Abstract
The calcium-activated protein phosphatase calcineurin is controlled by regulator of calcineurin (RCAN) in yeast up through mammals. The physiologic function of RCAN proteins remains an area of ongoing investigation because both positive and negative calcineurin regulatory effects have been reported. Here, we performed a yeast two-hybrid screen with RCAN1 as bait, identifying TAK1 binding protein 2 (TAB2) as an interacting partner. TAB2 directly interacted with RCAN1 in vitro and in vivo, recruiting TAK1, TAB1 and calcineurin, forming a macromolecular signaling complex. Overexpression of TAK1 and TAB1, or active TAK1-ΔN, promoted direct phosphorylation of RCAN1 in vitro and in vivo. TAK1 phosphorylated RCAN1 at two novel sites, serine 94 and 136, switching RCAN1 from an inhibitor to a facilitator of calcineurin-NFAT signaling, enhancing NFATc1 nuclear translocation, NFAT transcriptional activation and the hypertrophic growth of cultured cardiomyocytes. Remarkably, Rcan1/2 or Tab2 deficient mouse embryonic fibroblast (MEF) cultures each failed to show an interaction between the TAK1-TAB1-TAB2 and the calcineurin-NFAT signaling modules. We also observed a reciprocal negative feedback mechanism whereby sustained calcineurin activation inhibited TAK1 signaling through dephosphorylation of TAK1 and TAB1, an effect that was absent in Rcan1/2 deficient MEFs. Functionally, TAK1 was indispensable for the cardiomyocyte growth response induced by prohypertrophic stimuli. TAK1-dependent growth was also blocked by inhibition of calcineurin activity with Cain. Finally, a dominant interfering fragment of RCAN1 that disrupts the TAK1-TAB1-TAB2, calcineurin-NFAT complex also blocked cardiomyocyte hypertrophy to several stimuli. These results describe a novel signaling relationship between two central regulatory pathways whereby TAK1-TAB1-TAB2 selectively induces calcineurin-NFAT signaling through direct phosphorylation of RCAN1, while calcineurin activation diminishes TAK1 signaling by dephosphorylation of TAK1 and TAB1.
Introduction
Calcineurin (protein phosphatase 2B) is a calcium-calmodulin-activated, serine/threonine protein phosphatase that is activated by sustained elevations in intracellular calcium1,2. Once activated, calcineurin directly dephosphorylates nuclear factor of activated T cells (NFAT) transcription factors within the cytoplasm, promoting their translocation into the nucleus and the activation of gene expression2. Calcineurin–NFAT signaling is critically involved in regulating a diverse range of biologic processes including T lymphocyte development and reactivity, development of the nervous and vascular systems, fiber-type switching in skeletal muscle, development of heart valves, development of bone, and the control of cardiac hypertrophy1–3. Although calmodulin bound to Ca2+ is the only known activator of calcineurin, a wide range of regulatory proteins have been described that can alter calcineurin activity. One such protein is known as RCAN (previously known as MCIP/DSCR/calcipressin), which is a member of gene family that includes Rcan1, Rcan2, and Rcan3 4,5. In mammalian cells, overexpression of RCAN1 predominantly blocks NFAT activation by direct binding to the calcineurin active site6. However, in yeast the RCAN1 homologue RCN1/CBP1 have functional and phenotypic characteristics of a calcineurin activator7–9. Consistent with this observation, Rcan1−/− mice showed an impaired cardiac hypertrophic response to pressure overload or chronic adrenergic stimulation, suggesting that it may also function to enhance calcineurin-NFAT signaling in higher organisms10,11. It remains unclear how endogenous RCAN1 either inhibits or facilitates calcineurin activity towards target proteins, although direct phosphorylation of RCAN proteins may serve as a potential mechanism12.
In this study, we identified TAK1-binding protein 2 (TAB2) as an RCAN1-interacting factor in a yeast two-hybrid screen. TAB2 is an adaptor protein linking signals from the transforming growth factor-β (TGF-β) receptor and TRAF proteins to the TGF-β-activated kinase 1 (TAK1) signaling complex13. TAK1 is an upstream member of the mitogen-activated protein kinase (MAPK) superfamily that serves as a pivotal integrator of membrane-bound signals elicited by TGF-β, interleukin-1 (IL-1), IL-18, tumor necrosis factor (TNF), and receptor activator of nuclear factor κB (NF-κB) ligands (RANKL) 14,15. TAK1 forms a complex with TAB2 and TAB1 (TAK1-binding protein 1), where TAB1 functions as an activator of TAK116. The TAK1-TAB1-TAB2 complex directly activates MAPK kinase 3/6 (MKK3/6) and p38, MKK4 and c-Jun N-terminal kinase (JNK), as well as the NF-κB pathway17,18. TAK1 signaling has also been shown to critically regulate a number of important biologic processes, including the cardiac hypertrophic growth response19. The identification of RCAN1 as an interacting partner for the TAK1-TAB1-TAB2 complex suggested a novel interaction between two central regulatory pathways, which here we show serves to control the cardiomyocyte growth response.
Results
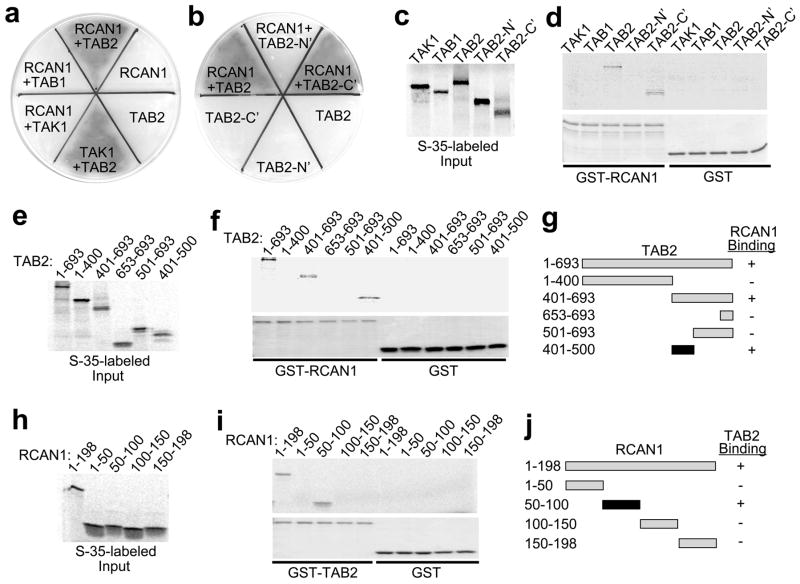
Given the central importance of calcineurin-NFAT signaling in controlling the cardiac growth response we sought to identify additional regulatory components by yeast two-hybrid screening for RCAN1 interacting proteins. An RCAN1 bait plasmid was transformed into yeast along with an adult mouse heart library of prey plasmids. Three independent clones containing a C-terminal (C’) fragment encoding amino acids 402 to 693 of mouse TAB2 were obtained from approximately 3.2 ×106 transformants. Re-transformation of RCAN1 bait with TAB2 prey gave a strong interaction that supported growth on selectable X-α-galactosidase medium in yeast, similar to a positive control consisting of TAK1 bait with a TAB2 prey plasmid (Fig. 1a). No interaction was observed with RCAN1 bait alone, TAB2 prey alone, or RCAN1 bait with either TAB1 or TAK1 prey (Fig. 1a). The interaction with RCAN1 was also observed with just a C-terminal fragment of TAB2, but not with the N-terminus (Fig. 1b). These data suggest that RCAN1 directly interacts with TAB2, but not with other members of the TAK1 complex. To further characterize this interaction, a GST pull-down assay was performed with GST or GST-RCAN1 resin in combination with radio-labeled TAK1, TAB1, TAB2 proteins (Fig. 1c). This assay showed a direct interaction between RCAN1 and full-length TAB2 and the C-terminus of TAB2, but not with GST alone (Fig. 1d). TAB2 fragments were radioactively labeled in a coupled transcription-translation reaction and subjected to pull-down with GST-RCAN1 or GST alone (Fig. 1e,f). GST-RCAN1, but not GST alone, interacted with full-length TAB2, TAB2 401–693, and TAB2 401–500. Equal content of GST-RCAN1 is shown by SDS-PAGE stained with Coomassie brilliant blue (Fig. 1f, lower panel). These data indicate that the TAB2 401–500 fragment is sufficient to bind to RCAN1 (Fig. 1g). In addition, a reciprocal pull-down assay was performed in which RCAN1 deletion proteins were radioactively labeled and subjected to pull-down with GST-TAB2 or GST alone (Fig. 1h,i). Equal content of the GST-TAB2 fusion protein was also shown by SDS-PAGE (Fig. 1i, lower panel). The RCAN1 50–100 fragment is the minimally required region for TAB2 interaction (Fig 1j).
Figure 1.
RCAN1 physically interacts with TAB2. (a,b) Yeast two-hybrid assay showing streak plates on selectable and colorimetric media. The dark gray area designates successful growth through interaction of the designated fusion proteins. (c) TAK1, TAB1 and TAB2 were [35S]methionine-labeled (d) and subjected to a pull-down assay with GST-RCAN1 or GST. A Coomassie brilliant blue-stained gel shows loading of the GST fusion proteins used (lower). (e) TAB2 truncations were [35S]methionine-labeled (f) and subjected to a pull-down assay with GST-RCAN1 or GST. A Coomassie brilliant blue-stained gel shows loading of the GST fusion proteins used (lower). (g) Schematic representation showing the RCAN1 interacting domain in TAB2. (h) RCAN1 truncations were [35S]methionine-labeled and (i) subjected to a pull-down assay with GST-TAB2 or GST. A Coomassie brilliant blue-stained gel shows the integrity of the GST fusion proteins used (lower). (j) Schematic representation showing the TAB2 interacting domain in RCAN1.
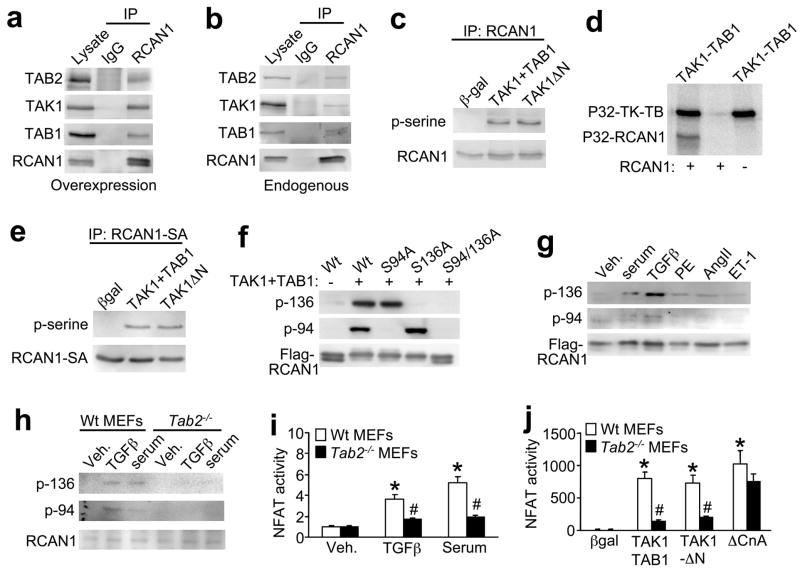
To confirm that the association between RCAN1 and TAB2 occurs in mammalian cells, RCAN1, TAB2, TAK1, and TAB1 were overexpressed in neonatal cardiomyocytes by infection with corresponding recombinant adenoviruses. Cell extracts were immunoprecipitated with an anti-RCAN1 antibody followed by Western blotting with an anti-TAB2, anti-TAK1, or anti-TAB1 antibody. The results show that TAB2 was immunoprecipitated with an RCAN1 antibody but not with a nonspecific IgG antibody (Fig. 2a). Moreover, immunoprecipitation of RCAN1 resulted in the isolation of TAK1 and TAB1, suggesting that RCAN1 associates with the entire TAK1-TAB1-TAB2 complex (Fig. 2a). Finally, it was also of interest to determine if the RCAN1-TAK1-TAB1-TAB2 interaction could be identified without overexpression of each protein, although we needed to overexpress activated calcineurin (ΔCnA) as a way of inducing sufficient RCAN1 expression in cardiomyocytes (calcineurin activation induces greater levels of endogenous RCAN1 expression). Endogenous TAK1, TAB1, and TAB2 each immunoprecipitated with RCAN1 in cardiomyocytes (Fig. 2b).
Figure 2.
TAK1 phosphorylates RCAN1 at serine 94 and 136 in vivo and in vitro. (a) Western blots after immunoprecipitation (IP) with anti-RCAN1 or pre-immune IgG from cultured neonatal cardiomyocytes infected with adenoviruses encoding RCAN1, TAB2, TAK1 and TAB1. (b) Western blots after immunoprecipitation showing that endogenous TAB2, TAB1 and TAK1 form a complex that interacts with RCAN1 in neonatal cardiomyocytes. (c) Western blots following immunoprecipitation (IP) with anti-RCAN1 from cultured neonatal cardiomyocytes infected with the indicated recombinant adenoviruses. (d) Autoradiographic gel showing direct phosphorylation of recombinant-purified RCAN1 when incubated in the presence of recombinant-purified TAK1-TAB1 fusion protein. (e) Western blots after immunoprecipitation of RCAN1-S108/112A from cardiomyocyte extracts co-infected with TAK1 + TAB1 or TAK1ΔN. (f) Western blotting with phospho-specific RCAN1 antibodies from HEK 293 cells transfected with plasmids encoding flag-tagged wild type (Wt) or mutants of RCAN1, in the presence or absence of TAK1 and TAB1 plasmids. (g) Western blot for phospho-RCAN1 at serine 136 or 94 from neonatal cardiomyocytes infected with RCAN1 adenovirus for 24 hours and then treated with serum (1% FBS), TGFβ (5 ng/ml), phenyleprine (PE, 50 μM), angiotensin II (AngII, 100 nM), or Endothelin-1 (ET-1, 1 μM). (h) Western blotting with phospho-specific RCAN1 antibodies or total endogenous RCAN1 from wildtype and Tab2−/− MEFs kept overnight in serum free media then stimulated with serum or TGFβ for 30 minutes. (i) NFAT-luciferase activity (AdNFAT-luc infection) from cultured wildtype (Wt) and Tab2−/− MEFs stimulated with TGFβ or serum as in panel h. Results were averaged from 3 independent experiments. Error bars represent s.e.m. *P < 0.05 versus vehicle (veh); #P < 0.05 versus Wt stimulated. (j) NFAT-luciferase activity (AdNFAT-luc infection) from cultured wildtype and Tab2−/− MEFs infected with the indicated adenoviruses to alter TAK1 or calcineurin signaling. Results were averaged from 3 independent experiments. Error bars represent s.e.m. *P < 0.05 versus βgal; #P < 0.05 versus Wt with TAK1+TAB1 or TAK1-ΔN.
The interaction between RCAN1 and the TAK1-TAB1-TAB2 complex led us to hypothesize that TAK1 might directly regulate RCAN1 through phosphorylation. To address this possibility, cardiomyocytes were infected with adenoviruses encoding RCAN1, TAK1 and its activator TAB1, constitutively active TAK1 (TAK1-ΔN), or β-galactosidase as an internal control (Fig. 2c). RCAN1 was then immunoprecipitated and analyzed by Western blotting with an anti-phosphoserine antibody, showing RCAN1 phosphorylation by TAK1 (Fig 2c). This effect was directly assessed in vitro using a kinase assay with recombinant-purified RCAN1 and a purified active TAK1-TAB1 fusion protein. The data show strong autophosphorylation of TAK1 (upper band), and detectable phosphorylation of RCAN1 in the presence of TAK1-TAB1 (Fig. 2d). As a side note, apoptosis signal-regulating kinase 1 (ASK1), another MAPKKK, did not phosphorylate RCAN1, suggesting specificity for TAK1 (data not shown).
A previous study identified two serine residues in RCAN1, serine 108 and 112 that were phosphorylated by glycogen synthase kinase 3β (GSK-3β) and MAPK, respectively7,20. To determine if these two serines are also phosphorylated by TAK1, cardiomyocytes were infected with adenoviruses encoding TAK1 and its activator TAB1, TAK1-ΔN, or β-galactosidase along with RCAN1-SA in which serine 108 and 112 were mutated to alanine (Fig. 2e). Immunoprecipitation of RCAN1-S108/112A followed by Western blotting with phospho-serine antibody revealed no reduction in phosphorylation, suggesting that TAK1 phosphorylated RCAN1 at other sites (Fig. 2e). An in vitro kinase assay with purified RCAN1-S108/112A also showed phosphorylation by TAK1 (data not shown). Mass spectrometry was performed to identify the putative TAK1 phosphorylation sites in RCAN1, revealing a site at serine 136 and serine 94 (see Methods). Phosphorylation-specific rabbit polyclonal antibodies were then generated to the newly identified sites in RCAN1. To verify specificity, HEK293 cells were transiently transfected with RCAN1-Wt, RCAN1-S94A, RCAN1-S136A, or RCAN1-S94/136A, with or without TAK1 and TAB1, and the cell extracts were subjected to western blotting. The assay showed that both the p-Ser-136 and p-Ser-94 antibodies were entirely specific for their respective phosphorylated sites in RCAN1 (Fig. 2f). To determine if RCAN1 is phosphorylated in vivo at Ser-136 and Ser-94, western blotting was performed from cardiomyocyte extracts after infection with RCAN1 adenovirus and treatment with serum, TGFβ, phenylephrine (PE), angiotensin II (Ang II), or endothelin-1 (ET-1). While each of the agonists produced detectable RCAN1 phosphorylation at both sites, TGFβ gave a more robust effect, especially at serine-136 (Fig. 2g). These results validate the concept that TAK1 can phosphorylate RCAN1 in vivo. However, we also wanted to determine if TAK1-mediated phosphorylation of RCAN1 depended on formation of the greater complex through TAB2. Remarkably, phosphorylation of endogenous RCAN1 at both serine 136 and 94 was blocked in Tab2−/− MEFs compared with wildtype MEFs (Fig. 2h).
As will be presented below, TAK1 activation promotes a remarkable increase in calcineurin-NFAT activity through RCAN1. This feature of TAK1 was used to further probe the necessity of TAB2 in mediating a physiologic response of TAK1 towards calcineurin-NFAT. For example, NFAT-luciferase activity was stimulated by TGFβ, serum, TAK1+TAB1 or TAK1-ΔN in wildtype MEFs, but this activation was dramatically reduced in Tab2−/− MEFs, while activated calcineurin still increased NFAT activity in both cell lines (Fig 2i,j). Similarly, TGFβ induced NFAT-luciferase reporter activity was blocked in Tak1−/− MEFs (see supplementary information, Fig S1a). These results suggest that TAK1 regulates endogenous calcineurin-NFAT signaling by forming a complex through TAB2, permitting phosphorylation of RCAN1 at serine 136 and 94.
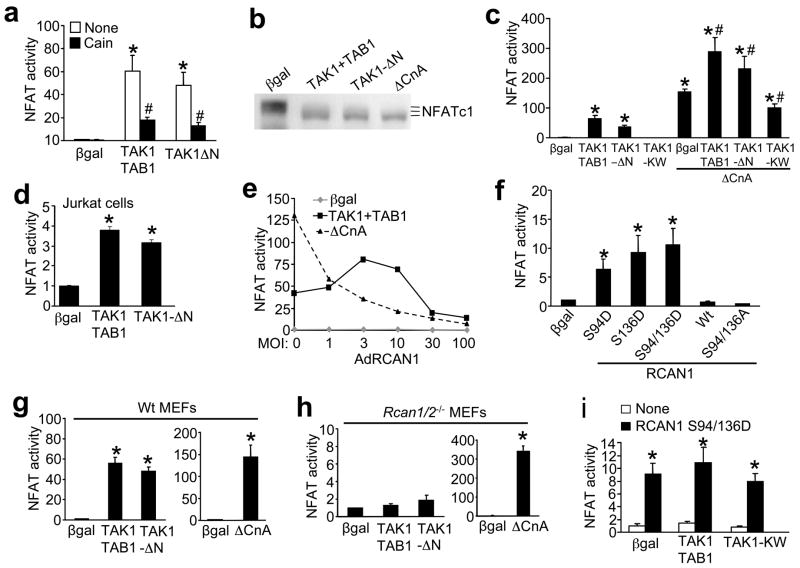
RCAN1 is a complicated regulator of calcineurin-NFAT, where it can activate or inhibit signaling depending on expression levels and/or post-translational modifications. To begin to examine the functional relevance of RCAN1 phosphorylation by TAK1 we examined calcineurin-NFAT activity in cultured neonatal cardiomyocytes in greater detail, as well as the hypertrophic growth response. Cardiomyocytes were infected with adenoviruses encoding an NFAT-luciferase reporter cassette, TAK1 and its activator TAB1, or TAK1-ΔN. TAK1 activated the NFAT-luciferase reporter by >50 fold (P < 0.05) in cardiomyocytes, an effect that was largely reversed by coinfection with AdCain, a calcineurin inhibitor (Fig. 3a). Similar infections were performed in cardiomyocytes along with an adenovirus encoding NFATc1-GFP, followed by western blotting for NFATc1 to monitor phosphorylation differences (Fig. 3b). Similar to activated calcineurin (ΔCnA), TAK1 enhanced NFATc1 dephosphorylation resulting in increased migration on SDS-PAGE. Collectively, these data indicate that TAK1 activates NFAT transcriptional activity through a calcineurin dependent mechanism. Reciprocally, infection with a dominant negative TAK1-KW adenovirus inhibited baseline NFAT-luciferase activity, suggesting that endogenous TAK1 regulates basal calcineurin-NFAT signaling (Fig. 3c). TAK1-TAB1 or TAK1-ΔN augmented, while dominant negative TAK1-KW diminished ΔCnA-induced NFAT-luciferase activity, further supporting the notion that TAK1 acts as a positive regulator of calcineurin-NFAT activity in cardiomyocytes (Fig 3c). Similar results were also observed in Jurkat T cells (Fig. 3d).
Figure 3.
TAK1 induces calcineurin-NFAT signaling in cardiomyocytes. (a) NFAT-luciferase activity from cultured neonatal cardiomyocytes infected with the indicated adenoviruses with or without the calcineurin inhibitor Cain. Results were averaged from 3 independent experiments. Error bars represent s.e.m. *P < 0.001 versus Adβgal; #P < 0.01 versus None. (b) Western blot for NFATc1 from neonatal cardiomyocytes infected with AdNFATc1-GFP and the additional indicated adenoviruses. (c) NFAT-luciferase activity from cardiomyocytes infected with the indicated adenoviruses, some of which were also co-infected with activated calcineurin (ΔCnA). Results were averaged from 3 independent experiments. Error bars represent s.e.m. *P < 0.001 versus Adβgal; #P < 0.01 versus ΔCnA only. (d) NFAT-luciferase activity from Jurkat T cells infected with the indicated adenoviruses. Results were averaged from 3 independent experiments. Error bars represent s.e.m. *P < 0.001 versus Adβgal. (e) NFAT-luciferase activity from cardiomyocytes infected with the indicated adenoviruses and increasing multiplicity of infection (MOI) of AdRCAN1. (f) NFAT-luciferase activity from cardiomyocytes transfected with the indicated RCAN1 Wt or mutant plasmids. Luciferase activity was determined 36 h after transfection. Results were averaged from 3 independent experiments. Error bars represent s.e.m. *P < 0.001 versus βgal. (g) NFAT-luciferase activity from Wildtype (Wt) and (h) Rcan1/2−/− MEFs that were infected with the indicated adenoviruses. Luciferase activity was determined 24 h after infection. Results were averaged from 3 independent experiments. Error bars represent s.e.m. *P < 0.001 versus Adβgal. (i) NFAT-luciferase activity from Rcan1/2−/− MEFs infected with AdNFAT-luc and then transfected with plasmids encoding RCAN1 S94/136D and expression vectors for the other indicated factors. Results were averaged from 3 independent experiments. Error bars represent s.e.m. *P < 0.001 versus βgal.
RCAN1 serves as an inhibitor of calcineurin when over-expressed21,22, consistent with our observations that both baseline and ΔCnA-induced NFAT-luciferase activity are inhibited by RCAN1 overexpression in a dose-dependent manner (Fig. 3e). However, co-infection of AdTAK1 with AdTAB1 produced an entirely different pattern of NFAT activity (Fig. 3e). Indeed, low doses of RCAN1 facilitated calcineurin-NFAT signaling in the presence of TAK1+TAB1 (Fig. 3e), suggesting that TAK1 augments calcineurin-NFAT signaling through RCAN1. To further examine the mechanism of this facilitation by TAK1, we assessed the effects of RCAN1 mutations on NFAT luciferase activity. Mutation of serine 94 or 136 to aspartic acid led to increased NFAT transcriptional activity, while mutation of both sites to alanine inhibited NFAT activity (Fig. 3f). These observations further suggest that phosphorylation at serine 94 and 136 in RCAN1 promotes calcineurin-NFAT activation. More importantly, TAK1 is no longer able to activate calcineurin-NFAT signaling in Rcan1/2−/− MEF cultures, while wildtype MEFs show robust activation of the AdNFAT-luciferase reporter with co-infection of AdTAK1 + AdTAB1 or with AdTAK1-ΔN (Fig 3g, h and see supplementary information, Fig. S1b). However, Rcan1/2−/− MEFs were still highly responsive to NFAT activation by an adenovirus encoding activated calcineurin, even greater than that observed in wildtype MEFs (Fig 3g,h, right graphs). Finally, the known augmentation in NFAT activity by RCAN1 S94/136D overexpression was not enhanced by TAK1 in Rcan1/2−/− MEFs, indicating that TAK1 specifically regulates calcineurin-NFAT signaling only through these two amino acids in RCAN1 (Fig. 3i). Collectively, these results indicate that TAK1-induced NFAT activation occurs entirely through its interaction with, and phosphorylation of RCAN1.
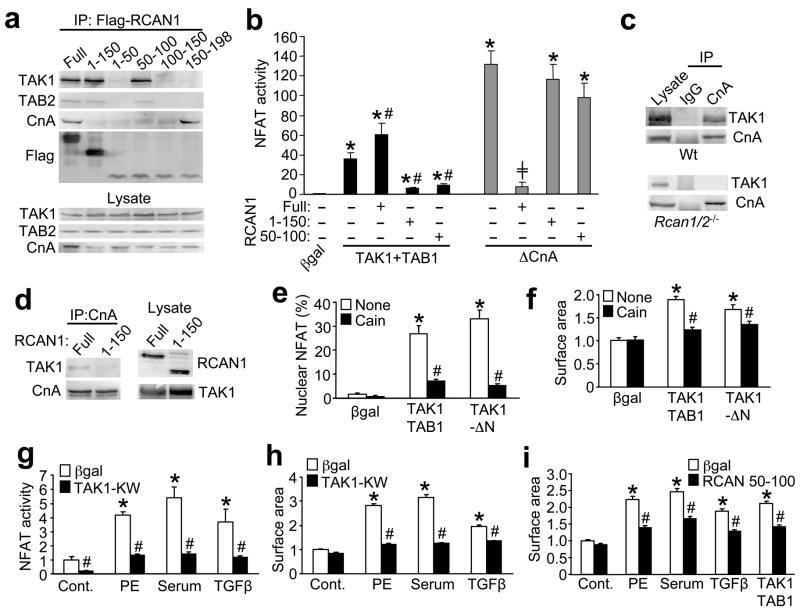
To further examine the functional ramifications associated with the TAK1-TAB1-TAB2 and RCAN1-calcineurin-NFAT complex, a series of biochemical and cellular assays were performed. Consistent with the GST-pull down assay described above, immunoprecipitation with several RCAN1 deletions showed that amino acids 50–100 of RCAN1 is sufficient to bind TAB2 and TAK1 in HEK293 cells (Fig. 4a). However, a different domain of RCAN1 (amino acids 150–198) was required for calcineurin association (Fig. 4a). These two deletion mutants were used to more carefully examine the effect of TAK1 on NFAT activation in vivo. Interestingly, both RCAN1 1–150 and RCAN1 50–100, which lack the calcineurin interacting domain, repressed NFAT activation induced by TAK1 + TAB1, while wildtype RCAN1 augmented NFAT activity (Fig. 4b). However, neither deletion mutant of RCAN1 altered the ability of activated calcineurin to induce NFAT, while wildtype RCAN1 was still fully inhibitory (Fig 4b). These results further suggest that TAK1 regulates calcineurin-NFAT signaling through an RCAN1 dependent mechanism. However, RCAN1 phosphorylation by TAK1 or TGFβ stimulation did not disrupt the ability of calcineurin to bind RCAN1 (data not shown).
Figure 4.
Calcineurin-RCAN1 is required for TAK1-induced hypertrophic growth signaling in cardiomyocytes. (a) Western blots with the indicated antibodies after immunoprecipitation of RCAN1 full-length or deletions (with anti-flag antibody) after transfection into HEK293 cells. (b) NFAT-luciferase activity from HEK293 cells transfected with the indicated plasmids. Luciferase activity was determined 36 h after transfection. Results were averaged from 3 independent experiments. Error bars represent s.e.m. *P < 0.001 versus Adβgal; #P < 0.01 versus TAK1+TAB1 only; +P < 0.01 versus ΔCnA only. (c) Western blotting for TAK1 or CnA from Wt or Rcan1/2−/− MEFs after immunoprecipitation with anti-CnA antibody or pre-immune IgG. (d) Western blotting for RCAN1 or TAK1 from cardiomyocytes infected with the indicated adenoviruses after CnA immunoprecipitation. (e) NFAT nuclear localization (AdNFATc1-GFP) in neonatal cardiomyocytes infected with the indicated adenoviruses for 24 h. Results were averaged from 3 independent experiments. Error bars represent s.e.m. *P < 0.001 versus Adβgal; #P < 0.001 versus None. (f) Cardiomyocyte surface areas for assessment of hypertrophy after infection with the indicated adenoviruses, with or without the calcineurin inhibitor Cain. Results were averaged from 3 independent experiments. Error bars represent s.e.m. *P < 0.001 versus Adβgal; #P < 0.001 versus none. (g) NFAT-luciferase activity in neonatal cardiomyocytes infected with βgal (control) or TAK1-KW adenoviruses. Cells were then stimulated with vehicle, PE (50 μM), 1% FBS, or TGFβ (5 ng/ml) for 24 h before luciferase activity was determined. Results were averaged from 3 independent experiments. Error bars represent s.e.m. *P < 0.001 versus control; #P < 0.001 versus Adβgal for each stimulant. (h) Cardiomyocyte surface areas after infection with βgal or TAK1-KW adenoviruses with or without PE (50 μM), 1% FBS, or TGFβ (5 ng/ml) stimulation for 24 h. Results were averaged from 3 independent experiments. Error bars represent s.e.m. *P < 0.001 versus Control; #P < 0.001 versus Adβgal of each stimulant. (i) Cardiomyocyte surface areas after infection with Adβgal or an adenovirus expressing RCAN1 amino acids 50–100. Twenty-four hrs later cells were stimulated with PE, 1% FBS, TGFβ, or infected with adenoviruses encoding TAK1+TAB1 for another 24 hrs. Results were averaged from 3 independent experiments. Error bars represent s.e.m. *P < 0.001 versus Control; #P < 0.001 versus Adβgal of each stimulant.
While the stability of the calcineurin-RCAN1 complex is not affected by TAK1, loss of RCAN1 prevents TAK1 participation in the calcineurin-NFAT complex. For example, TAK1 immunoprecipitated with calcineurin and RCAN1 (anti-CnA antibody) in wildtype MEFs but not in Rcan1/2−/− MEFs (Fig. 4c). Furthermore, TAK1 coimmunoprecipitated with calcineurin in cardiomyocytes infected with full-length AdRCAN1, but not with AdRCAN1 1–150 lacking the calcineurin-interacting domain (Fig. 4d). Collectively, these results indicate that RCAN1 serves as an adaptor for the formation of the entire macromolecular signaling complex.
To examine the functional consequences of TAK1 regulation of calcineurin-NFAT signaling we employed a growth assay in cultured neonatal cardiomyocytes, given that both calcineurin and TAK1 are known regulators of this process3,19. Consistent with our observations that TAK1 induced NFAT transcriptional activity, TAK1 + TAB1 or TAK1-ΔN also promoted NFATc1 nuclear translocation in cardiomyocytes, which was blocked with Cain (Fig. 4e). More importantly, overexpression of TAK1 + TAB1 or TAK1-ΔN enhanced the growth response of cardiomyocytes in culture through a calcineurin-dependent manner, given inhibition by Cain (Fig. 4f). Reciprocally, adenoviral mediated overexpression of TAK1-KW reduced NFAT-luciferase activity and myocyte growth in response to PE, serum and TGFβ, indicating that TAK1 is a necessary mediator of myocyte hypertrophy in culture in association with NFAT activation (Fig. 4g,h). Overexpression of decoy mutant of RCAN1 (aa 50–100), which binds TAB2-TAK1 but not calcineurin, inhibited agonist- and TAK1-induced hypertrophy of cardiac myocytes (Fig. 4i). Finally, transfection of an RNAi against TAK1, but not a control RNAi, blocked agonist-induced hypertrophy in neonatal cardiomyocyte cultures (see supplemental information, Fig. S2a,b). These results further support the importance of the TAK1-TAB1-TAB2 interaction with RCAN1-calcineurin-NFAT in controlling a critical biologic response in vivo.
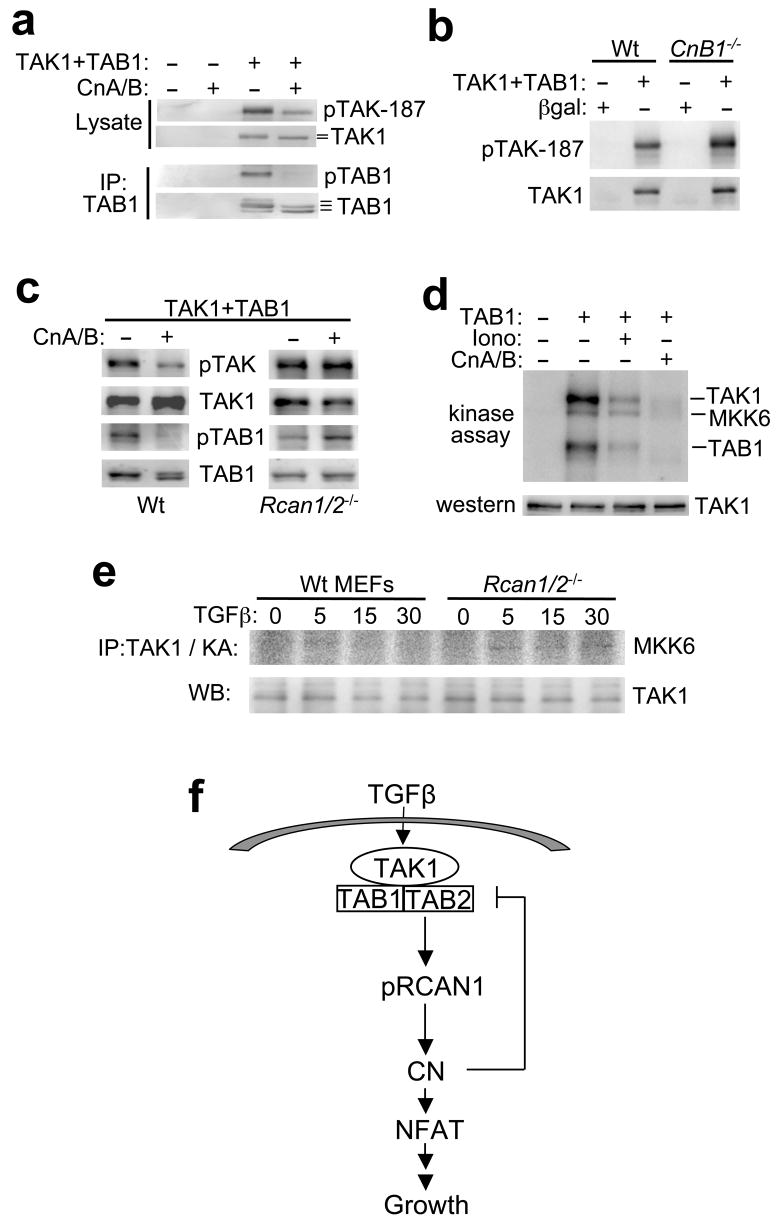
We also investigated the reciprocal regulatory relationship to determine if calcineurin might impart some type of regulation on TAK1, especially given that TAK1 requires phosphorylation for activation and calcineurin is a phosphatase. Cardiomyocytes were co-infected with adenoviruses expressing different combinations of calcineurin A and calcineurin B with TAK1 and TAB1, followed by Western blotting for phospho-TAK1 at threonine 187, or total phosphorylation of TAB1 using an immunoprecipitation assay with phospho-serine/threonine antibodies (Fig. 5a). The results show that TAK1-thr-187 is dramatically less phosphorylated in the presence of CnA/B, while TAB1 is completely dephosphorylated (Fig. 5a). Conversely, TAK1+TAB1-induced autophosphorylation of TAK1 at threonine 187 was enhanced in calcineurin deficient MEFs (lacking CnB1), suggesting that calcineurin basally regulates TAK1 phosphorylation (Fig. 5b). Consistent with our earlier results, the calcineurin-dependent dephosphorylation of TAK1 and TAB1 was completely inhibited in Rcan1/2−/− MEFs, while wildtype MEFs showed dephosphorylation comparable to that observed in cardiomyocytes (Fig. 5c). These results indicate that RCAN1 is required for calcineurin-dependent dephosphoryaltion of the TAK1 complex. Finally, we also observed that calcineurin-dependent dephosphorylation of the TAK1-TAB1 complex reduced TAK1 kinase activity (Fig. 5d). For this result an immunoprecipitation-kinase assays were performed with MKK6 as substrate from cardiomyocytes infected with adenoviruses expressing TAK1 (every reaction), TAB1, or CnA + CnB at baseline or after ionomycin stimulation. Similarly, TAK1 kinase activity towards MKK6 was increased in Rcan1/2−/− MEFs compared with wildtype MEFs after a time course of TGFβ stimulation, suggesting that endogenous calcineurin normally participates in TAK1 inactivation (Fig. 5e). The total amount of TAK1 immunoprecipitated in the kinase assay was the same under each condition (Fig. 5e). Collectively, the results in this section indicate that calcineurin can directly antagonize TAK1 signaling, suggesting a self-limiting feedback loop (Fig. 5f).
Figure 5.
Calcineurin reciprocally regulates TAK1 activity. (a) Western blots with the indicated antibodies from cardiomyocyte lysates after TAK1 immunoprecipitation. Cardiomyocytes were previously infected with the indicated recombinant adenoviruses. Altered migration patterns of TAK1 and TAB2 indicate differential phosphorylation (lines) (b) Western blots with the indicated antibodies from wildtype (Wt) or calcineurin B1 null (CnB1−/−) MEFs infected with the indicated adenoviruses. (c) Western blots with the indicated antibodies from Wt and Rcan1/2−/− MEFs infected with the indicated adenoviruses for 24 h. (d) Autoradiography of a kinase assay from cardiomyocytes infected with the indicated adenoviruses or treated with 1 μM ionomycin (Iono) for 30 min. TAK1 was also overexpressed and immunoprecipitated and incubated with GST-MKK6 in kinase assay buffer. (e) Immunoprecipitation (IP) of TAK1 followed by a kinase assay (KA) from Wt and Rcan1/2−/− MEFs stimulated with TGFβ for the indicated time in minutes. As a control total TAK1 from similar immunoprecipitates is shown. (f) Schematic representation of the TAK1-TAB1-TAB2 and RCAN1-calcineurin signaling network.
Discussion
The observation that TAK1-TAB1-TAB2 and RCAN1-calcineurin-NFAT signaling co-regulate one another has profound biologic ramifications considering the centrality that each signaling pathway plays on its own. TGFβ and other select cytokines are known activators of the TAK1 signaling complex, which serves as a focal integrating point for the activation of multiple downstream signaling effectors such as MKK3/6-p38, MKK4-JNK, and NF-κB17,18,23. The critical importance of TAK1-TAB1-TAB2 signaling is further supported by the known lethality associated with deletion of the genes encoding these three proteins in the mouse23,24. In addition to TAB1 and TAB2, TAB3 has recently been described as yet another critical co-factor for TAK1 that regulates its activity and interaction with other proteins25. TAK1 also integrates signals from multiple receptor systems, including the TGFβR, IL-1R, TNF1R, and toll-like receptors 3 and 4 23,26, collectively suggesting that TAK1 functions as a signaling coordination center. A similar “gateway-like” signaling function has been ascribed to calcineurin in many different cell types, where it appears to integrate the biologic responsiveness to diverse calcium releasing/mobilizing signal transduction pathways. Calcineurin-NFAT signaling is also critically regulated by a dedicated family of co-factors, referred to as RCANs4,5. Finally, calcineurin-NFAT responsiveness is counter-regulated by direct phosphorylation of NFAT factors in their N-termini through the activation of p38, JNK, GSK3β, casein kinase I &II, and protein kinase A27.
Here we showed that TAB2 directly interacts with RCAN1 in mammalian cells, generating a higher order complex between the TAK1-TAB1-TAB2 and the RCAN1-calcienurin-NFAT signaling modules. This interaction produced two independent regulatory effects, the first of which was a TAK1-dependent phosphorylation of RCAN1, resulting in greater calcineurin-NFAT coupling, greater NFAT-dependent gene activation and hypertrophy in neonatal cardiomyocytes. This result suggests that the TAK1-TAB1-TAB2 signaling module promotes the cardiac growth response through an enhancement in calcineurin-NFAT signaling, although TAK1-TAB1-TAB2 signaling likely affects the growth response through other effectors as well, such as NF-κB (Fig. 5e). Indeed, TAK1 overexpression in the heart induces the cardiac growth response, indicating that it functions as a central regulator of this process19,28. Our results further establish the importance of TAK1, showing that it is both sufficient and necessary for cardiomyocyte hypertrophic growth via an RCAN1/calcinerin signaling mechanism. Conversely, activation of calcineurin directly dampens TAK1 signaling through calcineurin-dependent dephosphorylation of both TAK1 and TAB1. This later observation suggests that calcium signals that mobilize calcineurin can modulate TGFβ, TLR and IL1R signals. However, signals that initiate TAK1 activation may or may not produce a negative feedback loop to dampen TAK1 signaling through RCAN-calcineurin. More specifically, we have only shown that RCAN1 facilitates NFAT activity downstream of calcineurin, presumably by enhancing coupling as previously shown (Fig 3 and ref. 10). Thus, when TAK1 phosphorylates RCAN1 resulting in greater calcineurin-NFAT coupling, it is uncertain if this also results in calcineurin activation towards NFAT-independent targets, such as TAB1 or TAK1.
Our data indicate that the effect of RCAN1 on calcineurin signaling depends on both post-translational modifications (i.e. phosphorylation) and expression level of RCAN1. At low TAK1 activity, dephosphorylated RCAN1 inhibits calcineurin activity by binding to the regulatory site on calcineurin, thus blocking NFAT activation. At high TAK1 activity RCAN1 is predominantly phosphorylated, causing a change in its function towards calcineurin such that it now facilitates activation of NFAT. When RCAN1 is overexpressed at low to intermediate levels, TAK1 can still enhance calcineurin-NFAT coupling through RCAN1. However, when RCAN1 is overexpressed at very high levels it acts as an inhibitor, likely through a non-physiologic blocking mechanism. It remains uncertain exactly how RCAN1 facilitates calcineurin-NFAT signaling, although inhibitor-2 and DARPP-32 may play a similar role in permitting PP1 activity29,30. Specifically, inhibitor-2 can undergo conformational changes upon phosphorylation and dephosphorylation that allosterically regulates PP1 activity31.
Here we showed that TAK1 directly phosphorylated RCAN1 at two novel sites (serine-94 and -136), resulting in activation of calcineurin-NFAT signaling. Two other groups reported that RCAN1 is phosphorylated at serine-108 and -112, although they disagreed on the functional effect associated with this event. Genesca et al. reported that phosphorylation of serine-108 and -112 increased the inhibitory capacity of RCAN1 towards calcineurin-NFAT12. In contrast, Abbasi and colleagues demonstrated that phosphorylated RCAN1 dissociates from calcineurin and binds with 14–3–3, thereby relieving its inhibitory effect32. In vivo, deletion of Rcan1/2 genes produced phenotypic effects that mimicked a loss of calcineurin signaling capacity, suggesting that the net biologic function of RCANs in vivo is to facilitate calcineurin-NFAT signaling10,11. Thus, the physiologic effect of TAK1 on calcineurin-NFAT signaling at endogenous levels of RCAN1 expression is most certainly supportive in effect. These results suggest an entirely novel signaling circuit whereby two major regulatory pathways interact to control at least one biological response, cardiomyocyte growth.
METHODS
Yeast two-hybrid assay
Yeast two-hybrid screening was performed using the Matchmaker Gal4 two-hybrid System 3 (Clontech, Palo Alto, CA) as described previously33. The bait construct consisted of full-length murine RCAN1 cloned in frame into the plasmid pGBKT7. Yeast strain AH109 was cotransformed with the bait and a murine adult cardiac cDNA library constructed into the EcoRI site of pGAD10 (custom-made library; Clontech). Confirmation of the initial interaction was performed by cotransforming each unique bacterial prey plasmid into AH109 with the RCAN1 bait construct and screening each combination on medium- and high-stringency media as previously described33.
Cell culture
Primary neonatal rat cardiomyocytes were prepared from hearts of 1- to 2-day-old Sprague-Dawley rat pups as previously described33. Rcan1/2−/− or wildtype MEFs were isolated at embryonic day 12.5 by trypsin digestion as described previously10. HEK293 cells were purchased from American Cell Culture Collection (Rockville, MD) and cultured as previously described33. Tab2−/− and Tak1−/− MEFs were a generous gift from Dr. Shizuo Akira, Osaka University, Japan. Cells were transfected with Fugene-6 reagent (Roche Applied Sciences, Indianapolis, Ind.). Adenoviral infections were performed as previously described at a multiplicity of infection of 10 to 100 plaque forming units per ml 33. Cultures were harvested 24 h after infection, and luciferase assays were preformed as described previously34.
Adenoviral constructs and expression vectors
Adβgal, AdΔCnA, AdCnB, Adcain, AdNFAT-Luc, and AdNFATc1-GFP have been previously described33. Mammalian expression vectors encoding TAB2, TAK1, TAK1-KW, and TAB1 were kind gifts from Kunihiro Matsumoto13. TAB1 and TAB2 cDNAs were subcloned into the pShuttle vector to generate AdTAB1 and AdTAB2 as described previously33. AdTAK1, AdTAK1-KW, and AdTAK1-ΔN were kind gifts from Paul D. Benya (UCLA-Orthopedic Hospital). Truncations of TAB2 and RCAN1 were amplified by PCR and subcloned into pCMVF, pGEX-4T-1, or pGAD10 vectors. RCAN1-S136A, RCAN1-S94A, RCAN1-S94/136A, RCAN1-S136D, RCAN1-S94D, RCAN1-S94/136D were generated using the QuickChange Site-Directed Mutagenesis Kit (Stratagene, La Jolla, CA).
GST pull-down assays
To generate GST fusion proteins, select DNA sequences from TAB2 and RCAN1 were amplified by PCR and subcloned into pGEX-4T-1 (Amersham Pharmacia Biotech, Piscataway, NJ). Binding assays were performed with [35S]methionine-labeled proteins synthesized in vitro using a transcription-translation-coupled reticulocyte lysate system (Promega, Madison, WI) as described previously33.
Immunoprecipitation and Western blot analysis
Immunoprecipitation was performed as previously described33. Proteins were resolved on 8–15% SDS-PAGE gels, transferred to PVDF membranes and immunoblotted using the following antibodies: anti-TAK1, anti-TAB1, anti-TAB2, and anti-pan CnA (Santa Cruz Biotechnology, Santa Cruz, CA); anti-phosphoserine (Chemicon International Inc., Temecula, CA); anti-Flag (Sigma, St. Louis, MO); anti-Myc-Tag, anti-phospho-TAK1(Thr187), anti-phosphothreonine (Cell Signaling, Beverly, MA). Custom rabbit polyclonal anti-phospho-RCAN1-serine 136 and anti-phospho-RCAN1-serine 94 were generated and affinity purified by YenZym (Burlingame, CA).
Immunoprecipitation kinase assay
TAK1 was immunoprecipitated with anti-TAK1 (Santa Cruz Biotechnology) and protein A/G-agarose. After washing the immunoprecipitates three times with lysis buffer33 and once with kinase assay buffer (20 mM Tris-Cl [pH 7.5], 10 mM MgCl2, 1 mM dithiothreitol, 1 mM phenylmethylsulfonyl fluoride), the immunoprecipitates were incubated with 1 μg of GST-MKK6 in 25 μl of kinase assay buffer with 0.3 μCi of [32P]ATP for 10 min at 30°C. Samples were subjected to SDS-PAGE (8%) and visualized by PhosphorImager analysis (Amersham Pharmacia Biotech).
Immunohistochemistry and hypertrophy analysis
To assess cardiomyocyte hypertrophy, cells were immunostained with an antibody against α-actinin (Sigma, St. Louis, MO). Quantitation of cardiomyocyte cell surface area was performed on α-actinin-stained cardiomyocytes by confocal laser microscopy and NIH Image Analysis software, version 1.63.
Detection of phosphorylation sites by mass spectrometry
Purified RCAN1 was incubated with the purified TAK-TAB1 fusion protein (Upstate Biotechnology, Lake Placid, NY) in reaction buffer with or without ATP at 30°C for 15 min. The samples were digested with trypsin overnight. An aliquot of each digest was analyzed using nanoflow-HPLC electrospray ionization directly coupled to an LTQ-Orbitrap (Thermo Electron, San Jose, CA) using top 5 data dependent mode MS/MS mode as previously described35. All MS/MS spectra were searched via Sequest using variable modification of +80 on serine and threonine for identification of phosphorylation. Data were loaded into Scaffold (Proteome Software, Portland, OR) for manual validation. Phosphorylated peptides in RCAN1 identified were QVEDATPVINYDLLYAI[p-S]K and LYFAQTLHIGS[p-S]HLAPPNPDK.
Supplementary Material
Acknowledgments
We are very grateful to Dr. Yang Xia for the generous gift of the S108/112A mutant RCAN1 plasmid, and Dr. Jun Tsuji for providing the Tak1−/− MEFs originated from Dr Akira’s laboratory. This work was supported by grants from the National Institutes of Health (J.D.M), the Fondation Leducq (Heart failure network grant to J.D.M), and the Howard Hughes Medical Institute.
References
- 1.Wu H, Peisley A, Graef IA, Crabtree GR. NFAT signaling and the invention of vertebrates. Trends Cell Biol. 2007;17:251–60. doi: 10.1016/j.tcb.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 2.Hogan PG, Chen L, Nardone J, Rao A. Transcriptional regulation by calcium, calcineurin, and NFAT. Genes Dev. 2003;17:2205–2232. doi: 10.1101/gad.1102703. [DOI] [PubMed] [Google Scholar]
- 3.Molkentin JD. A calcineurin-dependent transcriptional pathway for cardiac hypertrophy. Cell. 1998;93:215–228. doi: 10.1016/s0092-8674(00)81573-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hilioti Z, Cunningham KW. The RCN family of calcineurin regulators. Biochem Biophys Res Commun. 2003;311:1089–1093. doi: 10.1016/s0006-291x(03)01515-8. [DOI] [PubMed] [Google Scholar]
- 5.Davies KJ, et al. Renaming the DSCR1/Adapt78 gene family as RCAN: regulators of calcineurin. FASEB J. 2007;21:3023–3028. doi: 10.1096/fj.06-7246com. [DOI] [PubMed] [Google Scholar]
- 6.Chan B, Greenan G, McKeon F, Ellenberger T. Identification of a peptide fragment of DSCR1 that competitively inhibits calcineurin activity in vitro and in vivo. Proc Natl Acad Sci U S A. 2005;102:13075–13080. doi: 10.1073/pnas.0503846102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hilioti Z, et al. GSK-3 kinases enhance calcineurin signaling by phosphorylation of RCNs. Genes Dev. 2004;18:35–47. doi: 10.1101/gad.1159204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kingsbury TJ, Cunningham KW. A conserved family of calcineurin regulators. Genes Dev. 2000;14:1595–1604. [PMC free article] [PubMed] [Google Scholar]
- 9.Gorlach J, et al. Identification and characterization of a highly conserved calcineurin binding protein, CBP1/calcipressin, in Cryptococcus neoformans. EMBO J. 2000;19:3618–3629. doi: 10.1093/emboj/19.14.3618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sanna B, et al. Modulatory calcineurin-interacting proteins 1 and 2 function as calcineurin facilitators in vivo. Proc Natl Acad Sci U S A. 2006;103:7327–7332. doi: 10.1073/pnas.0509340103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vega RB, et al. Dual roles of modulatory calcineurin-interacting protein 1 in cardiac hypertrophy. Proc Natl Acad Sci USA. 2003;100:669–674. doi: 10.1073/pnas.0237225100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Genesca L, et al. Phosphorylation of calcipressin 1 increases its ability to inhibit calcineurin and decreases calcipressin half-life. Biochem J. 2003;374:567–575. doi: 10.1042/BJ20030267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takaesu G, et al. TAB2, a novel adaptor protein, mediates activation of TAK1 MAPKKK by linking TAK1 to TRAF6 in the IL-1 signal transduction pathway. Mol Cell. 2000;5:649–658. doi: 10.1016/s1097-2765(00)80244-0. [DOI] [PubMed] [Google Scholar]
- 14.Yamaguchi K, et al. Identification of a member of the MAPKKK family as a potential mediator of TGF-beta signal transduction. Science. 1995;270:2008–2011. doi: 10.1126/science.270.5244.2008. [DOI] [PubMed] [Google Scholar]
- 15.Besse A, et al. TAK1-dependent signaling requires functional interaction with TAB2/TAB3. J Biol Chem. 2007;282:3918–3928. doi: 10.1074/jbc.M608867200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shibuya H, et al. TAB1: an activator of the TAK1 MAPKKK in TGF-beta signal transduction. Science. 1996;272:1179–1182. doi: 10.1126/science.272.5265.1179. [DOI] [PubMed] [Google Scholar]
- 17.Ninomiya-Tsuji J, et al. The kinase TAK1 can activate the NIK-I kappaB as well as the MAP kinase cascade in the IL-1 signalling pathway. Nature. 1999;398:252–256. doi: 10.1038/18465. [DOI] [PubMed] [Google Scholar]
- 18.Wang C, et al. TAK1 is a ubiquitin-dependent kinase of MKK and IKK. Nature. 2001;412:346–351. doi: 10.1038/35085597. [DOI] [PubMed] [Google Scholar]
- 19.Zhang D, et al. TAK1 is activated in the myocardium after pressure overload and is sufficient to provoke heart failure in transgenic mice. Nat Med. 2000;6:556–563. doi: 10.1038/75037. [DOI] [PubMed] [Google Scholar]
- 20.Vega RB, Yang J, Rothermel BA, Bassel-Duby R, Williams RS. Multiple domains of MCIP1 contribute to inhibition of calcineurin activity. J Biol Chem. 2002;277:30401–30407. doi: 10.1074/jbc.M200123200. [DOI] [PubMed] [Google Scholar]
- 21.Rothermel B, et al. A protein encoded within the Down syndrome critical region is enriched in striated muscles and inhibits calcineurin signaling. J Biol Chem. 2000;275:8719–8725. doi: 10.1074/jbc.275.12.8719. [DOI] [PubMed] [Google Scholar]
- 22.Rothermel BA, et al. Myocyte-enriched calcineurin-interacting protein, MCIP1, inhibits cardiac hypertrophy in vivo. Proc Natl Acad Sci USA. 2001;98:3328–3333. doi: 10.1073/pnas.041614798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shim JH, et al. TAK1, but not TAB1 or TAB2, plays an essential role in multiple signaling pathways in vivo. Genes Dev. 2005;19:2668–2681. doi: 10.1101/gad.1360605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Komatsu Y, et al. Targeted disruption of the Tab1 gene causes embryonic lethality and defects in cardiovascular and lung morphogenesis. Mech Dev. 2002;119:239–249. doi: 10.1016/s0925-4773(02)00391-x. [DOI] [PubMed] [Google Scholar]
- 25.Ishitani T, et al. Role of the TAB2-related protein TAB3 in IL-1 and TNF signaling. EMBO J. 2003;22:6277–6288. doi: 10.1093/emboj/cdg605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sato S, et al. Essential function for the kinase TAK1 in innate and adaptive immune responses. Nat Immunol. 2005;6:1087–1095. doi: 10.1038/ni1255. [DOI] [PubMed] [Google Scholar]
- 27.Molkentin JD. Calcineurin-NFAT signaling regulates the cardiac hypertrophic response in coordination with the MAPKs. Cardiovasc Res. 2004;63:467–475. doi: 10.1016/j.cardiores.2004.01.021. [DOI] [PubMed] [Google Scholar]
- 28.Watkins SJ, Jonker L, Arthur HM. A direct interaction between TGFbeta activated kinase 1 and the TGFbeta type II receptor: implications for TGFbeta signalling and cardiac hypertrophy. Cardiovasc Res. 2006;69:432–439. doi: 10.1016/j.cardiores.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 29.Tung HY, Wangm W, Chan CS. Regulation of chromosome segregation by Glc8p, a structural homolog of mammalian inhibitor 2 that functions as both an activator and an inhibitor of yeast protein phosphatase 1. Mol Cell Biol. 1995;15:6064–6074. doi: 10.1128/mcb.15.11.6064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Greengard P, Allen PB, Nairn AC. Beyond the dopamine receptor: the DARPP-32/protein phosphatase-1 cascade. Neuron. 1999;23:435–447. doi: 10.1016/s0896-6273(00)80798-9. [DOI] [PubMed] [Google Scholar]
- 31.Cohen PT. Protein phosphatase 1--targeted in many directions. J Cell Sci. 2002;115:241–256. doi: 10.1242/jcs.115.2.241. [DOI] [PubMed] [Google Scholar]
- 32.Abbasi S, et al. Protein kinase-mediated regulation of calcineurin through the phosphorylation of modulatory calcineurin-interacting protein 1. J Biol Chem. 2006;281:7717–7726. doi: 10.1074/jbc.M510775200. [DOI] [PubMed] [Google Scholar]
- 33.Liu Q, Wilkins BJ, Lee YJ, Ichijo H, Molkentin JD. Direct interaction and reciprocal regulation between ASK1 and calcineurin-NFAT control cardiomyocyte death and growth. Mol Cell Biol. 2006;26:3785–3797. doi: 10.1128/MCB.26.10.3785-3797.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liang Q, et al. The transcription factor GATA4 is activated by extracellular signal-regulated kinase 1- and 2-mediated phosphorylation of serine 105 in cardiomyocytes. Mol Cell Biol. 2001;21:7460–7469. doi: 10.1128/MCB.21.21.7460-7469.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Godeny MD, et al. The N-terminal SH2 domain of the tyrosine phosphatase, SHP-2, is essential for Jak2-dependent signaling via the angiotensin II type AT1 receptor. Cell Signal. 2007;19:600–609. doi: 10.1016/j.cellsig.2006.08.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.