Abstract
The effect of early-life vulnerability factors on the subsequent pathophysiology of severe mood disorders has yet to be fully elucidated. This study examines the relationship between early adverse life experience, family history and hypothalamic-pituitary-adrenal (HPA) axis function. Childhood trauma questionnaire (CTQ) scores, family history data and the cortisol response to the dexamethasone/corticotrophin releasing hormone (dex/CRH) test were examined in 40 patients with severe mood disorder. Normative data for the CTQ was also obtained. The study demonstrated that mood disorder patients reporting high levels of childhood emotional neglect (n = 26) had an HPA axis response which did not differ from controls, whereas patients reporting low levels (n = 19) had an enhanced response (p = 0.011). A positive family history of mood disorder further enhanced this response. These data suggest that early adverse life events and genetic susceptibility have dissociable effects on glucocorticoid receptor-mediated negative feedback of the HPA axis in adult patients with severe mood disorders.
Keywords: childhood trauma, cortisol, bipolar disorder, chronic depression, family history
Background
The neurobiological underpinnings of the vulnerability to mood disorders conferred by childhood adversity and genetic predisposition have not been established. Here we present a brief overview of this topic with regard to hypothalamic-pituitary-adrenal (HPA) axis function and report on a recent study which attempted to elucidate the role of familial history of mood disorder and adverse events in childhood on HPA axis dysfunction in mood disorder patients.
Introduction
Dysfunction of the HPA axis is a widely reproduced finding in affective disorders (eg, Carroll 1982; Heuser et al 1994; Rush et al 1996; Gallagher et al 2007). Although elevated basal cortisol levels can be demonstrated peripherally in measures taken from saliva or urine, more sensitive integrated HPA axis challenges, such as the dexamethasone suppression test (DST) or the dexamethasone/corticotrophin releasing hormone (dex/CRH) test, reveal higher rates of abnormality (Heuser et al 1994). Such tests are more sensitive as they assess facets of the HPA axis which are considered to be primary causal factors, namely dysregulation of hypothalamic drive (Holsboer 2002) and reduced glucocorticoid receptor (GR) feedback (McQuade and Young 2000; Pariante and Miller 2001). We, and others have previously reported an enhanced response to the dex/CRH in the bipolar patients (Rybakowski and Twardowska 1999; Schmider et al 1995; Watson et al 2004). In chronic depression the results have been more equivocal, with a greater proportion of patients having a normal response to the dex/CRH test and to the DST (Watson et al 2002).
There is an established literature in rodents and non-human primates showing that early life stress influences the development of the HPA axis (Ladd et al 2004; Lightman et al 2002). The neurobiological consequences of early adversity have also been studied in man (Teicher et al 2003). Sexual abuse has been predominantly the focus of this work. These studies suggest that child sexual abuse may act via the HPA axis to mediate a vulnerability to both depression and post-traumatic stress disorder. It has been demonstrated that adult survivors of abuse who have depression or post-traumatic stress disorder (PTSD) have blunted adrenocorticotropic-releasing hormone (ACTH) and cortisol responses to CRF stimulation (Heim et al 2001) and it has been hypothesized that this is due to CRF receptor down regulation secondary to chronic CRF hypersecretion (Newport et al 2004). This group has also shown that depressed childhood abuse survivors have an enhanced HPA axis response to psychosocial stress (Heim et al 2000) and an attenuated ACTH and cortisol response to the low dose dexamethasone suppression test (DST) (Newport et al 2004). This latter finding is of importance as it implicates the glucocorticoid receptor as a potential mediating factor in the HPA disturbance. However, it should be noted that it was only evident in psychiatrically ill trauma survivors, but not in non-depressed abuse survivors, indicating that enhanced GR-feedback may not an invariable consequence of childhood trauma but may be related to consequent psychiatric illness that often occurs in traumatized individuals (Newport et al 2004).
The impact of a positive family history of mood disorder on HPA axis function in healthy subjects has been examined in several studies. Holsboer and colleagues found that healthy subjects with a high genetic load for affective disorders had an elevated cortisol response to the dex/CRH test which was between that of a control group and that of depressed patients (Holsboer et al 1995). These subjects were followed up 4 years later and their responses to the dex/CRH test were found to be relatively stable over this period (Modell et al 1998). Matsubara and colleagues recently found evidence of reduced mRNA expression of GR-alpha (the GR isoform which binds glucocorticoids) in peripheral blood cells of patients with bipolar disorder and in patients with major depressive disorder, both while depressed as well as in remission. Interestingly, first-degree relatives of patients with bipolar disorder also showed GR-alpha mRNA reduction (Matsubara et al 2006). Together these studies suggest that HPA axis abnormalities, especially at the GR level, may be a trait marker in severe psychiatric illness.
We have previously examined GR function in patients with DSM-IV confirmed chronic sub-type of major depressive disorder (cMDD) (Watson et al 2002) and bipolar disorder (BD) (Watson et al 2004) using delta (Δ) cortisol (peak minus 1500h (pre-CRH) cortisol) as the outcome measure on the dex/CRH test. In the present study we sought to examine childhood trauma and family history of mood disorder as potential mediators of this response. We hypothesized that in adult mood-disorder patients, childhood trauma and genetic vulnerability has an additive effect upon GR dysfunction. We examined this relationship by comparing childhood trauma questionnaire (CTQ) subscale scores, family history and the cortisol response on the dex/CRH test in patients with BD and cMDD.
Methods
Thirty DSM-IV confirmed BD patients (15 male, 15 female) and 10 cMDD patients (6 male, 4 female) participated in this study. Patients were recruited from out-patient clinics in secondary and tertiary care in the North-East of England and had previously participated in a program of research into neurobiological abnormalities in mood disorder in which the DST and dex/CRH tests were administered (Watson et al 2002, 2004). Neuroendocrine data was available for comparison from 28 healthy controls who had also participated in this program.
Illness characteristics for the patient groups are shown in Table 1. Of the BD patients, 5 were taking carbamazepine, 4 lamotrigine, 16 lithium, 4 gabapentin, 8 valproate, 5 an atypical and 4 a typical antipsychotic, 3 were taking trazodone, 1 venlafaxine, 5 an SSRI, 1 reboxetine and 1 L-tryptophan, of the cMDD patients 5 were taking venlafaxine, 3 mirtazepine, 2 an SSRI, 1 mianserin, 1 a tricyclic, 1 trazodone, 3 lithium, 2 valproate and 1 a typical and 1 an atypical antipsychotic.
Table 1.
Illness and demographic characteristics of patients
| cMDD (n = 10) | BD (n = 30) | |
|---|---|---|
| Mean (SD) | Mean (SD) | |
| Age (years) | 51.9(7.2) | 47.5(7.2) |
| Sex (n, male:female) | 6:4 | 15:15 |
| Duration of current episode (months) | 77.1(62.1) | 27.6(55.3) |
| Age of onset (years) | 33.1(9.4) | 27.9(8.8) |
| Number of psychiatric admissions | 2.8(5.0) | 5.2(4.8) |
| Number of previous depressive episodes | 1.9(3.0) | 39.5(66.5) |
| Lifetime number of months depressed | 90.7(77.7) | 64.9(68.4) |
| Number of previous manic episodes | – | 36.5(69.0) |
| Lifetime number of months manic | – | 16.2(21.5) |
| HDRS21 | 21.8(.5) | 6.5(7.6) |
| YMRS | 0.9(1.4) | 1.0(1.9) |
| GAF | 49.9(9.2) | 62.8(17.4) |
Abbreviations: HDRS21, 21 item Hamilton Depression Rating Scale; YMRS, Young Mania Rating Scale; GAF, Global Assessment of Functioning scale.
Procedure
Patients were asked to complete the childhood trauma questionnaire (CTQ). The CTQ is a valid and reliable 28-item retrospective self-report inventory (Bernstein and Fink 1998; Paivio and Cramer 2004). Items are scored 1 to 5 on a Likert scale. It has five subscales: emotional abuse, physical abuse, sexual abuse, emotional neglect and physical neglect. CTQ data was not available for the control subjects. However, normative CTQ data is available for comparison from a study by Scher et al (2001) of 1,000 male and female residents of the metropolitan Memphis, Tennessee area between the ages of 18 and 65 years (mean age 40).
Family history of affective disorder in first-degree relatives was determined by self-report during a semi-structured clinical interview for all patients. Family history of mood disorder was an exclusion criterion for controls.
Prior to participation, written informed consent was obtained from all participants following a complete description of the study. The study was approved by the Local Research Ethics Committee.
Statistical analysis
CTQ scores were compared between the clinical groups and with existing normative data (Scher et al 2001). Scores on each subscale were dichotomized into low (CTQ classifications: ‘none or minimal’ and ‘low to moderate’) or high (CTQ classifications: ‘moderate to severe’ and ‘severe to extreme’). CTQ sub-scale scores for bipolar patients were also compared with patients with cMDD.
The distribution of the neuroendocrine data led to a non-parametric statistical approach. Group differences were expressed as the median-difference and 95 % CI and analyzed by Mann-Whitney-U or Kruskal-Wallis-H tests. Δ-cortisol was compared between those with high and low levels of trauma on each of the CTQ sub-scales, for the total patient group and after dividing the group by gender and by diagnosis. To examine the relationship between family history, childhood trauma and HPA axis function, a Kruskal-Wallis-H test comparison of Δ-cortisol was made with four groups (i) high emotional neglect, with positive FH; (ii) high emotional neglect, with negative FH; (iii) low emotional neglect, with positive FH; (iv) low emotional neglect, with negative FH.
ROC analysis on the original data set (Watson et al 2004) suggested that a Δ-cortisol cut-point of 33.49 nmol/l had the highest discriminatory value. This cut-point was utilized to differentiate dex/CRH responders from non-responders. Fisher’s Exact test was used to compare rates of response to the dex/CRH test between patients with high and low levels of trauma for the CTQ sub-scales. Δ-cortisol reference data were available for comparison from 28 healthy controls (Watson et al 2004).
Results
CTQ data
CTQ total and sub-scale scores are presented in Table 2. Both male and female subjects reported higher levels of emotional abuse, emotional neglect, sexual abuse and physical neglect but not physical abuse than the normative sample. There was no significant group difference between BD and cMDD patients in total CTQ scores or any of the CTQ sub-scales (p > 0.4).
Table 2.
CTQ scores (data shown are mean with standard deviation in parenthesis) compared with normative data (Scher et al 2001)
| Subjects | Normative data | ||||||
|---|---|---|---|---|---|---|---|
| CTQ scale | Total group (n = 40) | Bipolar (n = 30) | cMDD (n = 10) | Male (n = 21) | Female (n = 19) | Male | Female |
| Total CTQ | 47.8(17.2) | 46.6(17.7) | 51.2(16.1) | 45.4(14.6)a | 50.4(19.7)a | 31.7(9.1) | 31.8(11.2) |
| Emotional abuse | 11.8(6.2) | 11.7(6.1) | 12.2(6.8) | 11.1(5.5)a | 12.6(6.9)a | 6.5(2.9) | 7.0(3.7) |
| Physical abuse | 7.1(2.8) | 7.4(3.0) | 6.3(2.3) | 6.8(2.5) | 7.5(3.2) | 6.6(2.3) | 6.5(2.5) |
| Sexual abuse | 8.6(6.4) | 8.5(6.3) | 9.1(7.3) | 7.6(5.4)a | 9.8(7.4)a | 5.2(1.4) | 5.7(2.6) |
| Emotional neglect | 12.7(5.9) | 12.1(6.2) | 14.5(5.0) | 12.0(5.0)a | 13.5(6.8)a | 7.0(3.6) | 6.8(3.5) |
| Physical neglect | 7.5(3.2) | 7.0(2.2) | 9.1(4.8) | 7.9(3.9)b | 7.1(2.1)c | 6.4(2.1) | 6.0(2.2) |
p < 0.0001;
p < 0.005;
p < 0.05
Neuroendocrine data
Patient groups vs control data
Δ-cortisol data are presented in Table 3. Kruskal-Wallis analysis reveals that the 3 groups (controls1 vs patients with high CTQ scores vs patients with low CTQ scores) differ in Δ-cortisol levels (H >18, df = 3, p< 0.0005). After separating by individual CTQ subscales, Mann-Whitney analyses revealed that both patient groups (ie, high and low groups) had significantly higher Δ-cortisol levels than controls within the emotional abuse, sexual abuse and physical neglect scales (p < 0.025). Within the physical abuse and emotional neglect scales, Δ-cortisol levels were also significantly higher than controls in the patient group with low (but not high) CTQ ratings (p < 0.025).
Table 3.
Δcortisol response to the dex/CRH test for each of the CTQ sub-scales for those with high and low levels of childhood trauma
| CTQ subscale | Lowa | Higha | Effect sizeb (Cohen’s d) | Median differenceb (95%CI) |
|---|---|---|---|---|
| Emotional abuse | 70.6(25.5 to 123.1)
n = 25 |
9.8(1.6 to 169.9)
n = 15 |
0.19 | 9.0(−19.4 to 79.7) |
| Physical abuse | 76.4(7.2 to 133.7)
n = 34 |
6.1(1.4 to 169.9)
n = 6 |
0.74 | 47.7(−2.6 to 132.1) |
| Sexual abuse | 40.9(5.2 to 112.9)
n = 30 |
114.8(5.1 to 169.9)
n = 10 |
0.09 | 20.1(−37.7 to 98.3) |
| Emotional neglect | 93.1(44.2 to 169.9)
n = 26 |
5.8(1.4 to 139.8)1 n = 14 |
0.81 | 68.2(3.8 to 120.7) |
| Physical neglect | 68.6(7.2 to 123.1)
n = 31 |
16.5(1.4 to 147.5)
n = 9 |
0.24 | 3.8(−51.5 to 83.6) |
Δ cortisol data reported as median (95%CI).
Comparison of patients with high vs low levels of abuse on the CTQ sub-scales.
Direct comparison of patients with high vs low CTQ scores
Δ cortisol levels are compared between patients with high and low levels of childhood trauma as recorded using the sub-scales of the CTQ. The effect size and median difference are reported Table 3. Significantly lower Δ-cortisol responses were observed in patients reporting high emotional neglect compared to low emotional neglect (median difference = 68.2, 95% CI = 3.8 to 120.7; U = 93, z = 2.52, p = 0.011). No differences were observed between the groups on the other CTQ subscales (p > 0.1 for all).
Effects of diagnosis or gender on response
The significant difference in Δ cortisol between patients with high and low levels of emotional neglect (EN) was present when females were examined alone (median difference = 109.3, 95% CI = 3.4 to 255.8; U = 12, z = 2.64, p = 0.008 [low EN: n = 11, median = 133.7; high EN: n = 8, median = 4.45]) but not when males were examined alone (median difference = 28.4, 95% CI = −65.4 to 114.1; U = 32, z = 1.01, p = 0.32 [low EN: n = 15, median = 82.1; high EN: n = 6, median = 10.8]). No differences were observed between groups classified as high vs low based on the other CTQ subscales after separation by gender.
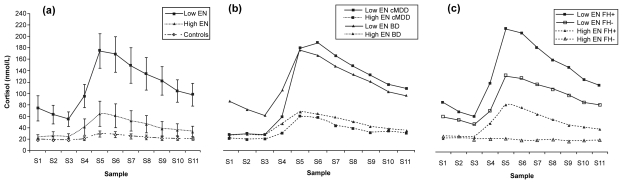
After dividing patients by diagnosis (see Figure 1b), Δ-cortisol was significantly lower in BD patients with high vs low ratings of emotional neglect (high EN: n = 9, median = 2.1 vs low EN: n = 21, median = 82.1; median difference = 62.1, 95% CI = 0 to 120.7; U = 60.5, z = 1.97, p = 0.05) but not in cMDD patients (high EN: n = 5, median = 9.8 vs low EN: n = 5, median = 172.9; median difference = 124.1, 95% CI = −34.1 to 261.8; U = 5.0, z = 1.6, p = 0.12).
Figure 1.
(a) Cortisol responses to the dex/CRH test in patients reporting high vs low emotional neglect (including control data for reference); (b) separated by diagnosis; (c) separated by positive or negative family history. Low EN, low emotional neglect; High EN, high emotional neglect; FH+, positive family history of mood disorder; FH−, negative family history of mood disorder; cMDD, chronic major depressive disorder; BD, bipolar disorder.
Effect of family history of mood disorder
Seven unipolar and 16 bipolar patients had a positive family history. Dividing patients by both family history and emotional neglect (see Figure 1c) revealed that the four consequent groups differed significantly in Δ-cortisol (H = 7.9, df = 3, p = 0.048). This difference was found in females (H = 8.9, df = 3, p = 0.036) but not in males.
Chi-squared analysis revealed that the proportion of patients who responded to the dex/CRH test was higher in those with low EN (73% of low EN patients were responders, 27% of low EN patients were non-responders) compared to those with high EN (29% responded, 71% were non-responders; Fisher’s Exact, p < 0.0005). This difference was statistically significant when females were considered alone (Fisher’s Exact, p = 0.024) but not when males were considered alone (Fisher’s Exact, p = 0.33). There were no significant differences in the proportion of responders for any of the other CTQ sub-scales.
Discussion
This is the first study to examine the relationship between retrospectively reported childhood trauma, family history and neuroendocrine function in mood disorder patients. Contrary to our hypothesis, patients reporting greater emotional neglect in childhood exhibited a cortisol response to the dex/CRH test which was indistinguishable from the controls response. Patients with lower reported levels of emotional neglect demonstrated an exaggerated response. When the sample was divided into sub-groups by diagnosis and sex, a similar pattern of response was seen, which was significant in BD patients but not in cMDD, and in females but not males. No differences were observed when the groups were separated by any other CTQ subscale. The relationship between emotional neglect and Δ-cortisol was different in those with a family history; a positive family history was associated with a greater HPA axis response (Figure 1c).
There are some potential confounds to the current data that should be considered when discussing the findings. First, data on family history and PTSD was collected by subject self-report and may therefore be non-robust. This could be improved in future studies using more specific structured interview. Also, the use of the CTQ to retrospectively assess childhood trauma may be subject to recall biases, especially in individuals who are currently depressed. The use of medication may have affected neuroendocrine responses and studies in drug-free patients would be desirable. Finally, CTQ data from patients in the study was compared to published normative data rather than to a healthy control population from a similar, matched demographic.
The exaggerated cortisol response to the dex/CRH test in mood disorder patients who do not report high levels of childhood emotional neglect suggests reduced GR mediated negative feedback in these patients. The greater cortisol response and by implication the reduced GR mediated feedback inhibition in patients with a positive family history (in both the high and low emotional neglect groups) suggests that genetic factors may underlie this neuroendocrine finding. This interpretation is supported by previous work. A post-mortem study of bipolar patients showed decreased expression of GR mRNA (Perlman et al 2004; Webster et al 2002) and an exaggerated responses to the dex/CRH test in healthy first degree relatives of mood disorder patients has been found (Lauer et al 1998).
The normal dex/CRH response in patients with high levels of emotional neglect suggests that emotional neglect in childhood either does not influence GR function or up-regulates GR function (most probably via persistent hypo-thalamic drive), offsetting the genetically induced GR down regulation seen in many bipolar patients and producing a null effect. This latter postulate is supported by studies using other indices of childhood trauma that show enhanced GR receptor function in survivors of sexual abuse, domestic violence and patients with post-traumatic-stress-disorder (Yehuda et al 1991; Heim and Nemeroff 2001; Griffin et al 2005).
Recent work suggests that childhood trauma relates not only to the vulnerability to mood disorder but also impacts on its course and prognosis with an increase in substance misuse co-morbidity, cycle frequency, lifetime suicide attempts and with an earlier onset of bipolar illness (Garno et al 2005). The data in the present study adds to this literature and shows the relationship between more moderate persistent degrees of childhood trauma and HPA axis function, in a population selected on the basis of diagnosis rather than abuse history.
In the present study cortisol levels differed between groups of patients characterized by severity of emotional neglect but not between patients divided by any of the other CTQ subscales. This may be because emotional neglect points to a pervasive deficiency in the parent-child relationship (Glaser 2002) and may therefore be more likely to exert a persistent effect on neurobiological stress systems. Emotional neglect in childhood is associated with low self-esteem, interpersonal problems, personality dysfunction, anxiety and major depressive disorder in adulthood. Low maternal warmth has been shown to predict faster relapse in bipolar disorder (Geller et al 2004) and comparative studies suggest that, compared with other forms of childhood trauma, emotional abuse and neglect may be greater predictors of adult psychopathology (Brown et al 2005), monoamine system dysregulation (Roy 2002) and increased CSF CRH (Lee et al 2005). The absence of a significant difference in cortisol levels between patients who reported high and low levels of trauma for the other CTQ subscales may be a type II error consequent on the relatively small sample size. There was, for instance, an apparently large difference in cortisol levels between patients with high compared to low reports of physical abuse which was sufficient to yield an effect size of 0.74 (see Table 3). Co-occurrence of multiple forms of abuse is common (Teicher et al 2006) and there are limitations to the inferences that we can make by studying individual forms of abuse in isolation.
The significant effect of childhood trauma on adult psychopathology has been most often reported in female (eg, Brown and Harris 1978) or mixed (eg, Kendler et al 2000; MacMillan et al 2001) samples. However, childhood disadvantage and adversity have been shown to be associated with adulthood psychopathology in males as well as females (eg, Sadowski et al 1999). Previous studies demonstrating a persistent effect of childhood adversity on HPA axis function have similarly been performed predominantly in women. However, enhanced cortisol responses to a cognitive challenge have been shown in both male and females with PTSD related to child sexual abuse (Bremner et al 2003). It is therefore noteworthy that, in keeping with the previous literature, data in this present study suggest that CTQ scores are elevated in both male and female mood disorder patients. However, it is only in females that we demonstrated a relationship between reported childhood emotional neglect and adulthood HPA axis function. The apparently greater impact of emotional neglect on neurobiological function in women may, perhaps in part, account for the gender bias in the prevalence of affective disorder. However, the specificity of this effect in the present study may also be due simply to statistical power and should therefore be examined further.
Patients in this cohort were a sub-sample of patients who had participated in a neuroendocrine investigation (Watson et al 2002, 2004). The data in this study suggests that the interpretation of future neuroendocrine studies would be enhanced by consideration of the effects of perceived childhood trauma. Future research should extend these preliminary findings to further verify the impact of genetic vulnerability and emotional neglect on neuroendocrine function, and the relationship between these factors and the subsequent development of mood disorders.
Acknowledgments
This study was supported financially by the Stanley Medical Research Institute. The sponsors of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. We are grateful for the assistance of Margaret Smith who was responsible for patient care.
Footnotes
Control reference data from Watson et al (2004): Δ cortisol mean = 12.8, SD = 28.4.
References
- Bernstein DP, Fink L. Manual for the Childhood Trauma Questionnaire: a retrospective self-report. The Psychological Corporation, Harcourt Brace; San Antonio: 1998. [Google Scholar]
- Bremner JD, Vythilingam M, Vermetten E, et al. Cortisol response to a cognitive stress challenge in posttraumatic stress disorder (PTSD) related to childhood abuse. Psychoneuroendocrinology. 2003;28:733–50. doi: 10.1016/s0306-4530(02)00067-7. [DOI] [PubMed] [Google Scholar]
- Brown GR, McBride L, Bauer MS, et al. Impact of childhood abuse on the course of bipolar disorder: A replication study in U.S. veterans. Journal of Affective Disorders. 2005;89:57–67. doi: 10.1016/j.jad.2005.06.012. [DOI] [PubMed] [Google Scholar]
- Brown GW, Harris T. Social origins of depression: a study of psychiatric disorder in women. London: Tavistock; 1978. [Google Scholar]
- Carroll BJ. The dexamethasone suppression test for melancholia. British Journal of Psychiatry. 1982;140:292–304. doi: 10.1192/bjp.140.3.292. [DOI] [PubMed] [Google Scholar]
- Gallagher P, Watson S, Smith MS, et al. Plasma cortisol-dehydro-epiandrosterone (DHEA) ratios in schizophrenia and bipolar disorder. Schizophrenia Research. 2007;90:258–65. doi: 10.1016/j.schres.2006.11.020. [DOI] [PubMed] [Google Scholar]
- Garno JL, Goldberg JF, Ramirez PM, et al. Impact of childhood abuse on the clinical course of bipolar disorder. British Journal of Psychiatry. 2005;186:121–5. doi: 10.1192/bjp.186.2.121. [DOI] [PubMed] [Google Scholar]
- Geller B, Tillman R, Craney JL, et al. Four-year prospective outcome and natural history of mania in children with a prepubertal and early adolescent bipolar disorder phenotype. Archives of General Psychiatry. 2004;61:459–67. doi: 10.1001/archpsyc.61.5.459. [DOI] [PubMed] [Google Scholar]
- Glaser D. Emotional abuse and neglect (psychological maltreatment): a conceptual framework. Child Abuse & Neglect. 2002;26:697–714. doi: 10.1016/s0145-2134(02)00342-3. [DOI] [PubMed] [Google Scholar]
- Griffin MG, Resick PA, Yehuda R. Enhanced cortisol suppression following dexamethasone administration in domestic violence survivors. American Journal of Psychiatry. 2005;162:1192–9. doi: 10.1176/appi.ajp.162.6.1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim C, Nemeroff CB. The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biological Psychiatry. 2001;49:1023–39. doi: 10.1016/s0006-3223(01)01157-x. [DOI] [PubMed] [Google Scholar]
- Heim C, Newport DJ, Bonsall R, et al. Altered pituitary-adrenal axis responses to provocative challenge tests in adult survivors of childhood abuse. American Journal of Psychiatry. 2001;158:575–81. doi: 10.1176/appi.ajp.158.4.575. [DOI] [PubMed] [Google Scholar]
- Heim C, Newport DJ, Heit S, et al. Pituitary-adrenal and autonomic responses to stress in women after sexual and physical abuse in childhood. JAMA. 2000;284:592–7. doi: 10.1001/jama.284.5.592. [DOI] [PubMed] [Google Scholar]
- Heuser I, Yassouridis A, Holsboer F. The combined dexamethasone/CRH test: a refined laboratory test for psychiatric disorders. Journal of Psychiatric Research. 1994;28:341–56. doi: 10.1016/0022-3956(94)90017-5. [DOI] [PubMed] [Google Scholar]
- Holsboer F. Neuroendocrinology of mood disorders. In: Davis KLCD, Coyle JT, et al., editors. Neuropsychopharmacology: the fourth generation of progess. Lippincott, Williams and Wilkins; Philadelphia, PA: 2002. [Google Scholar]
- Holsboer F, Lauer CJ, Schreiber W, et al. Altered hypothalamic-pituitary-adrenocortical regulation in healthy subjects at high familial risk for affective disorders. Neuroendocrinology. 1995;62:340–7. doi: 10.1159/000127023. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Bulik CM, Silberg J, et al. Childhood sexual abuse and adult psychiatric and substance use disorders in women: an epidemiological and cotwin control analysis. Archives of General Psychiatry. 2000;57:953–9. doi: 10.1001/archpsyc.57.10.953. [DOI] [PubMed] [Google Scholar]
- Ladd CO, Huot RL, Thrivikraman KV, et al. Long-term adaptations in glucocorticoid receptor and mineralocorticoid receptor mRNA and negative feedback on the hypothalamo-pituitary-adrenal axis following neonatal maternal separation. Biological Psychiatry. 2004;55:367–75. doi: 10.1016/j.biopsych.2003.10.007. [DOI] [PubMed] [Google Scholar]
- Lauer CJ, Schreiber W, Modell S, et al. The Munich vulnerability study on affective disorders: overview of the cross-sectional observations at index investigation. Journal of Psychiatric Research. 1998;32:393–401. doi: 10.1016/s0022-3956(98)00026-0. [DOI] [PubMed] [Google Scholar]
- Lee R, Geracioti TD, Jr, Kasckow JW, et al. Childhood trauma and personality disorder: positive correlation with adult CSF corticotropin-releasing factor concentrations. American Journal of Psychiatry. 2005;162:995–7. doi: 10.1176/appi.ajp.162.5.995. [DOI] [PubMed] [Google Scholar]
- Lightman SL, Windle RJ, Ma XM, et al. Hypothalamic-pituitary-adrenal function. Archives of Physiology and Biochemistry. 2002;110:90–3. doi: 10.1076/apab.110.1.90.899. [DOI] [PubMed] [Google Scholar]
- MacMillan HL, Fleming JE, Streiner DL, et al. Childhood abuse and lifetime psychopathology in a community sample. American Journal of Psychiatry. 2001;158:1878–83. doi: 10.1176/appi.ajp.158.11.1878. [DOI] [PubMed] [Google Scholar]
- Matsubara T, Funato H, Kobayashi A, et al. Reduced glucocorticoid receptor [alpha] expression in mood disorder patients and first-degree relatives. Biological Psychiatry. 2006;59:689–95. doi: 10.1016/j.biopsych.2005.09.026. [DOI] [PubMed] [Google Scholar]
- McQuade R, Young AH. Future therapeutic targets in mood disorders: the glucocorticoid receptor. British Journal of Psychiatry. 2000;177:390–5. doi: 10.1192/bjp.177.5.390. [DOI] [PubMed] [Google Scholar]
- Modell S, Lauer CJ, Schreiber W, et al. Hormonal response pattern in the combined DEX-CRH test is stable over time in subjects at high familial risk for affective disorders. Neuropsychopharmacology. 1998;18:253–62. doi: 10.1016/S0893-133X(97)00144-9. [DOI] [PubMed] [Google Scholar]
- Newport DJ, Heim C, Bonsall R, et al. Pituitary-adrenal responses to standard and low-dose dexamethasone suppression tests in adult survivors of child abuse. Biological Psychiatry. 2004;55:10–20. doi: 10.1016/s0006-3223(03)00692-9. [DOI] [PubMed] [Google Scholar]
- Paivio SC, Cramer KM. Factor structure and reliability of the Childhood Trauma Questionnaire in a Canadian undergraduate student sample. Child Abuse & Neglect. 2004;28:889–904. doi: 10.1016/j.chiabu.2004.01.011. [DOI] [PubMed] [Google Scholar]
- Pariante CM, Miller AH. Glucocorticoid receptors in major depression: relevance to pathophysiology and treatment. Biological Psychiatry. 2001;49:391–404. doi: 10.1016/s0006-3223(00)01088-x. [DOI] [PubMed] [Google Scholar]
- Perlman WR, Webster MJ, Kleinman JE, et al. Reduced glucocorticoid and estrogen receptor alpha messenger ribonucleic acid levels in the amygdala of patients with major mental illness. Biological Psychiatry. 2004;56:844–52. doi: 10.1016/j.biopsych.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Roy A. Self-rated childhood emotional neglect and CSF monoamine indices in abstinent cocaine-abusing adults: possible implications for suicidal behavior. Psychiatry Research. 2002;112:69–75. doi: 10.1016/s0165-1781(02)00176-2. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Giles DE, Schlesser MA, et al. The dexamethasone suppression test in patients with mood disorders. Journal of Clinical Psychiatry. 1996;57:470–84. doi: 10.4088/jcp.v57n1006. [DOI] [PubMed] [Google Scholar]
- Rybakowski JK, Twardowska K. The dexamethasone/corticotropin-releasing hormone test in depression in bipolar and unipolar affective illness. Journal of Psychiatric Research. 1999;33:363–70. doi: 10.1016/s0022-3956(99)00014-x. [DOI] [PubMed] [Google Scholar]
- Sadowski H, Ugarte B, Kolvin I, et al. Early life family disadvantages and major depression in adulthood. British Journal of Psychiatry. 1999;174:112–20. doi: 10.1192/bjp.174.2.112. [DOI] [PubMed] [Google Scholar]
- Scher CD, Stein MB, Asmundson GJ, et al. The childhood trauma questionnaire in a community sample: psychometric properties and normative data. Journal of Traumatic Stress. 2001;14:843–57. doi: 10.1023/A:1013058625719. [DOI] [PubMed] [Google Scholar]
- Schmider J, Lammers CH, Gotthardt U, et al. Combined dexamethasone/corticotropin-releasing hormone test in acute and remitted manic patients, in acute depression, and in normal controls: I. Biological Psychiatry. 1995;38:797–802. doi: 10.1016/0006-3223(95)00064-X. [DOI] [PubMed] [Google Scholar]
- Teicher MH, Andersen SL, Polcari A, et al. The neurobiological consequences of early stress and childhood maltreatment. Neuroscience & Biobehavioral Reviews. 2003;27:33–44. doi: 10.1016/s0149-7634(03)00007-1. [DOI] [PubMed] [Google Scholar]
- Teicher MH, Tomoda A, Andersen SL. Neurobiological consequences of early stress and childhood maltreatment: are results from human and animal studies comparable? Ann NY Acad Sci. 2006;1071:313–23. doi: 10.1196/annals.1364.024. [DOI] [PubMed] [Google Scholar]
- Watson S, Gallagher P, Del-Estal D, et al. Hypothalamic-pituitary-adrenal axis function in patients with chronic depression. Psychological Medicine. 2002;32:1021–8. doi: 10.1017/s0033291702005998. [DOI] [PubMed] [Google Scholar]
- Watson S, Gallagher P, Ritchie JC, et al. Hypothalamic-pituitary-adrenal axis function in patients with bipolar disorder. British Journal of Psychiatry. 2004;184:496–502. doi: 10.1192/bjp.184.6.496. [DOI] [PubMed] [Google Scholar]
- Webster MJ, Knable MB, O’Grady J, et al. Regional specificity of brain glucocorticoid receptor mRNA alterations in subjects with schizophrenia and mood disorders. Molecular Psychiatry. 2002;7:985–94. doi: 10.1038/sj.mp.4001139. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Lowy MT, Southwick SM, et al. Lymphocyte glucocorticoid receptor number in posttraumatic stress disorder. American Journal of Psychiatry. 1991;148:499–504. doi: 10.1176/ajp.148.4.499. [DOI] [PubMed] [Google Scholar]