Abstract
Tetrabenazine (TBZ), a catecholamine-depleting agent initially developed for the treatment of schizophrenia, when tested for other indications, has proven to be more useful for the treatment of a variety of hyperkinetic movement disorders. These disorders include neurological diseases characterized by abnormal involuntary movements such as chorea associated with Huntington’s disease, tics in Tourette’s syndrome, dyskinesias and dystonias in tardive dyskinesia, also primary dystonias and myoclonus. This review will include and discuss studies published during the period of 1960–2006 regarding the clinical efficacy and tolerability of TBZ in Huntington’s disease (HD). It will also review the chemistry, pharmacokinetics and dynamics of the drug and its mechanism of action compared to that of reserpine, the only similar compound. This review emphasizes the advantage of TBZ over dopamine-depleting compounds used in the treatment of chorea and reveals its clinical efficacy and side effects.
Keywords: tetrabenazine, Huntington’s disease, chorea
Introduction
Tetrabenazine (TBZ) was initially synthesized in the 1950s by O Schneider and A Brossi at the research laboratory of Hoffmann-La Roche in Basel. They created TBZ as an antipsychotic drug as part of their research into simpler chemical compounds with reserpine-like activity. TBZ was studied in a number of controlled trials in schizophrenia patients with equivocal results (Smith 1960; Weckorciez 1960; Kanjilal 1962). Later clinicians came to favor the use of dopamine receptor blocking drugs (DRBD), like phenothiazines, for treating psychosis given the evidence of better efficacy (Ashcroft 1961). However, as with many other drugs, when TBZ was tested for indications other than the original ones the preliminary results provided support for its use in disorders characterized by abnormal hyperkinetic involuntary movements, drug induced or primary. During the last two decades TBZ has been used in a multitude of movement disorders: tardive dyskinesia (Fahn 1985; Ondo 1999; Simpson 2000; Tarsy 2000) dystonia (Manji 1998; Raja 1998; Scott 2000) and tremor (Storey 1997), choreic syndromes (Gimenez-Rodan 1989; Ondo 2002), primary dystonia (Bartels 1984; Faulstich 1985; Manji 1998; Boghen 2000; Scott 2000), secondary dystonia (Duran 2001) and tic disorders (Vieregge 1987; Jankovic 1988; Scahill 2000).
Chemistry
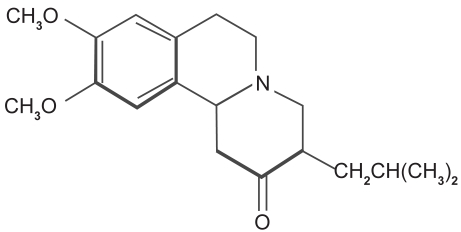
TBZ, also named Ro 1-9569 by Hoffmann-LaRoche, Inc., is a benzoquinolizine derivative with the following formula, 2-oxo-3-isobutyl-9,10-dimethoxy-1,2,3,4,6,7-hexahydrobenzo[a]quinolizine. It is a white crystalline substance with a bitter taste. The molecule is shown in Figure 1. It can be isolated from alkalinized biological material by extraction into heptane into diluted hydrochloric acid and assayed fluorometrically (Quinn 1959). Chromatographic analysis of TBZ reveals peak fluorescence at 282 nm (Roberts MS 1981). Based on the acid-basic transition, TBZ is characterized by a pKa of 6.0 (Scherman 1982).
Figure 1.
Tetrabenazine, a benzoquinolizine derivative with the chemical name, 2-oxo-3-isobutyl-9,10-dimethoxy-1,2,3,4,6,7-hexahydrobenzo[a]quinolizine.
Pharmacodynamics and pharmacokinetics
TBZ is readily absorbed from the intestinal tract. After intravenous administration of TBZ tagged with tritium in humans, 54% was excreted in urine after 48 hours (Stumpf 1960).
TBZ enters the rabbit brain rapidly after intravenous administration attaining maximal levels of about 34 micrograms per gram within 10 minutes. In other tissues maximal concentration is seen within 15–30 minutes, latest in fat tissue (with concentrations in fat tissue coming last). The half life in various tissues (when given intravenously to rabbits) ranges from 0.9–2.7 hours, with the longest half-life being in fat tissue. After 24 hours, TBZ will have disappeared from most tissues, brain tissue included (Quinn 1959). TBZ has a relatively low bioavailability, 0.049 ± 0.032 (Roberts 1986).
TBZ is metabolized into two compounds: α- and β-dihydrotetrabenazine (DTBZ); α-DTBZ being the active compound, whereas β-DTBZ is biochemically inert. α-DTBZ has a high bioavailability because it is less protein-bound (44%–59%) compared to TBZ (83%–88%) (Roberts 1986). α-DTBZ has higher plasma levels than TBZ and the half-life is longer, 10 hours versus 6 hours (in humans) (Roberts 1986). Therefore it has to be administered two to three times a day.
Acute toxicity in mice LD 50 is found to be 150 mg/kg by intravenous injection, 400 mg/kg by subcutaneous injection and about 550 mg/kg by oral administration, being about ten times less toxic than reserpine, another cathecolamine depletor). Toxic doses produce spasms with respiratory inhibition and opisthotonus. Chronic toxicity observed in rats given a daily dose of 8–15 mg/kg daily in their food for 13 weeks was well tolerated and growth was little influenced and neither blood nor organ examinations showed any abnormality (Lingierde 1963). TBZ crosses the placenta but no case of teratogenicity has been reported in humans. It is also excreted in breast milk and therefore breastfeeding should be avoided while taking TBZ (Roberts 1986).
Mechanism of action
The mechanism of action of TBZ is well known (Pettibone et al 1984). It acts mainly as a reversible high affinity inhibitor of monoamine uptake into granular vesicles of presynaptic neurons and secondary depletion at low doses, as well as a weak D2 postsynaptic receptor blocker in high doses (Reches 1983) TBZ depletes all three monoamines, but particularly dopamine (Pletscher et al 1962). One in vivo study of rats showed that TBZ decreased dopamine levels by 40%, serotonin by 44%, and norepinephrine by 41% in the brain (Lane et al 1976).
The effect of a seven-day repetitive administration of TBZ in rats was investigated by examining the locomotive behavior and histomorphological findings of substantia nigra in rats. These studies compared the effect of a single dose of TBZ which caused decrease of voluntary movements and no histological changes versus repetitive administration of TBZ which demonstrated irreversible and significant changes in spontaneous locomotion as well as histological changes in the neurons of the substantia nigra pars compacta. The results suggest that this could be a new and useful model for the behavioral characteristics and anatomical pathology of Parkinson’s disease as one of the oxidative stress models induced by abnormal dopamine metabolism (Satou et al 2001).
In an autopsy study of Huntington’s disease (HD) patients, those patients who had received TBZ displayed a greater overall depletion of monoamines than patients not exposed to TBZ, primarily in the caudate, but also, to a lesser degree, in the amygdala, hippocampus, and temporal lobe (Pearson and Reynolds 1988). PET studies w/11-C-raclopride show a 28% reduction in striatal binding after TBZ.
α-DTBZ has been shown to inhibit monoamine uptake driven by a transmembrane proton elecrochemical gradient generated by an ATP-ase proton pump using a special transporter (VMAT). In rat fibroblasts the central neural transporter VMAT2 is inhibited by TBZ, but the peripheral endocrine specific VMAT1 is not (Masuo et al 1990; Erickson et al 1996). VMAT2 is a large protein with 12 transmembrane helices encoded by the VMAT2 gene localized to chromosome 10q25. VMAT2 is expressed primarily in the brain. VMAT1 is encoded by a gene on chromosome 8p21.3 and is expressed in the periphery (Gonzalez et al 1994).
The dual effects of TBZ are thought to be responsible both for its therapeutic effects as well as its side effects.
Tetrabenazine versus reserpine
The pharmacologic agent most similar to TBZ is reserpine (R). Both TBZ and R seem to reduce cells’ capacity to store monoamines, therefore causing a depletion of the brain storage of these amines and in the case of Reserpine a depletion of the amines in the peripheral sites. Both drugs act centrally on VMAT2, but reserpine also inhibits VMAT1 peripherally, which may explain the higher frequency of hypotension and gastrointestinal side effects it causes. While reserpine binds irreversibly to both VMAT’s, TBZ binds reversibly only to VMAT2. TBZ displays a much shorter half life than reserpine (hours as opposed to days) and has a more rapid onset of action. This confers an advantage on TBZ in the clinical setting as its efficacy can be assessed more rapidly and its side effects abate more rapidly upon discontinuation of drug usage. Differences summarized in Table 1.
Table 1.
Difference between tetrabenazine and reserpine
| Tetrabenazine | Reserpine | |
|---|---|---|
| Monoamine depletion | Central through VMAT2 | Central and peripheral through VMAT1 and VMAT2 |
| Binding | Reversible | Non-reversible |
| Post-synaptic effects | YES(weak D2 blocker at high dose) | NO |
| T1/2 | 10 hrs | Several days |
| Side effects | NO | YES(gastrointestinal and hypotension) |
Huntington’s disease
HD is a genetic neurological disease, which manifests a triad of psychiatric, cognitive and movement disorders. HD is inherited in an autosomal dominant fashion with complete penetrance. The prevalence rate in the United States has been estimated at 5–10 per 100,000 (Jancovic et al 1995). Genetic studies of families with a very high prevalence and incidence of HD from the Lake Maracaibo area in Venezuela led to the discovery of an unstable trinucleotide (CAG) repeat present in the gene on chromosome 4. The number of CAG repeats in normal subjects is up to 29 repeats, while the presence of 36 or more CAG repeats ensures the development of HD (The Huntington’s Disease Collaborative Research Group 1993). The phenomena of anticipation and paternal imprinting have been well described in HD. The disease often presents with psychiatric problems or one type of hyperkinetic movement disorder, usually chorea. HD patients slowly progress over 15–20 years to a bedridden state. In advanced stages functional disability develops with dysphagia, dysarthria, prominent chorea with motor impersistence and in advanced stages a hypokinetic rigid state accompanied by dementia, depression and psychosis. HD represents the neurological disease with the highest rates of depression and suicide.
Unfortunately there is no treatment that can cure or slow the course of the disease. Only modest symptomatic treatment options exist for those suffering from HD, mostly focused on ameliorating depression, psychosis, and chorea. Many therapeutical modalities, most of them including dopamine receptor blockers or dopamine depletors have been evaluated over the years, most of them in open label studies or presented as case reports. Few randomized double-blind studies with TBZ have been done in HD. (Mc Lellan et al 1974; Sajatovic et al 1991; Van Vugt et al 1997; Huntington Study Group 2006).
Huntington’s disease and tetrabenazine treatment
One of the main reasons for using TBZ instead of dopamine receptor blockers is its relative safety, exemplified by the fact that TBZ has never been documented as having caused tardive dyskinesia (TD). This is a major advantage of TBZ over the typical neuroleptics, since between 25%–40% of those chronically treated with DRBD eventually develop TD (Smith and Baldessarini 1980).
The first reports of the therapeutic potential of TBZ in patients with HD were published in the 1960’s (Brandrup 1960; Sattes 1960; Sattes and Hase 1964). Since the 1970’s, numerous other clinical trials with relatively small numbers of patients have demonstrated the beneficial effects of TBZ for patients with chorea (Fog and Pakkenberg 1970; Gilligan et al 1972; Swash et al 1972; Huang 1976; Toglia et al 1978; Kingston 1979; Asher and Aminoff 1979; Jancovic 1982, 1983; Jancovic and Orman 1988; Gimenez-Roldan and Mateo 1989; Jancovic and Beach 1997; Ondo et al 2002; Paleacu et al 2004; Kenney and Jancovic 2006).
TBZ was approved for the treatment of chorea in the United Kingdom in 1971. It is also available in Canada and Australia, as well as in several European countries. TBZ is still unavailable in the United States, though it has been obtained by selected physicians via the Notice of Claimed Investigational Exemption for a New Drug (IND). However, in spite of its low availability, several long-term studies of TBZ in movement disorders, including HD, have been reported from the US.
There have been five major studies, which assessed the long-term efficacy of TBZ in chorea patients, most of them with HD. The most recent, a phase-III study assessing the safety, efficacy, and dose-tolerability of TBZ for ameliorating chorea in 84 patients with HD, was published by the Huntington Study Group (HSG) in 2006. HD patients were randomized to placebo (n = 30) or TBZ (n = 54) up to 100 mg/day at a titration rate of 12.5 mg per week. Based on the chorea score of the Unified Huntington Disease Rating Scale (UHDRS), TBZ significantly reduced the chorea score by 5.0 points compared to 1.5 for placebo (p < 0.0001). Likewise, the CGIC showed significantly higher improvement rates in patients treated with TBZ in comparison to those given placebo. There were five withdrawals in the TBZ treatment group and five serious adverse events (SAE) including one suicide, which was felt to be due to situational depression rather than TBZ-induced depression. There were no SAE in the placebo group.
A retrospective chart analysis of 76 hyperkinetic patients in a pediatric population treated at Baylor College of Medicine from 1996–2004 revealed significant improvement in chorea in 89% of the patients (Vuong et al 2004).
In a study comprising 118 patients with hyperkinetic movement disorders (28 diagnosed with HD), patients were assessed by phone interview using the Clinical Global Impression of Change (CGIC) and the overall efficacy of treatment was shown via a composite score made up of the patient’s score and the caregiver’s score. Significant improvement of hyperkinesias was seen in 61% of patients, with a subgroup of subjects with chorea and facial dyskinesias responding most favorably (Paleacu et al 2004).
In another small study of 19 HD patients treated with TBZ, the follow-up was carried out in a prospective fashion. The evaluation was done using the Abnormal Involuntary Movement Scale (AIMS), which was done by two separate investigators blinded to the drug administered. Eighteen patients completed and these patients were rated at 3.3 months at a final mean dose of 62.5 ± 37.4 mg/day. Fifteen patients scored better than before treatment, the scores of one remained the same and the scores of two others worsened (Ondo et al 2002).
The first published study assessing the long-term efficacy of TBZ in 400 hyperkinetic movement disorders patients selected from a large group of 526 included 29 HD patients. These patients improved by 82.8% on a scale of 1 to 5 (where 1 = improvement, 4 = no response and 5 = worsening). The average treatment duration was 28 ± 31.1 months (Jancovic and Beach 1997).
Kingston summarized the experience of 40 patients who had been receiving TBZ for almost 7 years for several movement disorders including chorea. 75% of these patients experienced marked or moderate improvement (Kingstone 1979).
A single-blind crossover study with a pretreatment phase, active drug, followed by placebo in 26 patients with HD, TD, and dystonia found that 54% experienced marked or moderate improvement of chorea with TBZ for the 3-week duration of the study (Asher and Aminoff 1979). Many studies that included a mixed group of movement disorders mention that the patients with chorea and TD responded better than patients with dystonia or tics.
A double-blind prospective crossover study of 20 patients assessed TBZ versus placebo for its effect on a variety of hyperkinetic movement disorders (Jancovic 1982). TBZ was found to improve the hyperkinesia score more than placebo to a statistically significant degree. Patients noted functional improvement, but this endpoint was not assessed in a quantitative manner. An open-label follow up of these same patients found that 62% of patients initially enrolled in the double-blind crossover study continued to display moderate improvement of the movement disorder 6–18 months later (Jancovic 1983). Major studies reporting on TBZ efficacy on chorea are summarized in Table 2.
Table 2.
Major studies in which chorea, including HD, patients were treated with TBZ showing treatment outcome
| Authors and year of publication | Number of patients | Outcome measures | Outcome |
|---|---|---|---|
| Huntington study group 2006 | 84 HD patients | Reduction in chorea score of the UHDRS | 5 point reduction in HD compared to 1.5 in placebo patients (p < 0.0001) |
| Vuong et al 2004 | 76 pediatric patients | Chorea improvement by CGIC | 89% of the patients improved |
| Paleacu et al 2004 | 118 patients with hyperkinetic movement disorders, 28 HD | CGIC | 61% of patients improved (chorea improved in 19/28 patients) |
| Ondo et al 2002 | 19 HD patients | AIMS | 79% of patients improved (15/19) |
| Jancovic and Beach 1997 | 400 hyperkinetic patients, 29 HD | Modified CGIC (from 1–5) | Overall improved by 82.8% 97% of chorea patients improved (28/29) |
| Jancovic 1982 | 20 hyperkinetic patients | Functional | 62% of patients improved |
| Asher and Aminoff 1979 | 26 patients with chorea, TD, tics | Clinical | 54% of chorea patients improved |
| Kingstone 1979 | 40 hyperkinetic patients | Clinical | 75% marked to moderate improvement |
Abbreviation: UHDRS, unified Huntington disease rating scale; CGIC, clinical global impression of change; AIMS, abnormal involuntary movement scale
Dosing issues
TBZ is usually initiated at a dose of 12.5 mg twice a day and is slowly titrated in two or three divided doses up to 150–200 mg/day in increments of 25 mg/week. In studies and in daily practice, given the short half-life, dosing TID is sometimes necessary. The dose escalation is stopped when the patient experiences a clear therapeutic effect or intolerable side effects. This technique might indeed create a bias towards an increase in side effects in many clinical studies on TBZ as most studies are designed to increase the dose until intolerable side effects are noted and then the dose is slightly decreased to alleviate the side effects. Most drug-related side effects can be alleviated by lowering the dose. Overdose in a case of self-poisoning with tetrabenazine was described in a 27-year-old female without any significant sequelae, except for sedation, after taking approximately 1 gram of TBZ (Kidd et al 1972).
Tolerability and side effects
The most common immediate side effects include drowsiness/sedation, weakness, parkinsonism, depression and acute akathisia, all of which are reversible with decreased dosing (Jancovic 1997; Paleacu 2004). Several studies have observed that younger patients tolerate TBZ better than the elderly. It is also notable that side effects vary slightly across different age groups: while younger patients showed a trend to experience more insomnia and depression, older patients seemed more likely to develop parkinsonism (Hunter 2002; Paleacu 2004). Other rare side effects include: insomnia, nervousness/anxiety, nausea and vomiting, tremor, memory problems, confusion, “trance-like/zombie”, orthostatic hypotension, balance and gait difficulties, dizziness, diarrhea, headaches, hallucinations, paresthesias, pharyngeal spasm and pain, blurred vision, panic attacks, paranoia (Jancovic and Beach 1997).
Changes in clinico-chemical tests during 12 months on TBZ were always minor, nearly always unsystematic and, on the whole, tended more towards a normalization of values. Even taking into account the limitations inherent in an uncontrolled trial, the conclusion was reached that long-term treatment with TBZ seems to be quite safe (Jancovic and Beach 1997). The extrapyramidal side effects of TBZ include parkinsonism, acute dystonia and akathisia and rarely neuroleptic malignant syndrome (NMS). It is particularly notable than not one single case of tardive dyskinesia has been reported with TBZ; it is this fact which confers its great advantage over dopamine receptor blockers. Concurrent use of antipsychotics with tetrabenazine can induce parkinsonism in HD patients (Moss and Stewart 1986) or acute dystonic reactions (Schott et al 1989). In one of the NMS cases, factors potentiating NMS included a high dosage of tetrabenazine exceeding the accepted therapeutic range together with co-medication with the dopamine-synthesis inhibitor alpha-methylparatyrosine, while in an other case, abrupt introduction of the drug and discontinuation of concomitant neuroleptics may have contributed to this adverse reaction. Uneventful recovery occurred in both cases without the need for drugs specifically enhancing dopaminergic transmission, while rechallenge by tetrabenazine with conventional doses and slow upward titration was not followed by recurrence of the NMS (Burke et al 1981; Mateo et al 1992; Osseman et al 1996). Other serious adverse events included pneumonia (Shoulson 1981) severe dysphagia (Jancovic and Beach 1997), and suicide (Gimenez-Roldan 1989). While it is well known that depression is extremely prevalent in HD and that suicide rates for this population are among the highest of those for all neurological diseases, TBZ, while capable of potentiating depression, did not seem to be directly involved in the suicide cases or in any other fatalities which occurred during treatment.
Conclusion
TBZ, a dopamine-depleting drug with a unique pharmacologic mechanism, has been found to be safe and efficacious for the treatment of a variety of hyperkinetic movement disorders, including HD. In the absence of modern disease modifying treatments for HD, symptomatic drug treatments such as TBZ remain important. These drugs, ideally, should be efficacious and devoid of debilitating side-effects.
Though most published studies are not double-blind, placebo-controlled trials, the sustained long-term benefit of patients speaks in favor of TBZ’s clinical efficacy in HD. Furthermore, TBZ does not cause TD, which is its major advantage over dopamine receptor blockers. It also has generally well tolerated side effects that are usually dose-related and therefore resolvable by decreasing doses or employing slow titration. Potentially fatal side effects such as dysphagia and depression are rare, though their existence mandates close monitoring of patients.
Based on his observations and previous open studies demonstrating TBZ efficacy in chorea Mc Lellan may have rightfully affirmed that “tetrabenazine is the drug of first choice for the suppression of chorea in patients with Huntington’s disease” (McLellan 1974).
References
- Ashcroft GW, Macdougall EJ, Barker PA. A comparison of tetrabenazine and chlorpromazine in chronic schizophrenia. J Ment Sci. 1961;107:287–93. doi: 10.1192/bjp.107.447.287. [DOI] [PubMed] [Google Scholar]
- Asher SW, Aminoff MJ. Tetrabenazine and movement disorders. Neurology. 1981;31:1051–4. doi: 10.1212/wnl.31.8.1051. [DOI] [PubMed] [Google Scholar]
- Bartels M, Zeller E. Tetrabenazine (Nitoman) therapy of chronic spontaneous oral dyskinesia. A video- and EMG-controlled study. Eur Arch Psychiatry Neurol Sci. 1984;234:172–4. doi: 10.1007/BF00461557. [DOI] [PubMed] [Google Scholar]
- Boghen DR, Lesser RL. Blepharospasm and hemifacial spasm. Curr Treat Options Neurol. 2000;2:393–400. doi: 10.1007/s11940-000-0037-7. [DOI] [PubMed] [Google Scholar]
- Brandrup E. Reserpin og tetrabenacin ved chorea Huntington. Nordisk Medicin. 1960;4:968–9. [Google Scholar]
- Burke RE, Fahn S, Mayeux R, et al. Neuroleptic malignant syndrome caused by dopamine-depleting drugs in a patient with Huntington disease. Neurology. 1981;31:1022–5. doi: 10.1212/wnl.31.8.1022. [DOI] [PubMed] [Google Scholar]
- Duran E, Chacon JR. Spasmodic torticollis and vertebral hemangioma. Rev Neurol. 2001;32:60–2. [PubMed] [Google Scholar]
- Erickson JD, Schafer MK, Bonner TI, et al. Distinct pharmacological properties and distribution in neurons and endocrine cells of two isoforms of the human vesicular monoamine transporter. Proc Natl Acad Sci USA. 1996;93:5166–71. doi: 10.1073/pnas.93.10.5166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahn S. A therapeutic approach to tardive dyskinesia. J Clin Psychiatry. 1985;46:19–24. [PubMed] [Google Scholar]
- Faulstich ME, Carnrike CL, Jr, Williamson DA. Blepharospasm and Meige syndrome: a review of diagnostic, aetiological and treatment approaches. Psychosom Res. 1985;29:89–94. doi: 10.1016/0022-3999(85)90012-1. [DOI] [PubMed] [Google Scholar]
- Fog R, Pakkenberg H. Combined nitoman-pimozide treatment of Huntington’s chorea and other hyperkinetic syndromes. Acta Neurol Scand. 1970;46:249–51. doi: 10.1111/j.1600-0404.1970.tb05621.x. [DOI] [PubMed] [Google Scholar]
- Gilligan BS, Wodak J, Veale Tetrabenazine in the treatment of extrapyramidal dyskinesias. Med J Aust. 1972;2:1054–6. doi: 10.5694/j.1326-5377.1972.tb103717.x. [DOI] [PubMed] [Google Scholar]
- Gimenez-Roldan S, Mateo D. Huntington disease: tetrabenazine compared to haloperidol in the reduction of involuntary movements. Neurologia. 1989;4:282–7. [PubMed] [Google Scholar]
- Gonzalez AM, Walther D, Pazos A, et al. Synaptic vesicular monoamine transporter expression: distribution and pharmacologic profile. Mol Brain Res. 1994;22:219–26. doi: 10.1016/0169-328x(94)90050-7. [DOI] [PubMed] [Google Scholar]
- Huang CY, McLeod JG, Holland, et al. Tetrabenazine in the treatment of Huntington’s chorea. Med J Aust. 1976;1:583–4. [PubMed] [Google Scholar]
- Hunter C, Wang A, Vuong K. Age-related tolerability of tetrabenazine. Mov Disord. 2002;17(Suppl 5):S41. [Google Scholar]
- Huntington Study Group. Tetrabenazine as an antichorea therapy in Huntington’s disease: a random controlled trial. Neurology. 2006;66:366–72. doi: 10.1212/01.wnl.0000198586.85250.13. [DOI] [PubMed] [Google Scholar]
- Jankovic J. Treatment of hyperkinetic movement disorders with tetrabenazine: a double-blind crossover study. Ann Neurol. 1982;11:41–7. doi: 10.1002/ana.410110108. [DOI] [PubMed] [Google Scholar]
- Jankovic J. Tetrabenazine in the treatment of hyperkinetic movement disorders. Adv Neurol. 1983;37:277–89. [PubMed] [Google Scholar]
- Jankovic J, Beach J. Long-term effects of tetrabenazine in hyperkinetic movement disorders. Neurology. 1997;48:358–62. doi: 10.1212/wnl.48.2.358. [DOI] [PubMed] [Google Scholar]
- Jankovic J, Beach J, Ashizawa T. Emotional and functional impact of DNA testing on patients with symptoms of Huntington’s disease. J Med Genet. 1995;32:516–18. doi: 10.1136/jmg.32.7.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankovic J, Glaze DG, Frost JD., Jr Effect of tetrabenazine on tics and sleep of Gilles de la Tourette’s syndrome. Neurology. 1984;34:688–92. doi: 10.1212/wnl.34.5.688. [DOI] [PubMed] [Google Scholar]
- Jankovic J, Orman J. Tetrabenazine therapy of dystonia, chorea, tics, and other dyskinesias. Neurology. 1988;38:391–4. doi: 10.1212/wnl.38.3.391. [DOI] [PubMed] [Google Scholar]
- Jankovic J, Rohaidy H. Motor, behavioral and pharmacologic findings in Tourette’s syndrome. Can J Neurol Sci. 1987;14(3 Suppl):541–6. doi: 10.1017/s0317167100038087. [DOI] [PubMed] [Google Scholar]
- Jimenez-Jimenez FJ, Garcia-Ruiz PJ. Pharmacological options for the treatment of Tourette’s disorder. Drugs. 2001;61:2207–20. doi: 10.2165/00003495-200161150-00005. [DOI] [PubMed] [Google Scholar]
- Kanjilal GC, Matheson B. A trial of tetrabenazine (Nituman) in disturbed mentally subnormal patients. J Ment Sci. 1962;108:225–8. doi: 10.1192/bjp.108.453.225. [DOI] [PubMed] [Google Scholar]
- Kenney C, Jankovic J. Tetrabenazine in the treatment of hyperkinetic movement disorders. Expert Rev Neurother. 2006;6:7–17. doi: 10.1586/14737175.6.1.7. [DOI] [PubMed] [Google Scholar]
- Kidd DW, McLellan DL. Self-poisoning with tetrabenazine. Br J Clin Pract. 1972;26:179–80. [PubMed] [Google Scholar]
- Kingston D. Tetrabenazine for involuntary movement disorders. Med J Aus, 1979;1:628–30. doi: 10.5694/j.1326-5377.1979.tb119426.x. [DOI] [PubMed] [Google Scholar]
- Lane JD, Smith JE, Shea PA. Neurochemical changes following the administration of depleters of biogenic monoamines. Life Sci. 1976;19:1663–7. doi: 10.1016/0024-3205(76)90071-0. [DOI] [PubMed] [Google Scholar]
- Lingjerde O. Tetrabenazine (Nitoman) in the treatment of psychoses. Acta Psychiatrica Scand. 1963;170(Suppl):158–209. [PubMed] [Google Scholar]
- Manji H, Howard RS, Miller DH, et al. Status dystonicus: the syndrome and its management. Brain. 1998;121:243–52. doi: 10.1093/brain/121.2.243. [DOI] [PubMed] [Google Scholar]
- Masuo Y, Pélaprat D, Scherman D, et al. [3H]Dihydrotetrabenazine, a new marker for the visualization of dopaminergic denervation in the rat striatum. Neurosci Lett. 1990;114:45–50. doi: 10.1016/0304-3940(90)90426-a. [DOI] [PubMed] [Google Scholar]
- Mateo D, Munoz-Blanco JL, Gimenez-Rodan S. Neuroleptic malignant syndrome related to tetrabenazine introduction and haloperidol discontinuation in Huntington’s disease. Clin Neuropharmacol. 1992;15:63–8. doi: 10.1097/00002826-199202000-00009. [DOI] [PubMed] [Google Scholar]
- Mc Lellan DL, Chalmers RJ, Johnson RH. A double blind trial of tetrabenazine, thiopropazate and placebo in patients with chorea. The Lancet. 1974;26:104–7. doi: 10.1016/s0140-6736(74)92338-1. [DOI] [PubMed] [Google Scholar]
- Mikkelsen BO. Tolerance of tetrabenazine during long-term treatment. Acta Neurol Scand. 1983;68:57–60. doi: 10.1111/j.1600-0404.1983.tb04816.x. [DOI] [PubMed] [Google Scholar]
- Moss JH, Stewart DE. Iatrogenic parkinsonism in Huntington’s chorea. Can J Psychiatry. 1986;31:865–6. doi: 10.1177/070674378603100916. [DOI] [PubMed] [Google Scholar]
- Ondo WG, Hanna PA, Jankovic J. Tetrabenazine treatment for tardive dyskinesia: assessment by randomized videotape protocol. Am J Psychiatry. 1999;156:1279–81. doi: 10.1176/ajp.156.8.1279. [DOI] [PubMed] [Google Scholar]
- Ondo WG, Tintner R, Thomas M, et al. Tetrabenazine Treatment for Huntington’s Disease-Associated Chorea. Clin Neuropharmacol. 2002;25:300–2. doi: 10.1097/00002826-200211000-00003. [DOI] [PubMed] [Google Scholar]
- Ossemann M, Sindic CJ, Laterre C. Tetrabenazine as a cause of neuroleptic malignant syndrome. Mov Disord. 1996;11:95. doi: 10.1002/mds.870110118. [DOI] [PubMed] [Google Scholar]
- Paleacu D, Giladi N, Moore O, et al. Tetrabenazine treatment in movement disorders. Clin Neuropharmacol. 2004;27:230–3. doi: 10.1097/01.wnf.0000136892.24629.96. [DOI] [PubMed] [Google Scholar]
- Pearson SJ, Reynolds G P. Depletion of monoamine transmitters by tetrabenazine in brain tissue in Huntington’s disease. Neuropharmacology. 1988;27:717–19. doi: 10.1016/0028-3908(88)90080-9. [DOI] [PubMed] [Google Scholar]
- Pettibone DJ, Totaro JA, Pflueger AB. Tetrabenazine-induced depletion of brain monoamines: characterization and interaction with selected antidepressants. Eur J Pharmacol. 1984;20:425–30. doi: 10.1016/0014-2999(84)90562-4. [DOI] [PubMed] [Google Scholar]
- Pletscher A, Brossi A, Gey K. Benzoquinolizine derivatives: a new class of monoamine decreasing drugs with psychotropic action. International Review of Neurobiology. 1962;4:275–306. [Google Scholar]
- Quinn GP, Shore PA, Brodie BB. Biochemical and pharmacological studies of RO 1-9569 (tetrabenazine), a nonindole tranquilizing agent with reserpine-like effects. J Pharmacol Exp Ther. 1959;127:103–9. [PubMed] [Google Scholar]
- Raja M. Managing antipsychotic-induced acute and tardive dystonia. Drug Saf. 1998;9:57–72. doi: 10.2165/00002018-199819010-00005. [DOI] [PubMed] [Google Scholar]
- Reches A, et al. Tetrabenazine, an amine-depleting drug, also blocks dopamine receptors in rat brain. J Pharmacol Exp Ther. 1983;225:515–21. [PubMed] [Google Scholar]
- Roberts MS, McLean S, Millingen KS, et al. The pharmacokinetics of tetrabenazine and its hydroxy metabolite in patients treated for involuntary movement disorders. Eur J Clin Pharmacol. 1986;29:703–8. doi: 10.1007/BF00615962. [DOI] [PubMed] [Google Scholar]
- Roberts MS, Watson HM, Mc Lean, et al. Determination of therapeutic plasma concentrations of tetrabenazine and an active metabolite by high-performance liquid chromatography. J Chromatogr. 1981;226:175–82. doi: 10.1016/s0378-4347(00)84218-8. [DOI] [PubMed] [Google Scholar]
- Sajatovic M, Verbanac P, Ramirez F, et al. Clozapine treatment of psychiatric syndromes resistant to neuroleptic treatment in patients with Huntington’s disease. Neurology. 1991;41:156–8. doi: 10.1212/wnl.41.1.156. [DOI] [PubMed] [Google Scholar]
- Satou T, Anderson AJ, Itoh T, et al. Repetitive administration of tetrabenazine induces irreversible changes in locomotion and morphology of the substantia nigra in rats. Exp Toxicol Pathol. 2001;53:303–8. doi: 10.1078/0940-2993-00195. [DOI] [PubMed] [Google Scholar]
- Sattes H. The treatment of chorea minor with the monoamine liberator “Nitoman. Psychiatr Neurol Neurochirurg (Basel) 1960;140:13. [PubMed] [Google Scholar]
- Sattes H, Hase E. Die Behandherd Extrapyramidaler Hypokinesien unto besonderer Beriicksichtigung der chorea Huntington. Psychiatr Neurol Neurochirurg (Aust) 1964;67:289. [PubMed] [Google Scholar]
- Scahill L, Chappell PB, King RA, et al. Pharmacologic treatment of tic disorders. Child Adolesc Psychiatr Clin N Am. 2000;9:99–117. [PubMed] [Google Scholar]
- Scherman D, Henry JP. Acido-basic properties of the catecholamine uptake inhibitors tetrabenazine and dihydrotetrabenazine. Biochimie. 1982;64:915–21. doi: 10.1016/s0300-9084(82)80354-4. [DOI] [PubMed] [Google Scholar]
- Schott K, Ried S, Stevens I, et al. Neuroleptically induced dystonia in Huntington’s disease: a case report. Eur Neurol. 1989;29:39–40. doi: 10.1159/000116375. [DOI] [PubMed] [Google Scholar]
- Scott BL. Evaluation and treatment of dystonia. South Med J. 2000;93:746–51. [PubMed] [Google Scholar]
- Shoulson I, Goldblatt D. Huntington’s disease (HD): effect of tetrabenazine and antipsychotic drugs on motoric features. Neurology. 1981;31:79. [Google Scholar]
- Simpson GM. The treatment of tardive dyskinesia and tardive dystonia. J Clin Psychiatry. 2000;61(Suppl 4):39–44. [PubMed] [Google Scholar]
- Smith M. Clinical Comparison of Tetrabenazine (Ro 1-9569), reserpine and placebo in chronic schizophrenia. Dis Nerv Syst. 1960;21:120–3. [PubMed] [Google Scholar]
- Smith JM, Baldessarini RJ. Changes in prevalence, severity and recovery in tardive dyskinesia with age. Arch Gen Psychiatry. 1980;37:1368–73. doi: 10.1001/archpsyc.1980.01780250054006. [DOI] [PubMed] [Google Scholar]
- Storey E, Lloyd J. Tardive tremor. Mov Disord. 1997;12:808–10. doi: 10.1002/mds.870120533. [DOI] [PubMed] [Google Scholar]
- Stumpf W. Untersuchungen uber ‘Nitoman’. Psychiatr Neurol. 1960;140:63–8. [Google Scholar]
- Swash M, Roberts AH, Zakko H, et al. Treatment of involuntary movement disorders with tetrabenazine. J Neurol Neurosurg Psychiatry. 1972;35:186–91. doi: 10.1136/jnnp.35.2.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarsy D. Tardive Dyskinesia. Curr Treat Options Neurol. 2000;2:205–14. doi: 10.1007/s11940-000-0003-4. [DOI] [PubMed] [Google Scholar]
- The Huntington’s Disease Collaborative Research Group. A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington’s disease chromosomes. Cell. 1993;72:971–83. doi: 10.1016/0092-8674(93)90585-e. [DOI] [PubMed] [Google Scholar]
- Toglia JU, McGlamery M, Sambandham RR. Tetrabenazine in the treatment of Huntington’s chorea and other hyperkinetic movement disorders. J Clin Psychiatry. 1978;39:81–7. [PubMed] [Google Scholar]
- Van Vugt JP, Siesling S, Vergeer M, et al. Clozapine versus placebo in Huntington’s disease: a double blind randomized comparative study. J Neurol Neurosurg Psychiatry. 1997;63:35–9. doi: 10.1136/jnnp.63.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]