C, Dandekar P, Fox M, Dhingra K, Bennet L, Hanson MA. The effects of anaemia on heart, placenta and body weight, and blood pressure in fetal and neonatal rats. Journal of Physiology. 1995;488:515–519. doi: 10.1113/jphysiol.1995.sp020986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delavaud C, Bocquier F, Chilliard Y, Keisler DH, Gertler A, Kann G. Effect of sheep nutritional status and body fatness on plasma leptin concentration assessed by a specific RIA. Journal of Endocrinology. 2000;165:519–526. doi: 10.1677/joe.0.1650519. [DOI] [PubMed] [Google Scholar]
- Dodic M, Hantzis V, Duncan J, Rees S, Koukoulas I, Johnson K, Wintour EM, Moritz K. Programming effects of short prenatal exposure to cortisol. FASEB Journal. 2002;16:1017–1026. doi: 10.1096/fj.01-1045com. [DOI] [PubMed] [Google Scholar]
- Eckberg DL. Carotid baroreflex function in young men with borderline blood pressure elevation. Circulation. 1979;59:632–636. doi: 10.1161/01.cir.59.4.632. [DOI] [PubMed] [Google Scholar]
- Edwards LJ, McMillen IC. Impact of maternal undernutrition during the periconceptional period, fetal number, and fetal sex on the development of the hypothalamo-pituitary adrenal axis in sheep during late gestation. Biology of Reproduction. 2002;66:1562–1569. doi: 10.1095/biolreprod66.5.1562. [DOI] [PubMed] [Google Scholar]
- Gambling L, Dunford S, Wallace DI, Zuur G, Solanky N, Srai KS, McArdle HJ. Iron deficiency during pregnancy affects postnatal blood pressure in the rat. Journal of Physiology. 2003;552.2:603–610. doi: 10.1113/jphysiol.2003.051383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner DS, Jamall E, Fletcher AJW, Fowden AL, Giussani DA. Adrenocortical responsiveness is blunted in twin relative to singleton ovine fetuses. Journal of Physiology, London. 2004a;557:1021–1032. doi: 10.1113/jphysiol.2004.061796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner DS, Pearce S, Dandrea J, Walker RM, Ramsey MM, Stephenson T, Symonds ME. Peri-implantation undernutrition programs blunted angiotensin II evoked baroreflex responses in young adult sheep. Hypertension. 2004b;43:1–7. doi: 10.1161/01.HYP.0000126991.67203.7b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert JS, Lang AL, Grant AR, Nijland MJ. Maternal nutrient restriction in sheep: hypertension, decreased nephron number in offspring at 9 months of age. Journal of Physiology. 2005;565.1:137–148. doi: 10.1113/jphysiol.2005.084202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopalakrishnan G, Gardner DS, Rhind SM, Rae MT, Kyle CE, Brooks AN, Walker RM, Ramsay MM, Keisler DH, Stephenson T, Symonds ME. Programming of adult cardiovascular function after early maternal undernutrition in sheep. American Journal of Physiology. 2004a;287:R12–20. doi: 10.1152/ajpregu.00687.2003. [DOI] [PubMed] [Google Scholar]
- Gopalakrishnan GS, Gardner DS, Kurlak L, Langley-Evans SC, Rhind SM, Rae MT, Kyle CE, Stephenson T, Symonds ME, Budge H. Programming of nephron number in adult sheep by maternal nutrient restriction in early gestation. Proceedings of the Physiological Society. 2004b;560P:C18. [Google Scholar]
- Hawkins P, Steyn C, McGarrigle HG, Calder NA, Saito T, Stratford LL, Noakes DE, Hanson MA. Cardiovascular and hypothalamic-pituitary-adrenal axis development in late gestation fetal sheep and young lambs following modest maternal nutrient restriction in early gestation. Reproduction, Fertility and Development. 2000;12:443–456. doi: 10.1071/rd99071. [DOI] [PubMed] [Google Scholar]
- Heasman L, Clarke L, Firth K, Stephenson T, Symonds ME. Influence of restricted maternal nutrition in early to mid gestation on placental and fetal development at term in sheep. Pediatric Research. 1998;44:546–551. doi: 10.1203/00006450-199810000-00013. [DOI] [PubMed] [Google Scholar]
- Jackson AA, Dunn RL, Marchand MC, Langley-Evans SC. Increased systolic blood pressure in rats induced by a maternal low-protein diet is reversed by dietary supplementation with glycine. Clinical Science. 2002;103:633–639. doi: 10.1042/cs1030633. [DOI] [PubMed] [Google Scholar]
- Langley SC, Jackson AA. Increased systolic blood pressure in adult rats induced by fetal exposure to maternal low protein diet. Clinical Science. 1994;86:217–222. doi: 10.1042/cs0860217. [DOI] [PubMed] [Google Scholar]
- Langley-Evans SC. Fetal programming of cardiovascular function through exposure to maternal undernutrition. Proceedings of the Nutrition Society. 2001;60:505–513. doi: 10.1079/pns2001111. [DOI] [PubMed] [Google Scholar]
- Langley-Evans SC, Welham SJM, Jackson AA. Fetal exposure to a maternal low protein diet impairs nephrogenesis and promotes hypertension in the rat. Life Sciences. 1999;64:965–974. doi: 10.1016/s0024-3205(99)00022-3. [DOI] [PubMed] [Google Scholar]
- Mackenzie HS, Brenner BM. Fewer nephrons at birth: a missing link in the etiology of essential hypertension? American Journal of Kidney Disease. 1995;91:98. doi: 10.1016/0272-6386(95)90161-2. [DOI] [PubMed] [Google Scholar]
- Matthews JN, Altman DG, Campbell MJ, Royston P. Analysis of serial measurements in medical research. British Medical Journal. 1990;300:230–235. doi: 10.1136/bmj.300.6719.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMullen S, Langley-Evans SC. Maternal low protein diet in rat pregnancy programmes blood pressure through sex-specific mechanisms. American Journal of Physiology. 2004;288:R85–R90. doi: 10.1152/ajpregu.00435.2004. [DOI] [PubMed] [Google Scholar]
- Mostyn A, Wilson V, Dandrea J, Yakubu DP, Budge H, Alves-Guerra MC, Pecqueur C, Miroux B, Symonds ME, Stephenson T. Ontogeny and nutritional manipulation of mitochondrial protein abundance in adipose tissue and the lungs of postnatal sheep. British Journal of Nutrition. 2003;90:323–328. doi: 10.1079/bjn2003912. [DOI] [PubMed] [Google Scholar]
- Ookuwa H, Takata S, Ogawa J, Iwase N, Ikeda T, Hattori N. Abnormal cardiopulmonary baroreflexes in normotensive young subjects with a family history of essential hypertension. Journal of Clinical Hypertension. 1987;3:596–604. [PubMed] [Google Scholar]
- Ozaki T, Nishina H, Hanson MA, Poston L. Dietary restriction in pregnant rats causes gender-related hypertension and vascular dysfunction in offspring. Journal of Physiology. 2001;530:141–152. doi: 10.1111/j.1469-7793.2001.0141m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson JS, Owens JA, DeBarro T, Lok F, Chidzanja S. Maternal nutrition and fetal growth. In: Ward RHT, Smith SK, Donnai D, editors. Early Fetal Growth and Development. RCOG Press; Lonodon: 1994. pp. 317–329. [Google Scholar]
- Russel AJF, Doney JM, Gunn RG. Subjective assessment of body fat in live sheep. Journal of Agricultural Science, Cambridge. 1969;72:451–454. [Google Scholar]
- Schwartz J, Rose JC. Development of the pituitary adrenal axis in fetal sheep twins. American Journal of Physiology. 1998;274:R1–R8. doi: 10.1152/ajpregu.1998.274.1.R1. [DOI] [PubMed] [Google Scholar]
- Smith FG, Chan S, De Wildt SN. Effects of renal denervation on cardiovascular and renal responses to ACE inhibition in conscious lambs. Journal of Applied Physiology. 1997;83:414–419. doi: 10.1152/jappl.1997.83.2.414. [DOI] [PubMed] [Google Scholar]
- Symonds ME, Bryant MJ, Clarke L, Darby CJ, Lomax MA. Effect of maternal cold exposure on brown adipose tissue and thermogenesis in the neonatal lamb. Journal of Physiology. 1992;455:487–502. doi: 10.1113/jphysiol.1992.sp019313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symonds ME, Bryant MJ, Lomax MA. The effect of shearing on the energy metabolism of the pregnant ewe. British Journal of Nutrition. 1986;56:635–643. doi: 10.1079/bjn19860144. [DOI] [PubMed] [Google Scholar]
- Wallace JM. Nutrient partitioning during pregnancy: adverse gestational outcome in overnourished adolescent dams. Proceedings of the Nutrition Society. 2000;59:107–117. doi: 10.1017/s0029665100000136. [DOI] [PubMed] [Google Scholar]
- Wallace JM, Aitken RP, Cheyne MA. Nutrient partitioning and fetal growth in rapidly growing adolescent ewes. Journal of Reproduction and Fertility. 1996;107:183–190. doi: 10.1530/jrf.0.1070183. [DOI] [PubMed] [Google Scholar]
- Wallace JM, Bourke DA, Aitken RP, Milne JS, Hay WW. Placental glucose transport in growth-restricted pregnancies induced by overnourishing adolescent sheep. Journal of Physiology. 2003;547:85–94. doi: 10.1113/jphysiol.2002.023333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welham SJM, Wade A, Woolf AS. Protein restriction in pregnancy is associated with increased apoptosis of mesenchymal cells at the start of rat metanephrogenesis. Kidney International. 2002;61:1231–1242. doi: 10.1046/j.1523-1755.2002.00264.x. [DOI] [PubMed] [Google Scholar]
- Whorwood CB, Firth KM, Budge H, Symonds ME. Maternal undernutrition during early- to mid-gestation programmes tissue-specific alterations in the expression of the glucocorticoid receptor, 11β-hydroxysteroid dehydrogenase isoforms and type 1 angiotensin II receptor in neonatal sheep. Endocrinology. 2001;142:1778–1785. doi: 10.1210/endo.142.7.8264. [DOI] [PubMed] [Google Scholar]
- Wintour EM, Moritz KM, Johnson K, Ricardo S, Samuel CS, Dodic M. Reduced nephron number in adult sheep, hypertensive as a result of prenatal glucocorticoid treatment. Journal of Physiology. 2003;549:929–935. doi: 10.1113/jphysiol.2003.042408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods LL, Ingelfinger JR, Nyengaard JR, Rasch R. Maternal protein restriction suppresses the newborn renin-angiotensin system and programs adult hypertension in rats. Pediatric Research. 2001;49:460–467. doi: 10.1203/00006450-200104000-00005. [DOI] [PubMed] [Google Scholar]
- Yang K, Berdusco ET, Challis JR. Opposite effects of glucocorticoid on hepatic 11β-hydroxysteroid dehydrogenase mRNA and activity in fetal and adult sheep. Journal of Endocrinology. 1994;143:121–126. doi: 10.1677/joe.0.1430121. [DOI] [PubMed] [Google Scholar]