Abstract
We have previously developed 2-D array transducers for many real-time volumetric imaging applications. These applications include transducers operating up to 7 MHz for transthoracic imaging, up to 15 MHz for intracardiac echocardiography (ICE), 5 MHz for transesophageal echocardiography (TEE) and intracranial imaging, and 7 MHz for laparoscopic ultrasound imaging (LUS). Now we have developed a new generation of miniature ring-array transducers integrated into the catheter deployment kits of interventional devices to enable real-time 3-D ultrasound scanning for improved guidance of minimally invasive procedures. We have constructed 3 new ring transducers. The first consists of 54 elements operating at 5 MHz. Typical measured transducer element bandwidth was 25%, and the 50 Ohm round trip insertion loss was −65 dB. Average nearest neighbor cross talk was −23.8 dB. The second is a prototype 108-element transducer operating at 5 MHz. The third is a prototype 108-element ring array with a transducer center frequency of 8.9 MHz and a −6 dB bandwidth of 25%. All transducers were integrated with an 8.5 French catheter sheath of a Cook Medical, Inc. vena cava filter deployment device.
I. INTRODUCTION
The past few decades have seen tremendous break-throughs in the development and application of interventional medical devices including inferior vena cava (IVC) filters, vascular stents, aortic aneurysm stent grafts, vascular occluders, cardiac occluders, prosthetic cardiac valves, and catheter and needle delivery of radio frequency ablation. However, imaging modalities have not kept pace as these procedures are typically performed under fluoroscopic guidance and make use of X-ray contrast agents. Although fluoroscopy has excellent visualization of these devices in the body, soft tissue structures are not easily visualized. A bolus injection of X-ray contrast agent provides only a momentary glimpse of vascular structures. Finally, fluoroscopy is not inherently a 3-D imaging modality. The ability to visualize the surrounding anatomy as well as the device in 3 dimensions could be a useful attribute in the placement of these devices.
Building upon our previous work [1]–[9], we describe the design, fabrication, and testing of new transducers to explore the feasibility of using real-time 3-D ultrasound to guide the deployment of 2 of these interventional devices: IVC filters and abdominal aortic aneurysm stent grafts.
A. Vena Cava Filters
Frequently when a patient is nonambulatory for a period of time or has physical trauma, a blood clot or thrombus can form in the lower extremities. This condition is known as deep vein thrombosis (DVT). Deep vein thrombosis of the lower extremities occurs in adults with a yearly incidence of 1.6 per thousand and a cumulative risk by age 80 of 11% in males [10]. These blood clots may then travel up the venous system to the heart and lungs causing a pulmonary embolism, which occurs in 20 to 30% of patients with DVT. Pulmonary embolism (PE) is a serious condition that occurs in 355,000 patients and causes an estimated quarter million deaths in the United States every year [11]. Multiple anticoagulation agents are now available for the initial treatment of DVT including heparin and oral agents such as the vitamin K antagonists. Patients with idiopathic DVT or persistent risk factors require anticoagulant therapy for a minimum of 6 to 12 months, with a cumulative risk of recurrence as high as 30% at 8 years [12].
Development of IVC filters to interrupt flow in the IVC and prevent pulmonary embolism began some 50 years ago. The use of these devices has increased dramatically during the last 2 decades, with estimates of 49,000 implants yearly in the United States by 1999 [12]. IVC filters are indicated in the following conditions [11]: 1) Documented DVT or pulmonary embolism with a contraindication to anticoagulation; 2) failed treatment with anticoagulation; 3) failure of another form of vena cava interruption; 4) prophylaxis for proximal free-floating thrombus; 5) high-risk patient populations such as those with pulmonary hypertension and reduced cardiac reserve who are undergoing surgery for morbid obesity or orthopedic trauma; and 6) pregnant women with DVT.
Prophylactic vena cava filter placement offers a protection rate of almost 99% against fatal pulmonary embolism. Vena cava filters have also protected patients with a history of pulmonary embolism who have contraindications for anticoagulation. IVC filters are placed using a percutaneous procedure generally from a femoral or jugular vein. Filters are usually placed in the inferior vena cava below the renal veins. Although IVC filters are usually placed under fluoroscopic guidance, there is increasing interest in placing them using ultrasound guidance. The extensive fluoroscopy, cavography, CT, and contrast agent associated with these procedures necessarily entail large X-ray dosage to the patient and medical personnel.
Several authors have described successful placement of IVC filters using 2-D transabdominal ultrasound (TAUS) [13], [14] or 2-D intravascular ultrasound (IVUS) [15], [16]. TAUS provides real-time images that often allow placement of an IVC filter, but this technique cannot be used in very large patients or in patients with bowel gas that obscures visualization of the IVC. IVUS overcomes these shortcomings, but it lacks the longitudinal overview of the IVC and filter introducer provided by TAUS.
IVUS provides better visualization of the vessel lumen than TAUS and overcomes the potential for TAUS guided filter placement attempts to fail due to the lack of an adequate window. However the trans-axial images provided by IVUS do not define the relationship of the IVC filter introducer sheath to the renal veins. Moreover, difficulties arise with advancing the IVUS probe into the IVC, an inability to localize the renal veins, or an inability to define potential abnormalities. Ideally the IVC filter should be placed in close proximity to the renal veins, but the axial view provided by most IVUS systems limits the operator’s ability to estimate the distance from the filter introducer to the renal veins.
Finally, we note there is increasing interest in bedside filter placement in critically ill patients [16]. Many of these patients are difficult to transport to an angiography suite for conventional fluoroscopically guided placement. In critically ill patients the rate of complications potentially attributable to transportation ranges from 5.9% to 15.5%.
A real-time 3-D IVUS probe providing simultaneous sagittal, coronal, and axial views would overcome the shortcomings of both TAUS and conventional IVUS and improve bedside filter placement. A 3-D IVUS provides several potential advantages. First, it is not blocked by abdominal structures like conventional TAUS. Second, it can produce axial and sagittal images of the IVC like TAUS, and it can also produce coronal images not available in either TAUS or IVUS. The coronal image may be the most useful single view for ultrasound guided filter placement. Potentially, in this one view, the operator can visualize both renal veins, the filter introducer sheath, and the IVC. As improvement in 3-D imaging occurs, real-time volume rendered images may be the best tool for ultrasound-guided IVC filter placement. A large field of view and deep penetration would give the operator a complete overview of the IVC, its branches, and the filter introducer.
B. Abdominal Aortic Aneurysm Stent Grafts
Abdominal aortic aneurysm (AAA) is a permanent focal dilatation of the artery to 1.5 times its normal diameter affecting approximately 1.5% of the United States population [17] with more than 200,000 Americans diagnosed each year. The natural history of AAAs is to expand and rupture. AAAs and aortic dissections are responsible for at least 15,000 deaths yearly and in 2000 were the 10th leading cause of death in white males 65 to 74 years of age in the United States [18]. The prevalence of AAA in older men ranges from 4.2 to 8.8%. With an aging population, the incidence and prevalence of AAA is certain to rise. Most AAAs are asymptomatic, and physical examination lacks sensitivity for detecting an aneurysm. Risk factors include age, 65 years or older, male sex, strong family history of AAA, and smoking at least 100 cigarettes in a lifetime [19]. What sets AAA apart from more common causes of death is that it is a preventable problem. The U.S. Preventive Services Task Force states that screening benefits patients who have a relatively high risk for dying from an aneurysm.
Thus, the major impetus for AAA repair is for prophylaxis against aneurysm-related death. The 2 primary methods of AAA repair are open and endovascular. Traditional open AAA repair involves direct access to the aorta through an incision in the abdomen. This repair method is well established as definitive, requiring essentially no follow-up radiologic studies. However, as with all other major abdominal surgical operations, associated significant morbidity and mortality exist along with prolonged recovery and various late complications. Furthermore, both mortality and morbidity increase significantly with advanced patient age and associated co-morbid disease states.
Endovascular AAA repair using covered stent-grafts offers a significantly less invasive alternative to conventional open-surgical repair. An endograft, typically a cloth graft with a stent exoskeleton, is placed within the lumen of the AAA extending distally into the iliac arteries. This serves as a bypass and decreases the pressure on the aortic wall, leading to a reduction in AAA size over time and a decrease in the risk of aortic rupture. A considerable reduction in hospital stay has been demonstrated for endovascular AAA repair, with early return to preoperative levels of activity. Patients previously considered unsuitable for open repair can often receive treatment for aneurysms with endovascular techniques.
The most important issue associated with endovascular repair is that of “endoleaks” of pressurized blood into the residual aneurysm sac arising in the stent graft both during and subsequent to implantation. The problem of endoleaks results in concerns about the ability of the stent graft to protect the patient successfully from subsequent rupture. These concerns point to the critical role of high-quality imaging during the course of stent graft deployment to detect patent sac vessels [10].
In a study of 38 implants of fenestrated stent grafts [20], the mean fluoroscopy time was 30 +/− 23 min, and 182 +/− 62 mL of potentially toxic iodinated contrast material was used per procedure. Typical deployment instructions list at least 4 angiographic examinations of the aorta and stent graft before, during, and after the implant procedure to verify patency of renal and iliac vessels and check for endoleaks [21]. The extensive fluoroscopy, angiography, CT, and contrast agent associated with these procedures necessarily entail risk to the patient and medical personnel. Our long-term goal is to reduce substantially these dosages by significant use of catheter 3-D ultrasound probes integrated into the graft deployment kits to guide device deployment, detect immediate endoleaks, and measure patency in the stented branch vessels.
II. METHODS
A. Real-Time 3-D Ultrasound Imaging
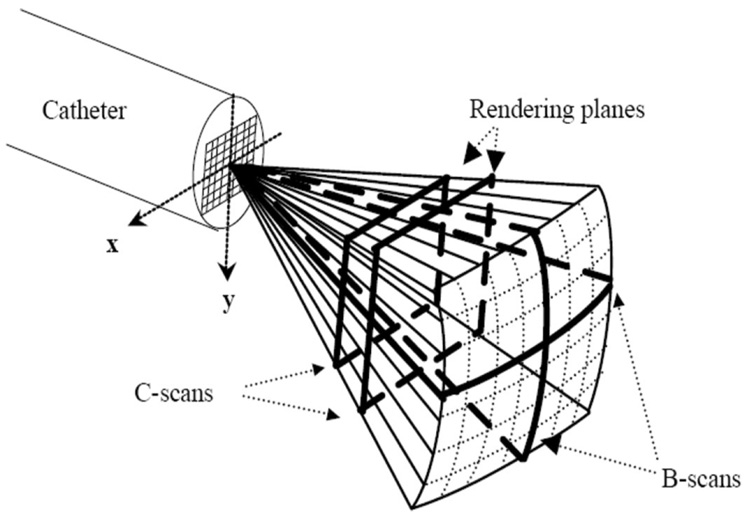
We used standard 3-D phased-array beaming forming techniques to generate our real-time 3-D images. Real-time 3-D ultrasound was developed in our laboratories at Duke University by von Ramm and Smith [22], [23] and first commercialized for cardiovascular applications by Volumetrics Medical Imaging, Inc. (VMI, Durham, NC). As shown in Fig. 1, the Duke/VMI 3-D system scans a 65° to 120° pyramid with matrix array transducers of up to 500 active channels to produce 3-D scans at rates up to 30 volumes/sec using 16:1 receive mode parallel processing. Although the system allows for different transmit apertures as a function of depth to keep a constant f number, we did not use that option in this work. Real-time display options include up to 5 image planes oriented at any desired angle, depth, and thickness within the pyramidal scan as well as real-time 3-D volume rendering, 3-D pulse wave Doppler, and 3-D color flow Doppler. Clinical and animal evaluations of the Duke/Volumetrics scanner have shown potential advantages over conventional 2-D scanners for measurement of cardiac function in terms of ventricular volumes [24], detection of perfusion defects [25], reduced scanning times in dobutamine stress echo exams [26], measurement of peak left ventricular flow velocities [27], guidance of right ventricular endomyocardial biopsy [28], and evaluation of congenital cardiac abnormalities [29].
Fig. 1.
Schematic of the pyramidal scan from catheter 2-D array transducer. Bold lines indicate possible display planes. By integrating and spatially filtering between 2 user-selected planes, real-time 3-D rendered images are displayed.
We used a VMI Model 1 scanner for this work. In our laboratories at Duke during the last few years, we have significantly modified the Volumetrics scanner to use our prototype 2-D arrays for applications such as real-time 3-D transesophageal imaging [7], laparoscopic imaging [9], and intracardiac and intravascular imaging with catheter transducers [5] at 5, 7, 10 [30], and 15 MHz [6]. No new modifications were needed for the transducers described in this paper to run on the VMI Model 1 scanner.
B. Transducer Design
We modified a Günther Tulip (Cook Medical Inc., Bloomington, IN) vena cava filter deployment kit for our ring transducer. This commercial product uses an 8.5 Fr sheath (ID) resulting in an approximately 11 French (OD) catheter to deploy the vena cava filter. To make connection to the piezoelectric elements, we used the MicroMiniature ribbon cables ( W. L. Gore and Associates, Newark, DE) incorporating parallel wires on 0.10 mm center to center spacing. We have used these cables previously in our 3-D transducer probes [5]–[9]. These cables can be used in 2 possible ways. First, every wire can be used as a signal wire to both conduct the transmit pulse to the element and the receive signal from the element to the ultrasound scanner. In this configuration, an integrated ground plane is added to the polyimide carrier of the cable. Second, every other wire is used to conduct the desired signal, while the interstitial wires are grounded to reduce electrical cross talk. We make use of both methods.
Ring-array transducers at frequencies up to 20 MHz are commercially used in radial scanning 2-D IVUS designs for vascular imaging especially the coronary arteries. In 2001, Wang et al. described a forward viewing ring array for IVUS applications [31] and in 2006, Degertekin et al. described a CMUT-based ring transducer for forward viewing IVUS [32]. The advantages of the ring geometry for 3-D scanning have been recently described by Ullate et al., who showed significantly reduced grating lobes of a segmented annulus array due to the effective nonuniform spacing of the array when projected on any steering axis [33].
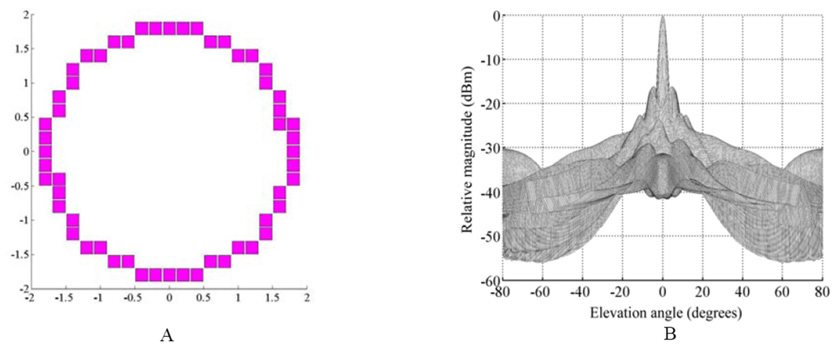
To explore our transducer aperture design and frequency parameters, we performed Field II [34] simulations of the ultrasound beam based on transducers with the cable specifications described above. Fig. 2(a) shows a 54-element ring aperture operating at 5 MHz with a circumferential element spacing of 0.20 mm. The transducer aperture OD is 3.8 mm. We approximated a true ring with rectilinear sampling corresponding to the cuts of the dicing saw. Fig. 2(b) shows the Field II on-axis beam response of the aperture in Fig. 2(a) with a 5 mm focal point for both transmit and receive modes over an angular range from −80° to +80° yielding a − 6dB beamwidth of 3.8° . At ± 80° we are just seeing the grating lobe enter the beam plot.
Fig. 2.
(a)The Field II simulated 54-element aperture and (b)beam response of a 5 MHz ring array transducer focused on axis at 5 mm. The predicted − 6dB beamwidth is 3.8° .
.
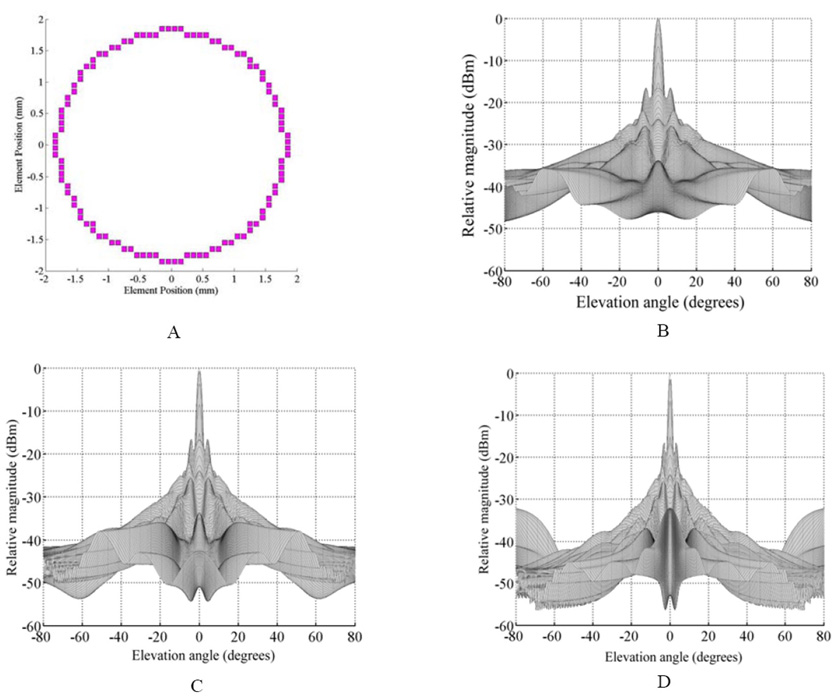
To explore frequencies above 5 MHz, the element size would have to decrease from the 5 MHz case. The PZT-5H transducer material (TRS Technologies, Inc., State College, PA ) that we use is about 0.30 mm thick to operate at 5 MHz for a 2-D array element. A 0.20 mm interelement spacing with a kerf of 0.020 mm results in an element that is 0.18 mm wide. This gives us a width to thickness ratio of 0.6. This is sufficient for the element to work in the desired bar mode. However, for a 7.5 MHz element, we typically use PZT that is 0.18 mm thick. To achieve the desired width to thickness ratio, we would like to have elements near 0.10 mm wide. With the limitation that the Gore cables are on 0.10 or 0.20 mm spacing, we chose an interelement spacing of 0.10 mm. A 0.020 mm kerf results in an element that is 0.08 mm wide. Because we halved the spacing, we doubled the number of transducer elements to 108. Fig. 3(a) shows the aperture; the simulated beam responses for 5 MHz are shown in Fig. 3(b); 7.5 MHz in 3(c); and 10 MHz in 3(d). In all cases, we focused on axis at 5 mm.
Fig. 3.
(a) Field II simulated 108-element aperture and the on axis beam response from that aperture at (b) 5 MHz, (c) 7.5 MHz, and (d) 10 MHz. Focus is at 5 mm. The elevation only plot is shown for simplicity .
.
Fig. 3 shows that for both 5 and 7.5 MHz, there is no sign of a grating lobe for the on-axis steering case out to +/−80°. Although one is starting to show in the 10 MHz simulation, it is well outside our typical pyramidal scan angle of 65°. In each case, the side lobe levels of the annular aperture are high, reducing contrast in the final image. Going to higher frequencies clearly improves the beam width and brings the side lobes much closer to the main peak.
Fig. 4 shows the results from simulating the aperture in Fig 3(a) steered to 45° in both azimuth and elevation. Steering our beam ± 45° would result in a 90° degree scan angle, larger than our typical 65° scan angle. As we can see in Fig. 4, there is no sign of a grating lobe for the 5 MHz case. At 7.5 and 10 MHz, some energy away from the steered beam is starting to show up in the beam plot. However, this energy is 29 dB down from the main peak for the 7.5 MHz transducer and is 25 dB down for the 10 MHz case.
Fig. 4.
(a) Field II simulated 108-element aperture and the on axis beam response from that aperture at (b) 5 MHz, (c) 7.5 MHz, and (d) 10 MHz. Focus is at 5 mm. The elevation only plot is shown for simplicity.
For ease of fabrication, we started building our prototypes with the 54-element design operating at 5 MHz. We chose this option because we have the most experience with soldering at the 0.20 mm pitch of the Gore cables. We have also constructed both 5.0 and 8.9 MHz 108-element transducers on the 0.10 mm spacing.
C. Transducer Fabrication
We have previously reported our transducer fabrication techniques of using wire guides [1], [2], multilayer ceramic interconnects [35], or multilayer flexible interconnects [36], [37] for building matrix arrays. Although wire guides are suitable for prototyping work, they tend to be very labor intensive. Multilayer ceramic interconnects have excellent electrical properties, but poor acoustic ones, and tend to be very expensive. Flexible interconnects have improved acoustic properties over ceramic ones, but we have had difficulty obtaining them in very high-density situations. To build the ring transducers, we took a different approach.
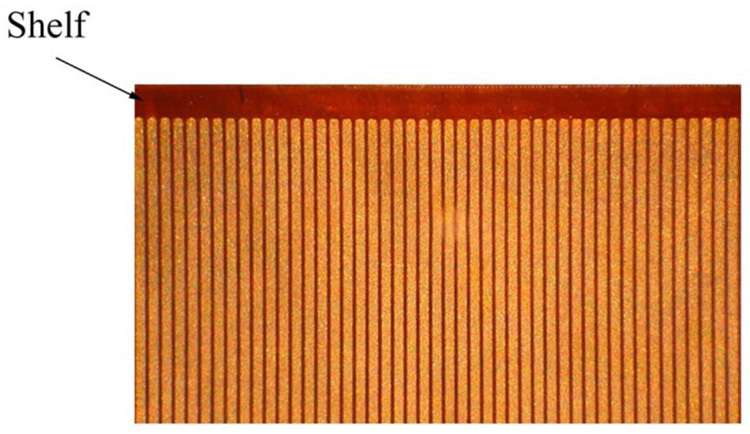
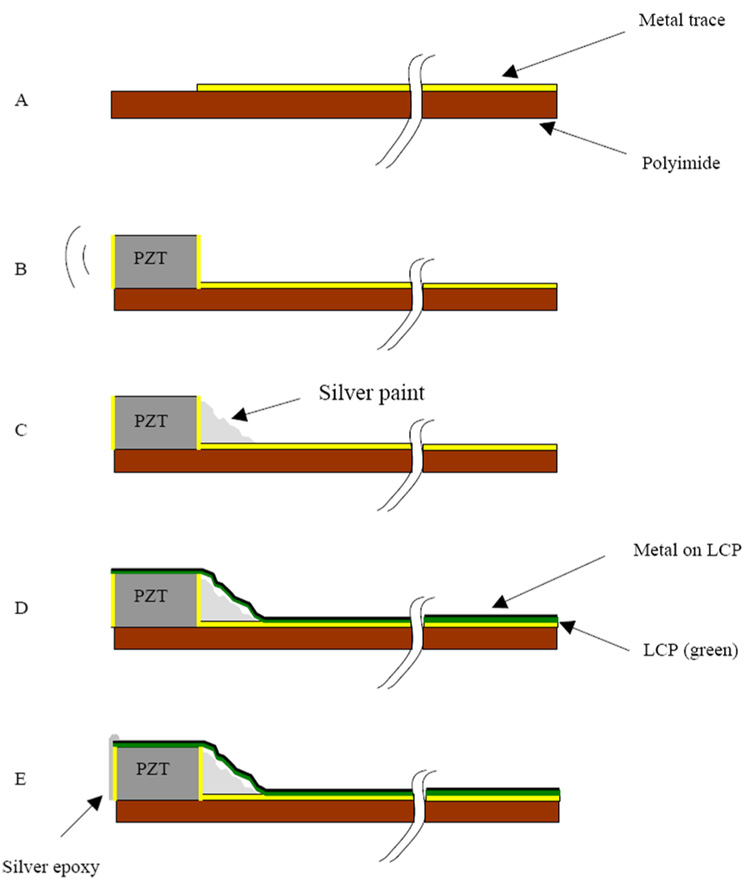
We started with 0.075 mm thick copper clad polyimide that was placed in a tinning solution to make it easier to solder to the traces later. The copper is an additional 0.025 mm thick. We used relatively thick polyimide for the first prototypes to make the dicing easier. The thicker substrate gives us a safety factor for the cut depth on the saw if the bonding to the carrier is not perfect. For the 54-element transducer, we used our diamond wheel dicing saw (Disco Hi-Tek America, Inc., Morrisville, NC) to cut the copper mechanically into traces at 0.20 mm spacing. An area at one end of the traces was prepared by removing all the copper, creating a shelf of polyimide on which the transducer elements would sit (Fig. 5). A beam of PZT ceramic (TRS Technologies, Inc., State College, PA ) was prepared that was 0.30 mm tall (thick), 0.18 mm wide, and 11 mm long. This beam was attached to the polyimide with a low viscosity epoxy (Epo-Tek 301, Epoxy Technology, Billerica, MA) such that the beam was on its side (Fig. 6). With the beam on its side, the electroded portions of the PZT are pointed in the forward direction. After attaching the PZT, silver paint was applied such that it contacted the bottom electrode of the PZT and the traces on the polyimide. The transducer beam was then diced. The whole structure was then wrapped around the 11 French catheter that is part of a Cook Medical Günther Tulip vena cava filter deployment kit. We attached the transducer using epoxy (Hysol E-60NC epoxy potting compound). The element kerfs were filled with the same epoxy. The MicroMiniature ribbon cables from W.L. Gore and Associates were then soldered to the traces and attached to the system cable for imaging. As described above, we used every other wire as a signal conductor and connected the interstitial wires to ground to improve electrical cross talk. A piece of 0.012 mm thick metallized liquid crystal polymer (LCP) was wrapped around the traces and the side of the PZT. The LCP is metallized on one side, and that side faces out so as not to contact the traces. Silver epoxy (Chomerics, Inc., Woburn, MA) was then applied to the face of the elements and to the metallized LCP and the grounded wires of the ribbon cables. This provided a front side ground to the PZT. Fig. 6 shows a step-by-step schematic of these processes.
Fig. 5.
A top view photograph showing a subset of metal traces on polyimide and the shelf area where the PZT is bonded. This part was made by MicroConnex, Inc., and the traces are on 0.10 mm spacing. The signal traces are the lighter color, the polyimide the darker .
.
Fig. 6.
Schematic showing the construction steps in building a ring-array transducer. (a) We start with a metallized polyimide substrate with an area without the metal to attach the PZT. (b) We next attach the PZT beam with nonconductive epoxy. (c) After the epoxy cures, silver paint is used to connect the back electrode of the PZT with the metal trace. The PZT is then diced and wrapped around the catheter lumen. (d) A 0.012 mm thick layer of liquid crystal polymer (LCP) is wrapped around the outer circumference of the PZT and polyimide substrate. This LCP layer is metallized with gold (shown in black) on one side only, the outer side, so that it does not risk shorting the traces together. (e) Finally, a layer of silver epoxy is spread on the face of the elements and connected to the gold on the outside of the LCP. This conductive layer is attached to the ground returns on the MicroMiniature ribbon cable from W. L. Gore and Associates .
.
For the 108-element 5MHz transducer, we started with 0.04 mm thick copper clad polyimide with laser-etched traces from MicroConnex, Inc. (Snoqualmie, WA), as shown in Fig. 5. The traces are spaced 0.10 mm center to center, and the parts included a shelf area without traces for attaching the PZT. Because we have a tighter interelement spacing, the PZT beam was 0.30 mm tall (thick), 0.10 mm wide × 11 mm long for the 5 MHz case. The attachment of the PZT was the same as the 54-element transducer. We diced the beam at the 0.10 mm spacing.
The 108-element, 8.9 MHZ transducer had only a few changes from the 5MHz 54-element transducer. The starting PZT beam had different dimensions: 0.183 mm tall (thick), 0.10 mm wide, and 11 mm long. It was also diced on 0.10 mm spacing. For both 108-element transducers, the MicroMiniature ribbon cables were soldered at the 0.10 mm pitch, none of the wires were connected to ground. Each ribbon included an integrated copper shield foil that was grounded. This shield was attached to metallized LCP to provide a ground connection to the front of the elements.
D. Transducer Measurement
We tested the transducers in a water tank by pulsing and receiving on a single element with a Model 5073PR pulser/receiver (Panametrics, Inc., Waltham, MA). The pulser was set for maximum amplitude. We had 39 dB of gain with the receiver and the low pass filter set with a 20 MHz cutoff. We used an aluminum block set at a depth of 1 cm as a reflector. We used the Panametrics Model 5605A Stepless Gate to gate the received pulse and then used a Model 3588A Spectrum Analyzer from Hewlett Packard (now Agilent Technologies, Inc., Santa Clara, CA) to look at the frequency response. A Model TDS 744A oscilloscope (Tektronix, Inc., Beaverton, OR) was used to display the received pulse. The pulse and spectrum was downloaded to a computer via a GPIB interface and Labview software (National Instruments, Inc., Austin, TX).
To measure the angular response, we used the same pulser/receiver and replaced the reflector with a point target. After aligning the transducer and the point target, we rotated the point in the circumferential direction in 2 degree steps. We took 30 measurements away from the peak in each direction, for 61 total data points.
E. Cook Medical, Inc. Devices
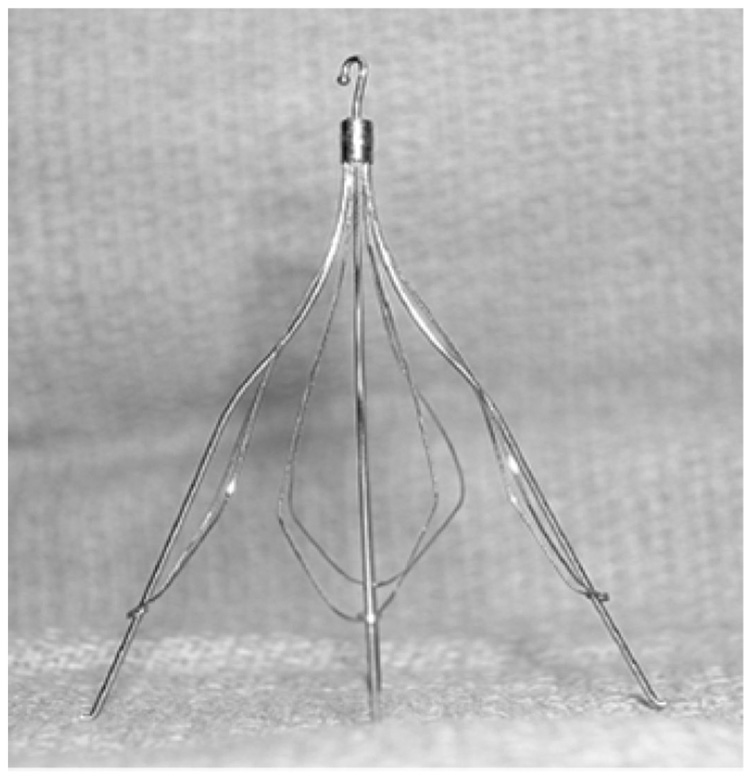
Fig. 7 shows a photograph of the Cook Medical Günther Tulip vena cava filter. The filter is made up of 4 main struts, each with fine petals that give it the characteristic tulip shape.
Fig. 7.
Photograph of Cook Medical Günther Tulip vena cava filter.
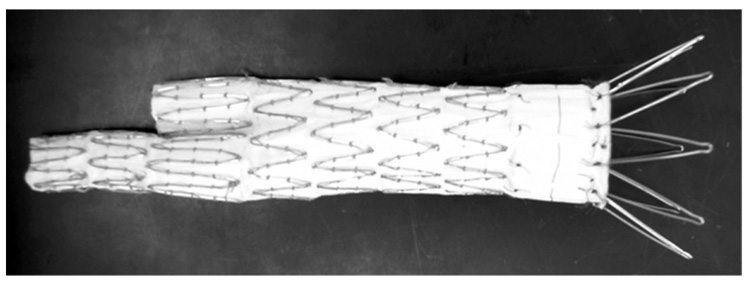
For the AA stent graft, we used the Cook Zenith AAA Endovascular Graft. The Zenith AAA Endovascular Graft [21] is a modular system consisting of 3 components, a bifurcated aortic main body, and 2 iliac legs (Fig. 8). The Zenith AAA Endovascular Graft main body is shipped preloaded onto the Introduction System. It has a sequential deployment method enabling positioning and allowing readjustment of the final graft position before deployment of the suprarenal barbs. The main body graft delivery system uses an 18 or 20 French catheter Introduction System.
Fig. 8.
Photograph of the Cook Zenith AAA Endovascular Graft.
III. RESULTS
A. 5MHz 54-Element Transducer
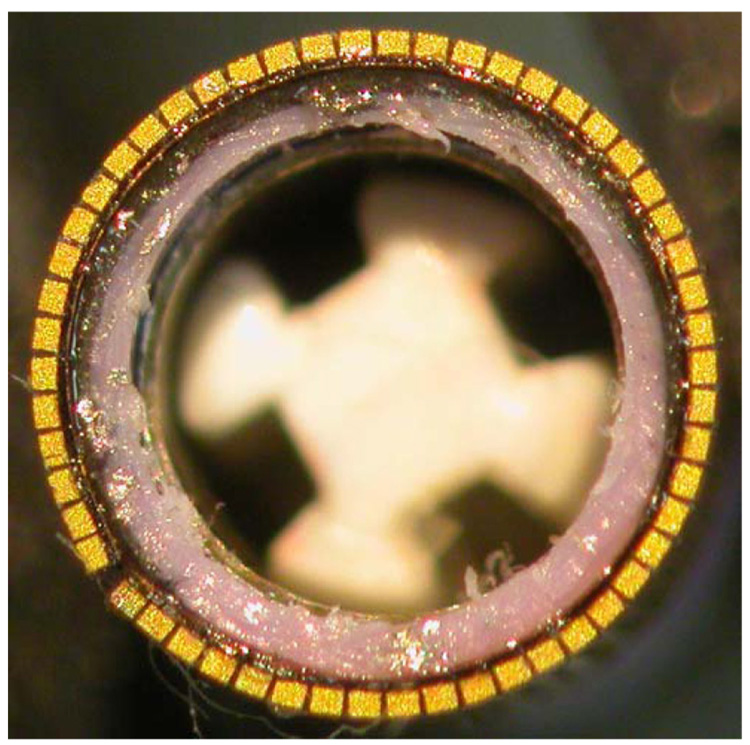
Fig. 9 shows a photograph of the 54-element ring array transducer after bonding to the catheter sheath, but before adding the electrical grounding. Fig. 10 shows the completed device, with the Cook Medical vena cava filter (labeled F in the photo) coming out the end of the catheter, but not deployed (open).
Fig. 9.
Closeup of ring array after dicing and bonding to the catheter lumen .
.
Fig. 10.
Completed integrated device showing the MicroMiniature ribbon cables (MC) and the Cook Medical vena cava filter (F) sticking out the lumen of the catheter .
.
Fig. 11 shows a typical pulse (a) and power spectrum (b) of this transducer. The center frequency is 4.9 MHz and the −6 dB bandwidth is 25%. There is essentially no acoustic backing behind the PZT elements and no matching layer in this prototype transducer. The average measured unloaded cross talk is −23.8 dB, and the round trip 50 Ohm insertion loss is −65 dB. Element yield was 96%.
Fig. 11.
(a) Typical pulse and (b) spectrum from the integrated transducer/VC filter deployment device. The center frequency is 4.9 MHz and the −6 dB bandwidth is 25%.
Fig. 12 shows a plot of the angular response of a single element from this transducer. The black circles and dashed lines represent the measured data, and the black line the theoretical. We measured the response in the radial direction, away from the center point of the ring. In this direction, we assume there is minimal impact on the angular response due to cross talk between elements.
Fig. 12.
Angular response of a single element (black circles and dashed line) from the 5 MHz, 54-element transducer plotted with the theoretical response (solid black line). These data were taken in the radial direction, not circumferential.
Fig. 13 shows a B-scan (a) and the simultaneous real-time inclined C-scan (b) of the filter (F, pictured in Fig. 7) in a water tank made with the 54-element integrated transducer/deployment kit and the 3-D scanner. The filter stands on a piece of rubber (R). Not only can we see the main struts (MS) of the filter in the C-scan image, but also the thinner wires that give this filter its tulip shape.
Fig. 13.
(a) A 7 cm deep B-scan and (b) a simultaneous real-time inclined C-scan of the vena cava filter (F) pictured in Fig. 7. The images were acquired in a water tank with a 54-element ring-array transducer integrated with the Cook Medical deployment kit. We see the filter (F) and rubber (R) on which it rests in both images. (b) also shows 3 of the main struts (MS) of the filter and the thinner wires that give it the tulip shape.
Fig. 14 shows results of a water tank experiment with simultaneous X-ray fluoroscopy and real-time 3-D ultrasound. The 54-element ring array transducer was positioned inside the Cook Medical aortic stent graft (labeled G in the figure). Agitated X-ray contrast agent was injected through the lumen of the catheter and imaged simultaneously in real-time with the X-ray system—see Fig. 14(a)—and the 3-D ultrasound system, which shows a cross-sectional volume rendered view of the graft bifurcation—see Fig. 14(b). The X-ray image seen in Fig. 14(a) shows the graft (G), the catheter (I), the tip of the transducer (T), and the jet of the X-ray contrast agent (CF).
Fig. 14.
(a) Simultaneous X-ray and (c)–(d) ultrasound images of agitated X-ray contrast. The X-ray image shows the Cook Medical aortic stent graft (G), our completed catheter (I) with its transducer tip (T), and the flow of the X-ray contrast (CF). (b) shows a cross-sectional rendered view of the stent graft (G) made with the 54-element 5 MHz transducer. Fig. 14(c) shows a 4 cm deep B-scan with color flow Doppler of the agitated contrast showing flow away from the transducer. (d) is a C-scan plane showing the 2 bifurcated legs of the graft (G) and Doppler flow away from the transducer. The plane was taken at the level indicated by the arrows in Fig. 14(c) .
.
In addition, in Fig. 14(c) we show 3-D color Doppler images of the contrast agent flowing through the graft including a 4 cm deep B-scan with flow away from the transducer and into one of the legs of the graft. In Fig. 14(d) we show the corresponding real-time C-scan showing flow in both legs of the graft taken at the plane indicated by the arrows. These images were produced simultaneously with Fig. 14(a) but not with Fig. 14(b).
B. 108-Element Transducers
Fig. 15 shows a photograph of the front of the prototype 8.9 MHz 108-element ring array. The elements have been diced and the transducer bonded to the catheter lumen. Because we use the same beam length (not thickness) and interelement spacing, the 5 MHz 108-element looks the same from this perspective. It is easy to see how much smaller the elements are compared with the 54-element array shown in Fig. 9. Fig. 16 shows typical pulse and spectrum from the 5 MHz 108-element ring array transducer. Fig. 17 shows a typical pulse and spectrum from the 8.9 MHz ring array transducer.
Fig. 15.
Photograph of the 108-element 8.9 MHz ring array after dicing and bonding to the 11 French catheter of the Cook Medical vena cava filter deployment kit .
.
Fig. 16.
(a) Typical pulse and (b) spectrum from the 5 MHz 108-element ring-array transducer. The center frequency is 4.7 MHz and the −6 dB bandwidth is 21%.
Fig. 17.
(a) Typical pulse and (b) spectrum from the 8.9 MHz 108-element ring array transducer. The center frequency is 8.9 MHz and the −6 dB bandwidth is 26%.
Because both of the 108-element arrays were initial prototypes, element yield in the completed transducers was lower than in the 54-element transducer. We had about 60% working elements for the 5 MHz 108-element transducer and 55% for the 8.9 MHz transducer. The average cross talk for the 5MHz 108-element transducer was −18.6 dB and −19.9 dB for the 8.9 MHz transducer.
IV. DISCUSSION
We successfully completed a working 5 MHz 54-element ring array transducer for real-time 3-D intravascular ultrasound and guidance of interventional devices. The images with this transducer are very promising. Although they are taken in a water tank and not in vivo, we can clearly see the major and minor struts of the Cook vena cava filter. The images in Fig. 13 look very much like the photograph shown in Fig. 7. Combined with our previous success imaging IVC filters in vivo with other catheter transducers we built [38], we are optimistic this transducer design will provide a solution for image guidance of these interventional devices.
The images in Fig. 14 of the stent graft are equally promising. To our knowledge, it is the first time a fluoroscopic contrast image was acquired simultaneously with 3-D ultrasound Doppler flow of the same contrast. We can clearly see the flow in the fluoroscopic image in Fig. 14(a), the B-scan in Fig. 14(c), and the C-scan in Fig. 14(d).
Both the 5 MHz transducers had a center frequency near their design frequencies. However, the 8.9 MHz transducer was originally designed for 7 MHz based on previous transducers [9]. The actual center frequency is 8.9 MHz. with a −6 dB bandwidth of 26%. Given the way these elements were constructed, we would need a 2-D simulation package to try to determine why this is. We suspect that the polyimide bonded to the side of the element has some effect on the resonance. The thickness of this polyimide is approximately 0.075 mm. The speed of sound for polyimide is 2200 m/s. Assuming the polyimide is a quarter wavelength resonator while attached to the PZT, this works out to a frequency of 7.3 MHz. There is a sharp null around 7 MHz in the spectrum for both this transducer and the 54-element 5 MHz transducer, also built on 0.075 mm polyimide. The 108-element 5 MHz transducer was built on the polyimide from MicroConnex, which was 0.04 mm thick. Unfortunately, the magnitude is so small it is hard to see the response at this frequency. However, this pulse is more symmetric than the 54-element transducer of the same frequency. We suspect that the polyimide is resonating out of phase with the PZT at this frequency, affecting the resulting pulse frequency.
Looking at the angular response for the 54-element transducer in Fig. 12, we see that we do not quite match the theoretical plot for an element of this size. The theoretical plot was based on the equation
where x is {(π * element size)/λ}sin(θ), λ is the wave-length in mm and θ is the angle of incidence with respect to the transducer face. These data were taken in the radial direction, the direction in which the element is bonded to the polyimide. Assuming that the element is well connected to the polyimide, we believe that the polyimide has effectively increased the size of the element from 0.18 mm to 0.255. Re-running the theoretical data with this element thickness, we obtain the plot shown in Fig. 18. We get much better agreement with these data, leading us to believe that the polyimide is effectively increasing the radial size of our elements.
Fig. 18.
Angular response of a single element (black circles and dashed line) from the 5 MHz, 54-element transducer plotted with the new theoretical response (solid black line). These new theoretical data assume a larger element size due to acoustic coupling with the 0.075 mm thick polyimide layer.
There is still room for much improvement in the ring transducers. For all the transducers the bandwidths were narrow, but this is expected in an element that is essentially air backed and has no matching layer. We have described previously matching layers from conductive materials when fabricating 2-D array elements [3]. The reason for using conductive materials is that it is difficult to access any part other than the face and the back of individual elements in a matrix array. One advantage of the ring configuration from a construction standpoint is that we have access to the side as well as the face and back of the 2-D elements. This should allow us to develop matching layers from nonconductive materials. Insulating the side of the PZT beam with a material such as parylene, we can then bring the top electrode part of the way down the side of the elements. This would allow an electrical connection to the front of the PZT element. Parylene has a very low dielectric constant (about 3), so any electricfield in a 5 µm layer should be very low. Matching layers of any material with the desired acoustic properties can be bonded to the face of the beam. In a batch style of manufacturing, the desired matching materials could be bonded to the face of a large plate of PZT material. The beams could be diced from this bulk material and bonded to the flex circuit as described in this work. After the silver paint is applied to connect to the back side of the elements to the traces, parylene can be sputtered onto the side of the beam that is showing. The edge of the top electrode would need to be masked off carefully in this process. Applying another mask and adding a conductive metal layer would then make electrical connection to the face electrode on the PZT. The desired backing material could then be castin place before dicing.
We also clearly have more work to do to improve the yield of the 108-element transducers. The 54-element transducer was not the first of it kind [39] and has a high yield for a research prototype. Both the 108-element transducers were first-run prototypes, and their poor yield reflects this. The 108-element arrays will increase the SNR of the device as a whole. Our Field II simulations show that we push the clutter floor down approximately 6 dB by doubling the number of elements. This will give us better contrast resolution, an important feature when trying to determine the surrounding anatomy of the inferior vena cava near the renal veins.
ACKNOWLEDGMENT
This research was supported by NIH grants HL 72840 and HL 64962, DTRI grant 1UL1 RR024128-01, and Cook Medical Inc.
We wish to acknowledge helpful conversations with Paul Suhocki.
Biographies

Edward D. Light (M’00) was born in Charlottesville,VA, in 1967. He received a B.S.E. degree in Biomedical Engineering and an M.S. in Biomedical Engineering in 1989 and 1997, respectively, both from Duke University, Durham, NC.
Since 1989 he has worked as an R&D Engineer at Duke, developing 2-D arrays for real-time volumetric imaging. He holds several patents in the field of catheter-based ultrasound imaging. He is currently pursuing his research interests in novel applications of 2-D arrays to the areas of catheter-based and endoscopic-based ultrasound imaging.

John “Fritz” Angle obtained his bachelor’s degree in electrical engineering from Cornell University in 1982. He did his Radiology Residency at the University of Missouri at Kansas City, and graduated from the University of Nebraska Medical College in 1986. Dr. Angle came to the University of Virginia in 1991 and completed his fellowship training in 1992. He is currently Associate Professor of Radiology at the University of Virginia, where he is Director of the Division of Interventional Radiology. He has authored over 65 peer reviewed articles and 75 abstracts.

Stephen W. Smith (M’91) was born in Covington, KY, on July 27, 1947. He received the B.A. degree in physics (summa cum laude) in 1967 from Thomas More College, Ft. Mitchell, KY, the M.S. degree in physics in 1969 from Iowa State University, Ames, and the Ph.D. degree in biomedical engineering in 1975 from Duke University, Durham, NC.
In 1969, he became a Commissioned Officer in the U.S. Public Health Service, assigned to the Food and Drug Administration, Center for Devices and Radiological Health, Rockville, MD, where he worked until 1990 in the study of medical imaging, particularly diagnostic ultrasound and in the development of performance standards for such equipment. In 1978, he became an adjunct associate professor of radiology at Duke University Medical Center. In 1990, he became an associate professor of biomedical engineering and radiology, and Director of Undergraduate Studies in Biomedical Engineering at Duke University. He holds 16 patents in medical ultrasound and has authored more than 100 publications in the field.
Dr. Smith is cofounder of Volumetrics Medical Imaging. He has served on the education committee of the American Institute of Ultrasound in Medicine, the executive board of the American Registry of Diagnostic Medical Sonographers, the editorial board of Ultrasonic Imaging, and the Technical Program Committee of IEEE-UFFC. He was co-recipient of the American Institute of Ultrasound in Medicine Matzuk Award in 1988 and 1990 and co-recipient of the IEEE-UFFC Outstanding Paper Award in 1983 and 1994.
Contributor Information
Edward D. Light, Department of Biomedical Engineering, Duke University, Durham, NC (e-mail: edl@duke.edu)..
John F. Angle, Department of Radiology, University of Virginia, Charlottesville, VA.
Stephen W. Smith, Department of Biomedical Engineering, Duke University, Durham, NC..
REFERENCES
- 1.Smith SW, Trahey GE, von Ramm OT. Two-dimensional arrays for medical ultrasound. Ultrason. Imag. 1992;vol. 14(no 3):213–233. doi: 10.1177/016173469201400301. [DOI] [PubMed] [Google Scholar]
- 2.Light ED, Davidsen RE, Hrushka TA, Smith SW. Progress in two-dimensional arrays for real time volumetric imaging. Ultrason. Imag. 1998;vol. 20(no 1):1–16. doi: 10.1177/016173469802000101. [DOI] [PubMed] [Google Scholar]
- 3.Light ED, Fiering JO, Hultman PA, Lee W, Smith SW. Update of two dimensional arrays for real time volumetric and real time intracardiac imaging; Proc. IEEE Trans. Ultrasonics Symp; 1999. pp. 1217–1220. [Google Scholar]
- 4.Light ED, Idriss SF, Wolf PD, Smith SW. Real time three dimensional intracardiac echocardiography. Ultrasound Med. Biol. 2001;vol. 27(no 9):1177–1183. doi: 10.1016/s0301-5629(01)00421-5. [DOI] [PubMed] [Google Scholar]
- 5.Lee W, Idriss SF, Wolf PD, Smith SW. A miniaturized catheter 2-D array for real-time 3D intracardiac echocardiography. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 2004;vol. 51(no 10):1334–1346. doi: 10.1109/tuffc.2004.1350962. [DOI] [PubMed] [Google Scholar]
- 6.Light ED, Smith SW. Two-dimensional arrays for real-time 3D intra-luminal ultrasound imaging; 30th Symp. Ultrasonic Imaging and Tissue Characterization; 2005. [Google Scholar]
- 7.Pua EC, Idriss SF, Wolf PD, Smith SW. Real-time 3D transesophageal echocardiography. Ultrason. Imag. 2004;vol. 27(no 4):217–232. doi: 10.1177/016173460402600402. [DOI] [PubMed] [Google Scholar]
- 8.Light ED, Mukundan S, Wolf PD, Smith SW. Real-time 3D intracranial ultrasound. Ultrasound Med. Biol. 2007;vol. 33(no 8):1277–1284. doi: 10.1016/j.ultrasmedbio.2007.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Light ED, Idriss SF, Sullivan KF, Wolf PD, Smith SW. Real-time 3D ultrasonic laparoscopy. Ultrason. Imag. 2005;vol. 27(no 3):129–144. doi: 10.1177/016173460502700301. [DOI] [PubMed] [Google Scholar]
- 10.Kaufman JA. Vascular and Interventional Radiology. Philadelphia, PA: Mosby; 2004. [Google Scholar]
- 11.Chiou AC, Biggs KL, Matsumura JS. Vena cava filters: why, when, what, how? Perspect. Vasc. Surg. Endovasc. Ther. 2005;vol. 17(no 4):329–339. doi: 10.1177/153100350501700407. [DOI] [PubMed] [Google Scholar]
- 12.Ansell J. Vena cava filters: do we know all that we need to know? Circulation. 2005;vol. 112(no 3):298–299. doi: 10.1161/CIRCULATIONAHA.105.547828. [DOI] [PubMed] [Google Scholar]
- 13.Neuzil DF, Garrard CL, Berkman RA, Pierce R, Naslund TC. Duplex-directed vena caval filter placement: report of initial experience. Surgery. 1998;vol. 123(no 4):470–474. [PubMed] [Google Scholar]
- 14.Sato DT, Robinson KD, Gregory RT, Gayle RG, Parent FN, DeMasi RJ, Meier GH, Sorrell KA, Goff CD, Weireter LJ, Jr, Riblet JL. Duplex directed caval filter insertion in multi-trauma and critically ill patients. Ann. Vasc. Surg. 1999;vol. 13(no 4):365–371. doi: 10.1007/s100169900270. [DOI] [PubMed] [Google Scholar]
- 15.Oppat WF, Chiou AC, Matsumura JS, Pearce WH. Intravascular ultrasound-guided vena cava filter placement. J. Endovasc. Surg. 1999;vol. 6(no 3):285–287. doi: 10.1177/152660289900600312. [DOI] [PubMed] [Google Scholar]
- 16.Ebaugh JL, Chiou AC, Morasch MD, Matsumura JS, Pearce WH. Bedside vena cava filter placement guided with intravascular ultrasound. J. Vasc. Surg. 2001;vol. 34(no 1):21–26. doi: 10.1067/mva.2001.115599. [DOI] [PubMed] [Google Scholar]
- 17.Sheehan MK, Marone L, Makaroun MS. Use of endoluminal aortic stent-grafts for the repair of abdominal aortic aneurysms. Perspect. Vasc. Surg. Endovasc. Ther. 2005;vol. 17(no 4):289–296. doi: 10.1177/153100350501700403. [DOI] [PubMed] [Google Scholar]
- 18.Upchurch GR, Jr, Schaub TA. Abdominal aortic aneurysm. Am. Fam. Physician. 2006;vol. 73(no 7):1195–1204. [PubMed] [Google Scholar]
- 19.Flemming C, Whitlock E, Beil T, Lederle F. Screening for abdominal aortic aneurysm: A best-evidence systematic review for the U.S. preventative task force. Ann. Intern. Med. 2005;vol. 142(no 3):203–211. doi: 10.7326/0003-4819-142-3-200502010-00012. [DOI] [PubMed] [Google Scholar]
- 20.Muhs BE, Verhoeven EL, Zeebregts CJ, Tielliu IF, Prins TR, Verhagen HJ, van den Dungen JJ. Mid-term results of endovascular aneurysm repair with branched and fenestrated endografts. J. Vasc. Surg. 2006;vol. 44(no 1):9–15. doi: 10.1016/j.jvs.2006.02.056. [DOI] [PubMed] [Google Scholar]
- 21.Cook Medical, Inc. 2006 [Online]. Available: http://www.cookmedical.com/ai/content/mmedia/T_ZAA_REV0.pdf,
- 22.Smith SW, Pavy HE, von Ramm OT. High speed ultrasound volumetric imaging system part I: Transducer design and beam steering. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 1991;vol. 32(no 2):100–108. doi: 10.1109/58.68466. [DOI] [PubMed] [Google Scholar]
- 23.von Ramm OT, Smith SW, Pavy HE. High speed ultra-sound volumetric imaging system part II: Parallel processing and display. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 1991;vol. 38(no 2):109–115. doi: 10.1109/58.68467. [DOI] [PubMed] [Google Scholar]
- 24.Schmidt MA, Ohazama CJ, Agyeman KO, Freidlin RZ, Jones M, Laurienzo JM, Brenneman CL, Arai AE, von Ramm OT, Panza JA. Real-time three-dimensional echocardiography for measurement of left ventricular volumes. Am. J. Cardiol. 1999;vol. 84(no 12):1434–1439. doi: 10.1016/s0002-9149(99)00591-3. [DOI] [PubMed] [Google Scholar]
- 25.Camarano G, Jones M, Freidlin RZ, Panza JA. Quantitative assessment of left ventricular perfusion defects using real-time three-dimensional myocardial contrast echocardiography. J. Am. Soc. Echocardiogr. 2002;vol. 15(no 3):206–213. doi: 10.1067/mje.2002.117338. [DOI] [PubMed] [Google Scholar]
- 26.Ahmad M, Xie TR, McCulloch M, Abreo G, Runge M. Real-time three-dimensional dobutamine stress echocardiography in assessment of ischemia: Comparison with two-dimensional dobutamine stress echocardiography. J. Am. Coll. Cardiol. 2001;vol. 37(no 5):1303–1309. doi: 10.1016/s0735-1097(01)01159-7. [DOI] [PubMed] [Google Scholar]
- 27.Tsujino H, Jones M, Shiota T, Oin JX, Greenberg NL, Cardon LA, Morehead AJ, Zetts AD, Travaglini A, Bauer F, Panza JA, Thomas JD. Real-time three-dimensional color Doppler echocardiography for characterizing the spatial velocity distribution and quantifying the peak flow rate in the left ventricular outflow tract. Ultrasound Med. Biol. 2001;vol. 27(no 1):69–74. doi: 10.1016/s0301-5629(00)00270-2. [DOI] [PubMed] [Google Scholar]
- 28.McCreery CJ, McCulloch M, Ahmad M, deFilippi CR. Real-time 3-dimensional echocardiography imaging for right ventricular endomyocardial biopsy: A comparison with fluoroscopy. J. Am. Soc. Echocardiogr. 2001;vol. 14(no 9):927–933. doi: 10.1067/mje.2001.113651. [DOI] [PubMed] [Google Scholar]
- 29.Fleishman CE, Li J, Ota T, Ohazama CJ, Stetten G, Adams D, von Ramm OT, Kisslo J. Identification of congenital heart defects using real time three-dimensional echo in pediatric patients. Circulation. 1996;vol. 94(no 8) suppl. I:416. [Google Scholar]
- 30.Light ED, Smith SW. Two dimensional arrays for real time 3D intravascular ultrasound. Ultrason. Imag. 2004;vol. 26(no 2):115–128. doi: 10.1177/016173460402600204. [DOI] [PubMed] [Google Scholar]
- 31.Wang Y, Stephens DN, O’Donnell M. A forward-viewing ring-annular ultrasound array for intravascular imaging; Proc. IEEE Ultrasonics Symp; 2001. pp. 1573–1576. [DOI] [PubMed] [Google Scholar]
- 32.Degertekin FL, Guldiken RO, Karaman M. Annular-ring CMUT arrays for forward-looking IVUS: Transducer characterization and imaging. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 2006;vol. 53(no 2):474–482. doi: 10.1109/tuffc.2006.1593387. [DOI] [PubMed] [Google Scholar]
- 33.Ullate LG, Godoy G, Martinez O, Sanchez T. Beam steering with segmented annular arrays. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 2006;vol. 53(no 10):1944–1954. [PubMed] [Google Scholar]
- 34.Jensen JA, Svensen NB. Calculation of pressure fields from arbitrarily shaped, apodized, and excited ultrasound transducers. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 1992;vol. 32(no 2):262–267. doi: 10.1109/58.139123. [DOI] [PubMed] [Google Scholar]
- 35.Smith SW, Light ED. Two-dimensional array transducers using thick film connection technology. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 1993;vol. 40(no 6):727–734. doi: 10.1109/58.248217. [DOI] [PubMed] [Google Scholar]
- 36.Fiering JO, Hultman P, Lee W, Light ED, Smith SW. High-density flexible interconnect for two-dimensional ultrasound arrays. IEEE Trans. Ultrason., Ferroelect., Freq. Contr. 2000;vol. 47(no 3):764–770. doi: 10.1109/58.842067. [DOI] [PubMed] [Google Scholar]
- 37.Strole J, Corbett S, Lee W, Light ED, Smith SW. A novel flex circuit area-array interconnect system for a catheter-based ultra sound transducer; Proc. IMAPS Int. MicroElectronic Packing Society Meeting; 2002. p. 2002. [Google Scholar]
- 38.Light ED, Angle JF, Smith SW. Advances in two dimensional arrays for real time 3D intravascular ultrasound; Proc. IEEE Trans. Ultrason. Symp; 2004. pp. 790–793. [DOI] [PubMed] [Google Scholar]
- 39.Light ED, Mukunda S, Wolf PD, Smith SW. Two dimensional arrays for real-time intracranial imaging of the brain; Proc. IEEE Trans. Ultrason. Symp; 2006. pp. 70–73. [Google Scholar]