Abstract
Objective
Selective serotonin reuptake inhibitors (SSRIs) have increased cognitive performance in some clinical studies of Alzheimer’s disease (AD), but it is has been difficult to dissociate whether this is due to direct effects on cognition (neurochemical or disease-modifying) or a secondary effect of mood stabilization. We performed a systematic review for preclinical and human clinical trial evidence to support the use of SSRIs specifically for the management of cognitive decline in AD.
Data sources
(1) PUBMED without language restrictions from 1950s until 2004 and updated August 2006, terms: “serotonin uptake inhibitors”[MeSH] AND (“Alzheimer disease”[MeSH] OR “Cognition Disorders”[MeSH]) NOT “Parkinson disease”[MeSH] AND (Clinical Trial[ptyp] OR Letter[ptyp] OR Meta-Analysis[ptyp] OR Randomized Controlled Trial[ptyp]) AND “alzheimer disease” [MESH] OR “Alzheimer*” combined with AND to “ssri*” OR “serotonin reuptake inhibitors” [MESH] NOT Review[ptyp]. (2) Cochrane Database of Systematic Reviews, keywords “SSRI” and “Alzheimer’s”.
Study selection
The PubMed search yielded 57 hits. Of these, 23 were included in this review for their specificity to SSRI use in AD or indications on efficacy beyond depressive symptoms. The other 34 citations were excluded because: (1) depression or other mood or behavioral disturbance severity was the reported outcome measure, (2) effects of SSRIs on cognition were confounded by concomitant use of other drugs, (3) subjects described were young adults, and/or (4) subjects had traumatic brain injury. The Cochrane Database of Systematic Reviews, 3rd Quarter 2006, yielded six citations related to SSRIs.
Data extraction
Data extracted from clinical trials included name of SSRI tested, cognitive outcome measures, and adverse events reported, which could include cognitive worsening.
Data synthesis
Preclinical evidence for use of SSRIs to enhance cognition in AD includes an effect at the hippocampus through carbonic anhydrase activation or stimulation of hippocampal neurogenesis. The chemical structure of paroxetine, and not intrinsic SSRI activity, may also affect APP ectodomain expression to reduce amyloid plaque formation. Clinical trials in AD generally have not assessed cognitive outcomes independently from mood or behavior stabilization. Currently, clinical studies in AD only indirectly support the use of SSRIs for disease modification by confirming a serotonergic deficit during the course of illness.
Conclusions
Lack of supportive evidence for SSRIs as cognition enhancers or disease modifiers in AD is the result of omissions in clinical trial design, as opposed to reporting of negative outcomes. The preclinical evidence warrants the study of SSRIs in AD using mood, behavior, cognition, neurochemistry, and possibly neuroimaging as outcome variables.
Keywords: Alzheimer’s disease, Amyloid precursor protein, APP ectodomain, carbonic anhydrase, selective serotonergic reuptake inhibitor
Introduction
Short term memory loss with associated hippocampal pathology is typically the earliest feature of Alzheimer’s disease (AD). Not only do the hippocampi atrophy as patients progress from mild cognitive impairment to AD (Jack et al 2005), but AD pathology begins in entorhinal cortex and hippocampus (Braak and Braak 1991). The evidence thus far focuses prevention and treatment for AD on preservation of hippocampal structure and function.
The neurotransmitter serotonin (5HT) has recently been linked to AD pathology in part because serotonergic receptors densely populate the hippocampus. Furthermore, extracellular 5HT levels have been correlated with memory performance. Serotonin depletion impairs memory encoding. (Schiapparelli et al 2005; Ueda et al 2005; van der Veen et al 2006; Khaliq et al 2006) The verbal memory impairment in former ecstasy (MDMA) users is believed to be the result of MDMA’s selective toxicity to serotonergic neurons.(Thomasius et al 2006) Cognitive improvement does not follow agonism across all 5HT receptor types (Meneses 1998; Meneses 2001; Meneses 2003; Meneses and Hong 1995; Meneses and Terron 2001; Meneses et al 1997). For example, 5HT6R blockade facilitates memory consolidation in rats. (Mitchell and Neumaier 2005)
Selective serotonin reuptake inhibitors (SSRIs) raise extracellular 5HT levels and have shown effects both on hippocampal plasticity and neurogenesis in animal models and treated patients. Hippocampal evoked potentials manifest enhanced plasticity after administration of fluvoxamine (Ohashi et al 2002) and fluoxetine (Smith and Lakoski 1998; Stewart and Reid 2000). Escitalopram, however, decreases long term potentiation (LTP) in CA1 neurons of the dorsal hippocampus (Mnie-Filali et al 2006), highlighting potential differences among the SSRIs for hippocampal effects. Paroxetine has led to increased hippocampal volumes in patients treated for post-traumatic stress disorder (PTSD) (Bremner and Vermetten 2004), implying promotion of neurogenesis.
Although patients with PTSD whose hippocampal volumes improved after treatment with paroxetine also showed improved memory performance (Bremner and Vermetten, 2004), enhancement of neuronal plasticity seems inversely related to memory performance. Fluoxetine shows no effect on spatial learning in rats (Stewart and Reid 2000) and even decreases memory retention in rats pretreated with the cognitive enhancing peptide, ghrelin (Carlini et al 2007). Similarly, despite escitalopram’s inhibitory effect on LTP, its less potent racemic form, citalopram, is reported to restore spatial memory to both rats and mice impaired by anticholinergic preparations (Egashira et al 2006). The discrepancy of results from these studies on SSRIs and memory performance may be related to differences in acute vs chronic use of SSRIs or administration of low vs high maintenance doses (Dumont et al 2005).
Most studies describe a less direct role for SSRIs in maintaining the hippocampus. SSRIs diminish factors that lead to apoptosis, such as stress-induced glucocorticoid levels (MacPherson et al 2005; Mattson et al 2004; Sapolsky 2003).
Inverse relationships between 5HT and neuropeptides may also impact hippocampal function. Neurokinin-1 (NK-1) agonists have effects on the dorsal raphe that can lead to decreased 5HT release, which would impact negatively on memory-related activity in the hippocampus. However, relevant to the cholinergic hypothesis of AD, enhancement of cholinergic activity within the mouse hippocampus by substance P is dependent upon NK-1 receptors. It is not yet clear what the relative costs to the AD patient would be if manipulation of NK-1 receptors leads to the loss of cholinergic facilitation vs. loss of serotonergic transmission (Gobbi and Blier 2005).
The impact of SSRIs on memory may stem from interactions with other modulators of memory that act through brain-derived neurotrophic factor (BDNF). Chronic fluoxetine and sertraline administration enhances cAMP response element binding protein expression in hippocampus, and this leads to increased BDNF expression. (Nibuya et al 1996) Chronic use of SSRIs also stimulates norepinephrine release, likely due to effects at the locus ceruleus (Page and Abercrombie 1997; Thomas et al 1998; Vizi et al 2004; Hajos-Korcsok et al 2000). The combination of increased extracellular 5HT and norepinephrine observed after administration of duloxetine but not fluoxetine enhances BDNF mRNA expression (Calabrese et al 2007), but the impact of BDNF on memory appears not to be related to hippocampal function. Instead, this increase in BDNF is observed in frontal lobes and not the hippocampus (Calabrese et al 2007). Improvements in memory performance are possible, if the frontal systems for sustained attention are facilitated.
Memory loss and hippocampal atrophy also can occur in depression (Sheline et al 1999; Burt et al 1995). Sheline et al (2002) reported preliminary data indicating an association among depression, memory loss, hippocampal atrophy, and decreased 5HT receptor binding. This has been modeled by Meeter et al (Meeter et al 2006). Clinicians commonly prescribe SSRIs to patients with AD, intending relief of secondary depression, anxiety, and agitation (Pollock et al 1999; Bains et al 2005; Nyth and Gottfries 1990). This raises a challenge in interpreting the results of both animal and human studies: does administering an SSRI prior to training enhance learning by decreasing the subject’s anxiety or depressive symptoms during testing? This illustrates the difficulty of distinguishing cognitive effects from behavioral or emotional ones and will be discussed in the review.
Whether as a symptomatic or disease-modifying intervention for AD, use of SSRIs is supported by autopsy data. Patients with AD compared against age-matched controls have serotonergic deficits manifested by degeneration of the raphé nuclei and decreased 5HT and metabolite 5-hydroxyindole acetic acid (5-HIAA) levels in brain parenchyma and the cerebrospinal fluid (CSF) (Chen et al 1996; Bowen et al 1988; Procter et al 1992; Nazarali and Reynolds 1992; Chen et al 2000; Sjogren et al 1998; Reinikainen et al 1988). CSF 5-HIAA levels are low during the AD patient’s life as well (Blennow et al 1992), with differences between early and late onset AD. 5-HIAA correlates positively with cognitive impairment in early onset AD but with motor impairment in late onset AD (Brane et al 1989).
We reviewed the preclinical and clinical evidence for benefits of SSRIs to cognition or to disease modification in AD. We report a systematic literature search for efficacy of SSRIs in AD outside of treatment for secondary depressive and anxious symptoms.
Methods
PUBMED was searched without language restrictions from the 1950s to 2004 and updated August 2006. Due to changes in the PubMed database, two different search strategies have been used. Initially the search was as follows: “serotonin uptake inhibitors”[MeSH] AND (“Alzheimer disease”[MeSH] OR “Cognition Disorders”[MeSH]) NOT “Parkinson disease”[MeSH] AND (Clinical Trial[ptyp] OR Letter[ptyp] OR Meta-Analysis[ptyp] OR Randomized Controlled Trial[ptyp]). Using specific drug names (eg, fluoxetine) instead of “Serotonin Uptake Inhibitors” did not add to the yield. The following two search statements were added at the update: “alzheimer disease” [MESH] OR “Alzheimer*” combined with AND to “ssri*” OR “serotonin reuptake inhibitors” [MESH] NOT Review[ptyp].
There is no MeSH term for “disease modification” or “neurodegeneration,” but we did conduct a search using “Hippocampus” [MeSH] AND “serotonin/pharmacology”[MeSH] AND “Alzheimer disease/drug therapy” [MeSH].
To cross-check for any further citations missed in our search strategy, we also checked references identified by reports from the Cochrane Database of Systematic Reviews using keywords “SSRI” and “Alzheimer’s,” which were more inclusive search terms than permutations of “serotonin reuptake inhibitor” and “Alzheimer’s disease” with either of the preceding keywords. It is possible that our search strategy omitted pertinent references from this review, but we believe we cast the widest and most reasonable net to find relevant papers.
The PubMed search yielded 57 hits. Of these, 23 were included in this review for their specificity to SSRI use in AD and indications on efficacy beyond depressive symptoms. The other 34 citations were excluded because: (1) depression or other neuropsychiatric symptom severity was the outcome measure, (2) effects of SSRIs on cognition were confounded by concomitant use of other drugs, (3) subjects described were young adults, and/or (4) subjects had traumatic brain injury. We did not actively search for adverse events in AD patients taking SSRIs but summarized relevant findings from papers that appeared within our search.
The search for MeSH terms “Hippocampus” AND “serotonin/pharmacology” AND “Alzheimer disease/drug therapy” revealed one citation (Schechter et al 2005) that supported a role for serotonin in hippocampal function but did not refer specifically to SSRIs.
The Cochrane Database of Systematic Reviews, 3rd Quarter 2006, yielded six citations related to SSRIs. Three of these citations referred to treatment of depression. The remaining three citations referred to relevant papers; one citation was added to the review, and the other two had been identified previously by our search strategy.
We have organized our review to present in vitro and animal experiments as Preclinical Evidence, followed by evidence from human clinical trials to support first cognition-enhancing, then disease modifying effects of the SSRIs.
Cognition-enhancing properties of SSRIs
Preclinical evidence for use of SSRIs to enhance cognition in AD
Aside from effects on 5HT receptors, SSRIs may have an effect at the hippocampus by acting as carbonic anhydrase activators (Sun and Alkon 2001). This hypothesis merits discussion because separate experiments indicate a mechanism of action beneficial to hippocampal function, but this effect has yet to be demonstrated in a clinical trial. Carbonic anhydrase catalyzes the production of bicarbonate (HCO3) from carbon dioxide and water. This in turn can increase the proportion of GABAergic interneurons triggering excitatory responses in rat hippocampal pyramidal cells. SSRIs would therefore incite GABAergic interneurons in a way that enhances synaptic efficacy, spatial learning, and memory (Sun and Alkon 2001). Although spatial learning and memory performance improved in a rat using another carbonic anhydrase activator (Sun and Alkon 2001), we are not able to extrapolate from this study what continuous administration of an SSRI might do for the carbonic anhydrase hypothesis in elderly patients with AD. Casini et al showed a more direct connection between SSRIs and carbonic anhydrase with an in vitro experiment, in which the action of SSRIs upon carbonic anhydrase was blocked with acetazolamide (Casini et al 2003). The authors identify fluoxetine, sertraline, and citalopram as carbonic anhydrase isozyme I and II activators, but they are less ambitious about cognitive effects on patients with AD than Sun and Alkon (2001). By contrast, Casini et al (2003) suggest that the carbonic anhydrase mechanism explains the antidepressant efficacy of SSRIs in AD.
As above, some SSRIs may stimulate hippocampal neurogenesis (Bremner and Vermetten 2004; MacPherson et al 2005; Mattson et al 2004; Sapolsky 2003); which might then translate to cognitive enhancement, as well as disease modification. We review studies in this area in the “Disease Modification Properties” section below.
The hypotheses carried forward in preclinical trials give a rationale to expect SSRIs to have cognitive benefits in AD. However, clinical trials have not been designed to follow the preclinical results above. This is probably because most of the evidence supporting cognition-enhancing effects of SSRIs was reported well after the publication of Phase II clinical trials of SSRIs in patients with AD. As will be discussed in the next section, clinical trials of SSRIs have focused primarily on mood or behavioral effects and have not rigorously tested memory or learning, measures of hippocampal neuronal activity, or changes in hippocampal volume as outcome measures.
Clinical evidence of SSRIs as cognitive enhancers
Although cognitive benefits are rarely central aims, they have been observed in several studies. Cutler et al enrolled four non-depressed patients with AD in a double-blind crossover study of serotonin reuptake blocker zimeldine and found that although there were drug responsive alterations in central and peripheral serotonergic function, they were unaccompanied by measurable changes in memory and/or reaction time (Cutler et al 1985). Taragano et al (1997) reported improvement in MMSE scores among depressed patients with AD who were treated with SSRI fluoxetine but attributed this improvement to the lifting of the depressive symptoms. In what may be the last clinical trial for subjects with AD excluding concomitant cholinesterase inhibitor therapy, the Depression in Alzheimer’s Disease Study (DIADS) clinical trial did not show differences on the MMSE for sertraline vs. placebo (Lyketsos et al 2003). A similar 16-week monotherapy trial of citalopram in early- and late-onset AD patients showed no improvement on the cognitive portions of the Gottfries-Brane-Steen geriatric rating scale (GBS) (Nyth and Gottfries 1990).
Munro et al (2004) found mixed SSRI effects on cognition when they split the DIADS data into gender groups: women with AD taking sertraline showed improvement on verbal learning and block design tasks compared to those on placebo; men worsened on these measures when taking sertraline. Lanctot et al (2002) report a greater prolactin release response to the non-selective 5HT reuptake inhibitor fenfluramine among women with higher Neuropsychiatric Inventory and BEHAVE-AD aggression scores and low MMSE scores at baseline. This may lend further support to gender differences in a beneficial role for SSRIs in AD, but there were no cognitive outcome measures for this fenfluramine challenge study.
Like Taragano, Mintzer (2003) concludes that cognitive benefits of SSRIs, if there are any, are likely to be secondary to their effect on mood or behavioral disturbances. This view is supported in a clinical drug trial by Pollock et al (2002). Patients with dementia (not necessarily due to AD) but not depression were admitted to the hospital for acute onset of severe agitation and treated with (1) the SSRI citalopram, (2) the neuroleptic perphenazine, or (3) placebo. One of the secondary outcome measures of the study was a cognitive subscale of the Neurobehavior Rating Scale. Patients in the citalopram arm of the study, in contrast to those randomized to perphenazine or placebo, showed significant improvement on the cognitive subscale. The number needed to treat (NNT) to achieve either a partial or full response to citalopram in Pollock’s study was 3; for a full response the NNT was 6. By comparison, the perphenazine NNT was 17 (Bruce Pollock, personal communication, February 17, 2005). Of note, the NNT for cholinesterase inhibitors to achieve a cognitive response in AD is 10 (Lanctot et al 2003). The NNT is impressive when compared to the widely used cholinesterase inhibitors, which are arguably the standard of care for AD (Courtney et al 2004). Although cognitive outcomes have been reported in a small number of studies, neither the cognitive subscales of the Neurobehavior Rating Scale nor the GBS nor the MMSE is a rigorous measure of memory nor learning at the standard of the Wechsler Memory Scale-Revised Logical I and II (Wechsler 1987) or California Verbal Learning Test (Delis et al 1987).
Other than the negative study on a very small sample by Cutler et al (1985), no clinical trial has distinguished SSRI cognitive benefits from mood or behavioral stabilization. Even if one were to separate cognition-enhancement from behavior control, Predictive value of the rodent improvements reported in preclinical trials has yet to be adequately assessed in clinical trials.
The only adverse cognitive events reported in AD have been “decreased concentration” in no more than six of 98 subjects taking citalopram in the Nyth and Gottfries study (1990). Case reports describe an 87 year old woman and four members of a separate family whose memory impairment began with fluoxetine administration for depression and reversed after withdrawal of the drug. (Joss et al 2003, Huang et al 2004) There are insufficient neuropsychological testing data from any of these reports to indicate common features of the cognitive worsening, eg, attentional vs encoding impairment.
SSRIs are frequently used concomitantly with cholinesterase inhibitors in patients with AD (Pollock et al 1999). The positive study cited above allowed subjects to take acetylcholinesterase inhibitors (AChE-Is) concurrently, so long as they were on stable doses for a month prior to study enrolment (Pollock et al 2002); this amounted to approximately 30% of the sample. Finkel et al reported no specific cognitive improvements on the MMSE or ADAS-Cog when augmenting donepezil with sertraline in a double-blind study, although there were modest improvements on the Clinician’s Global Impression scale for the augmented group (Finkel et al 2004).
Tacrine, the first cholinesterase inhibitor approved by the FDA for treatment of AD, has been shown to have non-competitive 5HT reuptake inhibition properties (Jossan et al 1992). McKenna et al (1997), following up on this report, explored analogues of tacrine for inhibition of serotonin uptake. Although they identified one compound as a relatively potent SSRI, follow-up clinical trial data are not available. Another group, noting the frequent concomitant use of SSRIs and AChE-Is, created a combination drug by combining fluoxetine and rivastigmine (Kogen et al 2002). They hypothesized that, “the antidepressive effect of serotonin transport inhibitors might reduce a demand on cholinergic systems in the brain to ameliorate cognitive effects.” The drug was developed in 2002, but neither pre-clinical nor clinical trial data have been published. It is not clear whether SSRIs enhance AD patient function by enhancing cholinesterase inhibitor effects or vice versa. Meneses (2003) suggests that AChE-Is potentiate SSRIs when the SSRI is given at a sub-effective dose.
Two more studies identified by our literature search were clinical trials of SSRIs in patients with AD, but their designs focused on showing a serotonergic deficit. Neither of the studies replicated the typical delivery of an SSRI to an AD patient or monitored cognition or disease modification as outcome measures (Tohgi et al 1995). Tohgi et al (1995) reported that patients with AD or subcortical ischemic vascular dementia have lower baseline CSF 5HT levels than age-matched controls. After a challenge with citalopram, AD CSF total 5HT levels doubled but, nevertheless, remained lower than the control group baseline CSF. This result confirms the postmortem evidence of 5HT deficit, as well as suggesting a 5HT deficit in living subjects with AD. However, the study had no mood or behavioral or cognitive outcome measures, and thus does not provide evidence of a clinical benefit. Furthermore, the investigators administered a citalopram challenge of 20 mg daily over a two-week period. Patients with AD may further adjust their CSF 5HT levels if the citalopram administration were prolonged; most patients are given SSRI trials for at least 6 weeks at a time. Also, this was not a controlled challenge study; control subjects received neither placebo nor citalopram.
In summary, positive studies supporting SSRIs as cognition enhancers have not been able to differentiate their results from effects on mood and behavioral control as a matter of study design. The one study (Cutler et al 1985) to assess cognitive effects of an SSRI in AD without depression had negative results, but the same study design has not been replicated with more commonly prescribed SSRIs. Existing studies in living AD patients indirectly support the use of SSRIs by confirming a serotonergic deficit during the course of illness and suggesting that patents with AD are particularly responsive to SSRIs.
Disease modification properties of SSRIs in AD
Preclinical evidence
In addition to providing symptomatic relief through compensation for a 5HT deficit in AD, SSRIs may also reduce amyloid plaque formation, which likely has a causative role in the development of the cognitive impairment in AD. The amyloid plaque is made up of Aβ 1–42 oligomers, which are formed after clipping of amyloid precursor protein by β-secretase and then γ-secretase. The ectodomain portion of APP shunts more of the APP away from the γ-secretase pathway and thereby promotes the production of “benign” amyloid. Two papers showed positive effects on APP ectodomain expression after agonism at 5HT2a and 5HT2c receptors: Nitsch et al (1996) demonstrated this in vitro, and the rodent model of Arjona et al (2002) shows increases in APP ectodomain levels in the CSF after intraperitoneal injections of the 5HTR agonists. The guinea pigs used for this experiment were young and not cognitively impaired specimens, and no cognitive or behavioral testing was included in the study design (Arjona et al 2002). In both of these studies, the effects of the 5HT2a or 5HT2c agonist were reversed with ritanserin, but it has not yet been established that administration of SSRIs will have similar effects in AD patients.
Morse et al (2003) and Payton et al (2004) have screened drugs for their ability to stimulate expression of the APP ectodomain. Six of 1200 drugs screened for interactions with the ectodomain suppressed the appropriate target: one such drug was paroxetine (Payton et al 2003). Although the screen included several SSRIs. Morse et al (2004) found that the effect of paroxetine on the APP ectodomain seems related to its secondary iron chelator properties, similar to dimercaptopropanol, the mercury chelator, identified in the Payton drug search. Thus, APP ectodomain modulation may not be a universal property of SSRIs. Tucker et al (2006) have since confirmed the beneficial effect of paroxetine on Aβ levels in an AD mouse model: adding paroxetine to the animal’s drinking water reduced cortical Aβ peptide levels.
Pakaski et al (2005) report an increase in APP secretion resulting from in vitro treatment of rat basal forebrain neurons with a different SSRI, citalopram, but the benefit of this result seems counterintuitive. Unlike the studies above, SSRI administration enhances secretion of entire APPs and does not specifically alter expression of the APP ectodomain segment.
When Palotas et al (2004) used lymphocytes in a cDNA microarray to screen for a link between SSRIs and AD, they found support for a different disease modification role for citalopram. Lymphocytes from patients with AD have impaired Aβ processing (Palotas et al 2002; Etcheberrigaray and Bhagavan 1999; Eckert et al 1998). These lymphocytes show ionic and signal transduction alterations, increasing susceptibility to apoptosis via oxidation. Challenge with citalopram for 4 weeks led to enhanced mRNA expression of the genes to prevent apoptosis in lymphocytes from both AD and non-AD control subjects (Palotas et al 2004). Messenger RNA expression and not cell survival was the outcome variable for this experiment. It is not yet clear what predictive validity a treated lymphocyte population may have for the CNS in AD or its microglial system, but this may be one way in which SSRIs could contribute to disease modification in AD.
Another potential disease-modifying effect of SSRIs is hippocampal neurogenesis. Hippocampal atrophy is a frequent finding on magnetic resonance imaging in AD, although not required for clinical diagnosis due to lack of specificity for AD. Neuropathologists use findings of neuritic plaques with amyloid cores and neurofibrillary tangles in the hippocampi of patients to diagnose AD (National Institute on Aging 1997). Agents that rejuvenate hippocampal neuronal activity or neurogenesis might enhance cognition and/or modify the AD process. Our literature search elicited one study on the effect of fluoxetine on hippocampal injury through isolation stress in a transgenic mouse model for AD (Dong et al 2004). The authors found that fluoxetine reversed the negative effects of isolation stress on hippocampal cell proliferation and normalized the performance on a memory task. Malberg (2000) had previously reported that chronic treatment with antidepressants facilitates hippocampal neurogenesis in rats. The effect was not specific to SSRIs: rats treated with the SSRI fluoxetine or an MAO-inhibitor or a norepinephrine uptake inhibitor had similarly increased hippocampal neuronal counts.
One study reporting apoptosis caused by an SSRI counters their potential benefit for AD. Neurotrophic cytokine S100B enhances neuronal survival in vitro (Reeves et al 1994). Administration of fluoxetine to the young adult rat increases hippocampal levels of S100B (Haring et al 1993), but aging humans have increased levels of S100B mRNA expression (Sheng et al 1996), and excessive amounts of CNS S100B paradoxically promote apoptosis in mice prone to accelerated senescence (Griffin et al 1998). Akhisaroglu et al (2003) compared the responses of young and old mice after administration of fluoxetine 10 mg/kg daily for 2 weeks. The older mice had higher levels of S100B. Fluoxetine further increased that abnormal level of S100B. However, there were no cognitive or mood or behavioral descriptions of the older mice with or without fluoxetine administration and therefore no clinical implications of the increased S100B. In addition, the authors did not mention effects of fluoxetine on morbidity, mortality or brain pathology in the mice.
Overall, studies have shown that selected SSRIs could modify disease through effects on amyloid plaque formation or hippocampal neurogenesis. These benefits are not specific to SSRIs. Not all SSRIs are implicated in APP ectodomain expression; the chemical structure of paroxetine appears more relevant than its intrinsic SSRI activity. Facilitation of neurogenesis is seen with other non-SSRI psychotropics. Preclinical testing for hippocampal neurogenesis in animal models with higher predictive validity may be warranted, but other antidepressants may be just as likely to provide this benefit. At this time, the preclinical evidence supports SSRIs more specifically as a symptomatic treatment and less convincingly as a unique type of disease modifier.
Clinical evidence
Our search revealed no clinical trials reporting disease modifying benefits of SSRIs, whether in CSF levels of AD biomarkers (eg, beta-amyloid, hyperphosporylated tau), neuropathological findings, or retention or normalization of hippocampal volume or functional imaging characteristics.
Conclusion
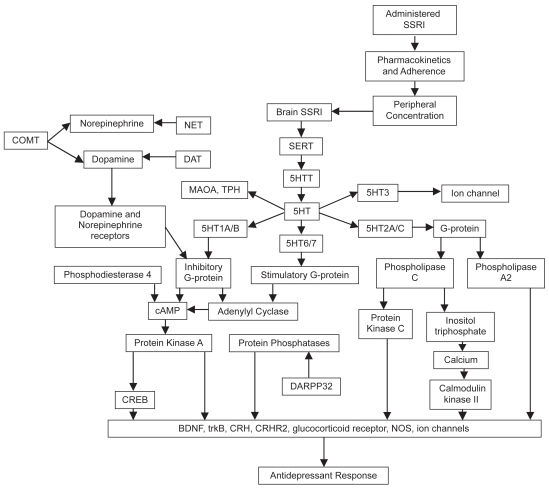
Figure 1 illustrates the wide range of SSRI interactions with other modulators of the CNS, many of which probably impact the neurodegenerative process of AD. Preclinical studies extend promises that the benefits of SSRIs to patients with AD could depend on promotion of hippocampal-dependent learning by carbonic anhydrase activation or reduction of Aβ deposition in the brain. Although there have been clinical trials of SSRIs on AD, outcome measures have not examined the potential effects indicated by preclinical work. The clinical evidence reviewed gives more positive than negative evidence that SSRIs have beneficial effects on cognitive function in AD patients, but these have not been shown to be independent of mood or behavioral improvement. Clinically, this may not be an important point to prove if mood and behavioral improvement add to the patient’s quality of life, but an important question remains unanswered: would SSRIs be of benefit for AD patients who are not actively manifesting mood or behavioral problems? The one negative study designed to address this question warrants replication in a larger sample with one of the SSRIs supported by the preclinical evidence to have effects on AD pathology, eg, fluoxetine, paroxetine, or citalopram.
Figure 1.
Potential cascade of events mediating and moderating SSRI effects. Used with permission from Adis International (Lotrich and Pollock 2005).
Abbreviations: BDNF. Brain-derived neuprtrophic factor; COMT, catecholamine-Omethyl-transferase; CRH, corticotrophin-releasing hormone; CRH2, CFH receptor 2; cAMP, cyclic adenosine monophosphate; CRED, cAMP response element binding protein; DARPP-32, dopamine and cAMP regulated phosphoprotein; DAT, dopamine transporter; MAOA, monoamine oxidase A; NOS, nitric oxide synthase; NET, norepinephrine transporter; 5-HT, serotonin; SERT, serotonin transport; SSRI, selective serotonin reuptake inhibitor; TPH, tryptophan hydroxylase.
Because SSRIs are already widely available to the AD population, an alternative to full development of preclinical evidence would be the addition of appropriate outcome measures for showing disease modification. Clinical trials may not be able to distill cognitive benefit independent of mood or behavior effects unless they incorporate functional neuroimaging to identify brain activity enhanced differentially in cognitive responders vs. non-responders. Ideally, studies should include neurochemical, neuroimaging, mood, behavioral, and cognitive outcome measures.
Acknowledgments
The authors are grateful to Christina Pataky and Joanna Szewczyk for literature search and formatting assistance with this manuscript. This work was funded by NIA grant F32AG022802 (TWC). Dr. Chow serves as a consultant to Janssen-Ortho and CanCog Technologies, Inc. Bruce Pollock has received research support or honoraria from Forest Laboratories, GlaxoSmithKline, Janssen Pharmaceuticals, Lundbeck Inc., Organon, and Sepracor.
References
- Arjona AA, Pooler AM, Lee RK, Wurtman RJ. Effect of a 5-HT(2C) serotonin agonist, dexnorfenfluramine, on amyloid precursor protein metabolism in guinea pigs. Brain Research. 2002;951:135–40. doi: 10.1016/s0006-8993(02)03153-0. [DOI] [PubMed] [Google Scholar]
- Bains J, Birks JS, Dening TR. Antidepressants for treating depression in dementia. Cochrane Database of Systematic Reviews. 2005 doi: 10.1002/14651858.CD003944. [DOI] [PubMed] [Google Scholar]
- Blennow K, Wallin A, Gottfries CG, Lekman A, Karlsson I, Skoog I, Svennerholm L. Significance of decreased lumbar CSF levels of HVA and 5-HIAA in Alzheimer’s disease. Neurobiology of Aging. 1992;13:107–113. doi: 10.1016/0197-4580(92)90017-r. [DOI] [PubMed] [Google Scholar]
- Bowen DM, Palmer AM, Francis PT, Procter AW, Lowe SL. “Classical” neurotransmitters in Alzheimer disease” . In: Terry RD, editor. Aging and the Brain. Raven Press; New York: 1988. p. 115. [Google Scholar]
- Braak H, Braak E. Neuropathological staging of Alzheimer-related changes. Acta Neuropathologica. 1991;82:239–59. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Brane G, Gottfries CG, Blennow K, Karlsson I, Lekman A, Parnetti L, Svennerholm L, Wallin A. Monoamine metabolites in cerebrospinal fluid and behavioral ratings in patients with early and late onset of Alzheimer dementia. Alzheimer Dis Assoc Disord. 1989;3:148–56. doi: 10.1097/00002093-198903030-00004. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Vermetten E. Neuroanatomical changes associated with pharmacotherapy in posttraumatic stress disorder. Annals of the New York Academy of Sciences. 2004;1032:154–7. doi: 10.1196/annals.1314.012. [DOI] [PubMed] [Google Scholar]
- Burt DB, Zembar MJ, Niederehe G. Depression and memory impairment: a meta-analysis of the association, its pattern, and specificity. Psychological Bulletin. 1995;117:285–305. doi: 10.1037/0033-2909.117.2.285. [DOI] [PubMed] [Google Scholar]
- Calabrese F, Molteni R, Maj PF, Cattaneo A, Gennarelli M, Racagni G, Riva MA. Chronic Duloxetine Treatment Induces Specific Changes in the Expression of BDNF Transcripts and in the Subcellular Localization of the Neurotrophin Protein. Neuropsychopharmacology. 2007 doi: 10.1038/sj.npp.1301360. [DOI] [PubMed] [Google Scholar]
- Carlini VP, Gaydou RC, Schioth HB, de Barioglio SR. Selective serotonin reuptake inhibitor (fluoxetine) decreases the effects of ghrelin on memory retention and food intake. Regulatory Peptides. 2007;140:65–73. doi: 10.1016/j.regpep.2006.11.012. [DOI] [PubMed] [Google Scholar]
- Casini A, Caccia S, Scozzafava A, Supuran CT. Carbonic anhydrase activators. The selective serotonin reuptake inhibitors fluoxetine, sertraline and citalopram are strong activators of isozymes I and II. Bioorganic & Medicinal Chemistry Letters. 2003;13:2765–8. doi: 10.1016/s0960-894x(03)00507-9. [DOI] [PubMed] [Google Scholar]
- Chen CP, Eastwood SL, Hope T, McDonald B, Francis PT, Esiri MM. Immunocytochemical study of the dorsal and median raphe nuclei in patients with Alzheimer’s disease prospectively assessed for behavioural changes. Neuropathology and Applied Neurobiology. 2000;26:571–2. doi: 10.1046/j.1365-2990.2000.00254.x. [DOI] [PubMed] [Google Scholar]
- Chen CPLH, Adler JT, Bowen DM. Presynaptic serotonergic markers in community-acquired cases of Alzheimer’s disease: correlations with depression and medication. Journal of Neurochemistry. 1996;66:1592–8. doi: 10.1046/j.1471-4159.1996.66041592.x. [DOI] [PubMed] [Google Scholar]
- Courtney C, Farrell D, Gray R, Hills R, Lynch L, Sellwood E, Edwards S, Hardyman W, Raftery J, Crome P, Lendon C, Shaw H, Bentham PAD. Collaborative Group 2004. Long-term donepezil treatment in 565 patients with Alzheimer’s disease (AD2000): randomised double-blind trial. Lancet. 2000;363:2105–15. doi: 10.1016/S0140-6736(04)16499-4. [DOI] [PubMed] [Google Scholar]
- Cutler NR, Haxby J, Kay AD, Narang PK, Lesko LJ, Costa JL, Ninos M, Linnoila M, Potter WZ, Renfrew J, et al. Evaluation of zimeldine in Alzheimer’s disease. Cognitive and biochemical measures. Arch Neurol. 1985;42:744–8. doi: 10.1001/archneur.1985.04210090008003. [DOI] [PubMed] [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Ober BA. Harcourt Brace Jovanovich, Inc; San Antonio: 1987. [Google Scholar]
- Dong H, Goico B, Martin M, Csernansky CA, Bertchume A, Csernansky JG. Modulation of hippocampal cell proliferation, memory, and amyloid plaque deposition in APPsw (Tg2576) mutant mice by isolation stress. Neuroscience. 2004;127:601–9. doi: 10.1016/j.neuroscience.2004.05.040. [DOI] [PubMed] [Google Scholar]
- Dumont GJ, de Visser SJ, Cohen AF, van Gerven JM Biomarker Working Group of the German Association for Applied Human Pharmacology. Biomarkers for the effects of selective serotonin reuptake inhibitors (SSRIs) in healthy subjects. British Journal of Clinical Pharmacology. 2005;59:495–510. doi: 10.1111/j.1365-2125.2005.02342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert A, Cotman CW, Zerfass R, Hennerici M, Muller WE. Lymphocytes as cell model to study apoptosis in Alzheimer’s disease: vulnerability to programmed cell death appears to be altered. Journal of Neural Transmission Supplement. 1998;54:259–67. doi: 10.1007/978-3-7091-7508-8_25. [DOI] [PubMed] [Google Scholar]
- Egashira N, Matsumoto Y, Mishima K, Iwasaki K, Fujioka M, Matsushita M, Shoyama Y, Nishimura R, Fujiwara M. Low dose citalopram reverses memory impairment and electroconvulsive shock-induced immobilization. Pharmacology, Biochemistry and Behavior. 2006;83:161–7. doi: 10.1016/j.pbb.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Etcheberrigaray R, Bhagavan S. Ionic and signal transduction alterations in Alzheimer’s disease: relevance of studies on peripheral cells. Molecular Neurobiology. 1999;20:93–109. doi: 10.1007/BF02742436. [DOI] [PubMed] [Google Scholar]
- Finkel SI, Mintzer JE, Dysken M, Krishnan KR, Burt T, McRae T. A randomized, placebo-controlled study of the efficacy and safety of sertraline in the treatment of the behavioral manifestations of Alzheimer’s disease in outpatients treated with donepezil. International journal of geriatric psychiatry. 2004;19:9–18. doi: 10.1002/gps.998. [DOI] [PubMed] [Google Scholar]
- Gobbi G, Blier P. Effect of neurokinin-1 receptor antagonists on serotoninergic, noradrenergic and hippocampal neurons: comparison with antidepressant drugs. Peptides. 2005;26:1383–93. doi: 10.1016/j.peptides.2005.03.032. [DOI] [PubMed] [Google Scholar]
- Griffin WS, Sheng JG, Mrak RE. Senescence-accelerated overexpression of S100beta in brain of SAMP6 mice. Neurobiology of Aging. 1998;19:71–6. doi: 10.1016/s0197-4580(97)00167-x. [DOI] [PubMed] [Google Scholar]
- Hajos-Korcsok E, McTavish SF, Sharp T. Effect of a selective 5-hydroxytryptamine reuptake inhibitor on brain extracellular noradrenaline: microdialysis studies using paroxetine. European Journal of Pharmacology. 2000;407:101–7. doi: 10.1016/s0014-2999(00)00723-8. [DOI] [PubMed] [Google Scholar]
- Haring JH, Hagan A, Olson J, Rodgers B. Hippocampal serotonin levels influence the expression of S100 beta detected by immunocytochemistry. Brain Research. 1993;631:119–23. doi: 10.1016/0006-8993(93)91195-x. [DOI] [PubMed] [Google Scholar]
- Huang SC, Tsai SJ, Chang JC. Fluoxetine-induced memory impairment in four family members. International Journal of Psychiatry in Medicine. 2004;34:197–200. doi: 10.2190/DY6D-33BP-5WPB-1VDX. [DOI] [PubMed] [Google Scholar]
- Jack CRJ, Shiung MM, Weigand SD, O’Brien PC, Gunter JL, Boeve BF, Knopman DS, Smith GE, Ivnik RJ, Tangalos EG, Petersen RC. Brain atrophy rates predict subsequent clinical conversion in normal elderly and amnestic MCI. Neurology. 2005;65:1227–31. doi: 10.1212/01.wnl.0000180958.22678.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joss JD, Burton RM, Keller CA. Memory loss in a patient treated with fluoxetine. The Annals of Pharmacotherapy. 2003;37:1800–3. doi: 10.1345/aph.1D154. [DOI] [PubMed] [Google Scholar]
- Jossan SS, Adem A, Winblad B, Oreland L. Characterisation of dopamine and serotonin uptake inhibitory effects of tetrahydroaminoacridine in rat brain. Pharmacol Toxicol. 1992;71:213–5. doi: 10.1111/j.1600-0773.1992.tb00548.x. [DOI] [PubMed] [Google Scholar]
- Khaliq S, Haider S, Ahmed SP, Perveen T, Haleem DJ. Relationship of brain tryptophan and serotonin in improving cognitive performance in rats. Pakistan Journal of Pharmaceutical Sciences. 2006;19:11–5. [PubMed] [Google Scholar]
- Kogen H, Toda N, Tago K, Marumoto S, Takami K, Ori M, Yamada N, Koyama K, Naruto S, Abe K, Yamazaki R, Hara T, Aoyagi A, Abe Y, Kaneko T. Design and synthesis of dual inhibitors of acetylcholinesterase and serotonin transporter targeting potential agents for Alzheimer’s disease. Organic Letters. 2002;4:3359–62. doi: 10.1021/ol026418e. [DOI] [PubMed] [Google Scholar]
- Lanctot KL, Herrmann N, Yau KK, Khan LR, Liu BA, LouLou MM, Einarson TR. Efficacy and safety of cholinesterase inhibitors in Alzheimer’s disease: a meta-analysis. Canadian Medical Association Journal. 2003;169:557–64. [PMC free article] [PubMed] [Google Scholar]
- Lotrich FE, Pollock BG. Candidate genes for antidepressant response to selective serotonin reuptate inhibitors. Neuropsychiatr Dis Treat. 2005;1:17–35. doi: 10.2147/nedt.1.1.17.52301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyketsos CG, DelCampo L, Steinberg M, Miles Q, Steele CD, Munro C, Baker AS, Sheppard JM, Frangakis C, Brandt J, Rabins PV. Treating depression in Alzheimer disease: efficacy and safety of sertraline therapy, and the benefits of depression reduction: the DIADS. Arch Gen Psychiatry. 2003;60:737–46. doi: 10.1001/archpsyc.60.7.737. [DOI] [PubMed] [Google Scholar]
- MacPherson A, Dinkel K, Sapolsky R. Glucocorticoids worsen excitotoxin-induced expression of pro-inflammatory cytokines in hippocampal cultures. Experimental Neurology. 2005;194:376–83. doi: 10.1016/j.expneurol.2005.02.021. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Maudsley S, Martin B. BDNF and 5-HT: a dynamic duo in age-related neuronal plasticity and neurodegerative disorders. Trends in Neuroscience. 2004;27:589–94. doi: 10.1016/j.tins.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Meeter M, Talamini L, Schmitt JA, Riedel WJ. Effects of 5-HT on memory and the hippocampus: model and data. Neuropsychopharmacology. 2006;31:712–20. doi: 10.1038/sj.npp.1300869. [DOI] [PubMed] [Google Scholar]
- Meneses A. Physiological, pathophysiological and therapeutic roles of 5-HT systems in learning and memory. Reviews in the Neurosciences. 1998;9:275–89. doi: 10.1515/revneuro.1998.9.4.275. [DOI] [PubMed] [Google Scholar]
- Meneses A. Effects of the 5-HT(6) receptor antagonist Ro 04-6790 on learning consolidation. Behav Brain Res. 2001;118:107–10. doi: 10.1016/s0166-4328(00)00316-8. [DOI] [PubMed] [Google Scholar]
- Meneses A. A pharmacological analysis of an associative learning task: 5-HT1 to 5-HT7 receptor subtypes function on a Pavlovian/instrumental autoshaped memory. Learning and Memory. 2003;10:363–72. doi: 10.1101/lm.60503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meneses A, Hong E. Effect of fluoxetine on learning and memory involves multiple 5-HT systems. Pharmacol Biochem Behav. 1995;52:341–6. doi: 10.1016/0091-3057(95)00102-3. [DOI] [PubMed] [Google Scholar]
- Meneses A, Terron JA. Role of 5-HT(1A) and 5-HT(7) receptors in the facilitatory response induced by 8-OH-DPAT on learning consolidation. Behav Brain Res. 2001;121:21–8. doi: 10.1016/s0166-4328(00)00378-8. [DOI] [PubMed] [Google Scholar]
- Meneses A, Terron JA, Hong E. Effects of the 5-HT receptor antagonists GR127935 (5-HT1B/1D) and MDL100907 (5-HT2A) in the consolidation of learning. Behav Brain Res. 1997;89:217–23. doi: 10.1016/s0166-4328(97)00055-7. [DOI] [PubMed] [Google Scholar]
- Mitchell ES, Neumaier JF. 5-HT6 receptors: a novel target for cognitive enhancement. Pharmacology and Therapeutics. 2005;108:320–33. doi: 10.1016/j.pharmthera.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Mnie-Filali O, El Mansari M, Espana A, Sanchez C, Haddjeri N. Allosteric modulation of the effects of the 5-HT reuptake inhibitor escitalopram on the rat hippocampal synaptic plasticity. Neuroscience Letters. 2006;395:23–7. doi: 10.1016/j.neulet.2005.10.044. [DOI] [PubMed] [Google Scholar]
- Morse LJ, Payton SM, Cuny GD, Rogers JT. FDA-preapproved drugs targeted to the translational regulation and processing of the amyloid precursor protein. Journal of Molecular Neuroscience. 2004;24:129–36. doi: 10.1385/JMN:24:1:129. [DOI] [PubMed] [Google Scholar]
- National Institute on Aging, aRIWGoDCftNAoAsD. Consensus recommendations for the postmortem diagnosis of Alzheimer’s disease. Neurobiology of Aging. 1997;18:S1–2. [PubMed] [Google Scholar]
- Nazarali AJ, Reynolds GP. Monoamine neurotransmitters and their metabolites in brain regions in Alzheimer’s disease: a post-mortem study. Cellular and Molecular Neurobiology. 1992;12:581–7. doi: 10.1007/BF00711237. [DOI] [PubMed] [Google Scholar]
- Nibuya M, Nestler EJ, Duman RS. Chronic antidepressant administration increases the expression of cAMP response element binding protein (CREB) in rat hippocampus. Journal of Neuroscience. 1996;16:2365–72. doi: 10.1523/JNEUROSCI.16-07-02365.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyth AL, Gottfries CG. The clinical efficacy of citalopram in treatment of emotional disturbances in dementia disorders. A Nordic multicentre study. Br J Psychiatry. 1990;157:894–901. doi: 10.1192/bjp.157.6.894. [DOI] [PubMed] [Google Scholar]
- Ohashi S, Matsumoto M, Otani H, Mori K, Togashi H, Ueno K, Kaku A, Yoshioka M. Changes in synaptic plasticity in the rat hippocampomedial prefrontal cortex pathway induced by repeated treatments with fluvoxamine. Brain Research. 2002;949:131–8. doi: 10.1016/s0006-8993(02)02973-6. [DOI] [PubMed] [Google Scholar]
- Page ME, Abercrombie ED. An analysis of the effects of acute and chronic fluoxetine on extracellular norepinephrine in the rat hippocampus during stress. Neuropsychopharmacology. 1997;16:419–25. doi: 10.1016/S0893-133X(96)00281-3. [DOI] [PubMed] [Google Scholar]
- Palotas A, Kalman J, Palotas M, Juhasz A, Janka Z, Penke B. Beta-amyloid induced increase in the resting intracellular calcium concentration gives support to tell Alzheimer lymphocytes from control ones. Brain Research Bulletin. 2002;58:203–5. doi: 10.1016/s0361-9230(02)00773-6. [DOI] [PubMed] [Google Scholar]
- Palotas A, Puskas LG, Kitajka K, Palotas M, Molnar J, Pakaski M, Janka Z, Penke B, Kalman J. The effect of citalopram on gene expression profile of Alzheimer lymphocytes. Neurochemical Research. 2004;29:1563–70. doi: 10.1023/b:nere.0000029570.57903.74. [DOI] [PubMed] [Google Scholar]
- Payton S, Cahill CM, Randall JD, Gullans SR, Rogers JT. Drug discovery targeted to the Alzheimer’s APP mRNA 5’-untranslated region: the action of paroxetine and dimercaptopropanol. Journal of Molecular Neuroscience. 2003;20:267–75. doi: 10.1385/JMN:20:3:267. [DOI] [PubMed] [Google Scholar]
- Pollock BG, Mulsant BH, Rosen J, Sweet R, Mazumdar S, Bharucha A, Marin R, Jacob NJ, Huber KA, Kastango KB, Chew ML. Comparison of Citalopram, Perphenazine, and placebo for the acute treatment of psychosis and behavioral disturbances in hospitalized, demented patients. American Journal of Psychiatry. 2002;159:460–65. doi: 10.1176/appi.ajp.159.3.460. [DOI] [PubMed] [Google Scholar]
- Pollock BG, Rosen J, Mulsant BH. Antipsychotics and selective serotonin reuptake inhibitors for the treatment of behavioral disturbances in dementia of the Alzheimer type: a review of clinical data. Consultant Pharmacist. 1999;11:1251–58. [Google Scholar]
- Procter AW, Francis PT, Stratmann GC, Bowen DM. Serotonergic pathology is not widespread in Alzheimer’s patients without prominent aggressive symptoms. Neurochemical Research. 1992;17:917–22. doi: 10.1007/BF00993268. [DOI] [PubMed] [Google Scholar]
- Reeves R, Yao JMRC, Buck S, Zhang X, Yarowsky P, Gearhart JD, Hilt DC. Astrocytosis and axonal proliferation in the hippocampus of S100b transgenic mice. Proceedings of the National Academy of Science U S A. 1994;91:5359–63. doi: 10.1073/pnas.91.12.5359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinikainen KJ, Soininen H, Riekkinen PJ. A post-mortem study of noradrenergic, serotonergic and gabaergic neurons in Alzheimer’s disease. Journal of the Neurological Sciences. 1988;84:101–16. doi: 10.1016/0022-510x(88)90179-7. [DOI] [PubMed] [Google Scholar]
- Sapolsky R. Taming stress. Scientific American. 2003;289:86–95. doi: 10.1038/scientificamerican0903-86. [DOI] [PubMed] [Google Scholar]
- Schechter LE, Smith DL, Rosenzweig-Lipson S, Sukoff SJ, Dawson LA, Marquis K, Jones D, Piesla M, Andree T, Nawoschik S, Harder JA, Womack MD, Buccafusco J, Terry AV, Hoebel B, Rada P, Kelly M, Abou-Gharbia M, Barrett JE, Childers WJ. Lecozotan (SRA-333): a selective serotonin 1A receptor antagonist that enhances the stimulated release of glutamate and acetylcholine in the hippocampus and possesses cognitive-enhancing properties. Pharmacol Exp Ther. 2005;314:1274–89. doi: 10.1124/jpet.105.086363. [DOI] [PubMed] [Google Scholar]
- Schiapparelli L, Del Rio J, Frechilla D. Serotonin 5-HT receptor blockade enhances Ca(2+)/calmodulin-dependent protein kinase II function and membrane expression of AMPA receptor subunits in the rat hippocampus: implications for memory formation. Journal of Nerochemistry. 2005;94:884–95. doi: 10.1111/j.1471-4159.2005.03193.x. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Sanghavi M, Mintun MA, Gado MH. Depression duration but not age predicts hippocampal volume loss in medically healthy women with recurrent major depression. Journal of Neuroscience. 1999;19:5034–43. doi: 10.1523/JNEUROSCI.19-12-05034.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng JG, Mrak RE, Rovnaghi CR, Kozlowska E, Van Eldik LJ, Griffin WS. Human brain S100 beta and S100 beta mRNA expression increases with age: pathogenic implications for Alzheimer’s disease. Neurobiology of Aging. 1996;17:359–63. doi: 10.1016/0197-4580(96)00037-1. [DOI] [PubMed] [Google Scholar]
- Sjogren M, Minthon L, Passant U, Blennow K, Wallin A. Decreased monoamine metabolites in frontotemporal dementia and Alzheimer’s disease. Neurobiology of Aging. 1998;19:379–84. doi: 10.1016/s0197-4580(98)00086-4. [DOI] [PubMed] [Google Scholar]
- Smith JE, Lakoski JM. Cellular electrophysiological effects of chronic fluoxetine and duloxetine administration on serotonergic responses in the aging hippocampus. Synapse. 1998;30:318–28. doi: 10.1002/(SICI)1098-2396(199811)30:3<318::AID-SYN9>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Stewart CA, Reid IC. Repeated ECS and fluoxetine administration have equivalent effects on hippocampal synaptic plasticity. Psychopharmacology. 2000;148:217–23. doi: 10.1007/s002130050045. [DOI] [PubMed] [Google Scholar]
- Sun M-K, Alkon DL. Pharmacological enhancement of synaptic efficacy, spatial learning, and memory through carbonic anhydrase activation in rats. The Journal of Pharmacology and Experimental Therapeutics. 2001;297:961–67. [PubMed] [Google Scholar]
- Thomas DN, Nutt DJ, Holman RB. Sertraline, a selective serotonin reuptake inhibitor modulates extracellular noradrenaline in the rat frontal cortex. Journal of Psychopharmacology. 1998;12:366–70. doi: 10.1177/026988119801200406. [DOI] [PubMed] [Google Scholar]
- Thomasius R, Zapletalova P, Petersen K, Buchert R, Andresen B, Wartberg L, Nebeling B, Schmoldt A. Mood, cognition and serotonin transporter availability in current and former ecstasy (MDMA) users: the longitudinal perspective. Journal of Psychopharmacology. 2006;20:211–25. doi: 10.1177/0269881106059486. [DOI] [PubMed] [Google Scholar]
- Tohgi H, Abe T, Takahashi S, Saheki M, Kimura M. Indoleamine concentrations in cerebrospinal fluid from patients with Alzheimer type and Binswanger type dementias before and after administration of citalopram, a synthetic serotonin uptake inhibitor. Journal of Neural Transmission. Parkinsons Disease and Dementia Section. 1995;9:121–31. doi: 10.1007/BF02259654. [DOI] [PubMed] [Google Scholar]
- Ueda S, Sakakibara S, Yoshimoto K. Effect of long-lasting serotonin depletion on environmental enrichment-induced neurogenesis in adult rat hippocampus and spatial learning. Neuroscience. 2005;135:395–402. doi: 10.1016/j.neuroscience.2005.06.065. [DOI] [PubMed] [Google Scholar]
- van der Veen FM, Evers EA, van Deursen JA, Deutz NE, Backes WH, Schmitt JA. Acute tryptophan depletion reduces activation in the right hippocampus during encoding in an episodic memory task. Neuroimage. 2006;31:1188–96. doi: 10.1016/j.neuroimage.2006.01.014. [DOI] [PubMed] [Google Scholar]
- Vizi ES, Zsilla G, Caron MG, Kiss JP. Uptake and release of norepinephrine by serotonergic terminals in norepinephrine transporter knock-out mice: implications for the action of selective serotonin reuptake inhibitors. Journal of Neuroscience. 2004;24:7888–94. doi: 10.1523/JNEUROSCI.1506-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Harcourt, Brace, Jovanovich, Inc; San Antonio: 1987. [Google Scholar]