Abstract
We report a case of acute demyelinating encephalitis that occurred after viral vaccination against hepatitis A-, hepatitis B-, and poliovirus and vaccination against bacterial toxins of diphtheria and tetanus. After different diagnosis had been excluded, we diagnosed postvaccinal demyelinating encephalitis and started treatment with high dose intravenous methylprednisolone, followed by peroral application in decreasing dosages for three weeks. A few days after the treatment with methylprednisolone had been finished, the patient’s medical condition deteriorated again. Thus, we initiated plasma exchange at an advanced state of illness, which led to significant continuous improvement. The role of plasma exchange is discussed controversially, in particular the issue of timing. We report a case that shows improvement due to plasmapheresis several weeks after symptom onset.
Keywords: ADEM, vaccination, encephalitis, plasmapheresis, demyelination, plasma exchange
The incidence and significance of autoimmune central nervous system (CNS) manifestations after vaccination remain unknown (Schattner 2005). Acute disseminated encephalomyelitis (ADEM), an acute or subacute disease characterized by the occurrence of multifocal neurological deficits, was observed from one up to several weeks after vaccination (Gout 2001). Several cases of ADEM were described after active vaccinations against hepatitis B (Kaplanski et al 1995; Pirmohamed and Winstanley 1997; Tourbah et al 1999; Cabrera-Gomez et al 2002), pertussis (Sriram and Steinman 1984), diphtheria-tetanus-poliomyelitis (Mancini et al 1996), smallpox, yellow fever, typhoid fever, tuberculosis, and rabies (Gamboa et al 1983; Miller et al 1967). Here, we report a patient with acute demyelinating encephalitis that occurred after active vaccination.
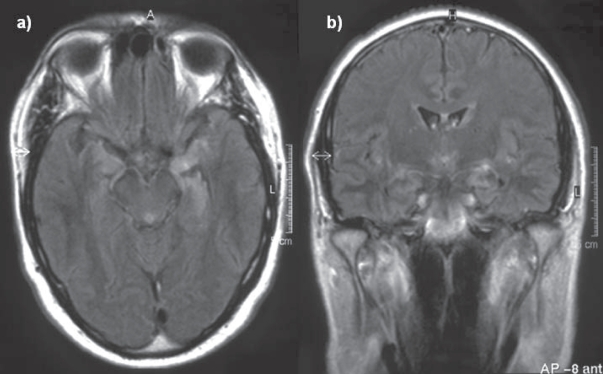
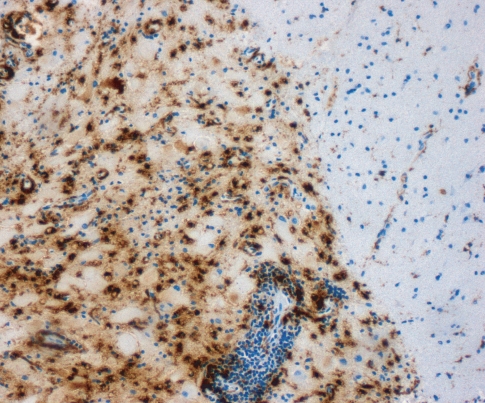
A 46-year-old male patient presented in a community hospital with fever, visual hallucinations, tonic-clonic seizures and progressive amnestic impairment three weeks after active vaccination with recombinant hepatitis A- and hepatitis B-virus, diphteria, tetanus, and poliovirus antigen. These multiple vaccinations were given on one day due to the urgent travel plans of the patient. The patient’s medical- and family history was unremarkable without any history of multiple sclerosis, seizures, or other neurological diseases. Tentative diagnosis during first external hospitalization was limbic encephalitis. The patient received acyclovir and anticonvulsive medication with carbamazepine. He did not obtain any immunomodulatory treatment and the further diagnostic examination did not reveal any signs of malignancy. Due to further progression, the patient was presented in our hospital ten weeks after symptom onset. The neurological examination revealed a central facial paresis, tongue deviation to the right side, dysarthria, and severe dysphagia, which required tracheostomy. Cranial magnetic resonance imaging (MRI) showed multiple white matter lesions with Gadolinium enhancement in the left temporal lobe, but lack of MRI changes in the brainstem (see Figure 2). Analysis of cerebrospinal fluid (CSF) yielded pleocytosis with 12 lymphocytes per microlitre with autochthone oligoclonal bands compared to serum bands. A specific causative pathogen by extensive microbiological CSF analysis could not be obtained. 18-fluorodeoxyglucose PET/CT showed a focal metabolic enhancement of the white matter lesions in the temporal lobe. Metabolism of the left temporal and parietal lobes was reduced. Stereotactic biopsy of one of the white matter lesions identified inflammatory demyelination without any signs of specific inflammation or malignancy (see Figure 1). We repeatedly performed thorax and abdomen CT scan, abdomen sonography, gastroscopy, colonoscopy, bronchoscopy, urological examination, and determination of serum analytic markers including tumor markers and specific antibodies to exclude paraneoplastic genesis (see Table 1). We repeatedly performed microbiological blood and CSF assays to exclude parainfectious disease. Any laboratory tests were without pathological findings.
Figure 2.
Axial (a) and coronar (b) FLAIR sequence showing a hyperintensive signal with gadolinium enhancement (b) in the left temporal lobe. Relevant radiological differential diagnosis are herpes simplex encephalitis, Hashimoto encephalitis, and limbic encephalitis.
Figure 1.
Histopathological examination of the biopsy sample revealed an inflammatory demyelinating lesion with perivascular lymphocytes and numerous macrophages (immunohistochemical staining for CD68).
Table 1.
Specific serum and CSF markers determined in patient. None of the performed tests was specific for a pathogen/pathological condition
| Serum | CSF |
|---|---|
| Paraneoplastic antibodies (anti-Yo; anti-Hu, anti-Ri), anti-amphiphysin antibodies, anti-MA antibodies, Carcino-Embryonic Antigen (CEA), Neuron Specific Enolase (NSE), Total Prostate Specific Antigen (t-PSA), Carcinoma related antibodies (CA 125, CA 19-9), Alpha-Feto proteine (AFP), Human chorionic gonadotrophin (Beta-HCG), Anti-voltage-gated potassium channel antibodies (VGKC), Waaler-Rose-Test; Antinuclear antibody test (ANA), anti-HIV 1+2, HIV-RNA, T lymphocytes CD4/CD8 ratio, hepatitis B surface antigen (HbsAg), Anti-HBc, anti hepatitis C (anti-HCV), CMV-PCR, immunelectrophoresis, cryoglobulines, parvovirus B-19 IgG-/IgM-antibodies, parvovirus B-19 PCR, herpesvirus (PCR-screening), CMV-PCR, antibodies against Treponema pallidum, antibodies against Borrelia burgdorferi, antibodies against Toxoplasma gondii, EBV IgG-/IgM-antibodies, CMV-pp65 antigene in lymphocytes, anti thyreoglobuline antibodies, anti TSH-receptor antibodies, anti-TPO-antibodies. | (total protein 482 mg/l) No inthrathecal synthesis of IgM-, IgG-, IgA-antibodies, anti-CV2 (cAMP 5), a-beta-amyloid (1–42) and tau, hyperphosphorylated (181)-tau, enterovirus-PCR, herpesvirus (PCR-screening), parvovirus B-19 PCR, measles/mumps/rubella titres, CMV-PCR, Listeria monocytogenes, mycobacterium tuberculosis SDA, CMV-IgG titre, EBV-IgG titre, HSV IgG titre, VZV IgG titre, herpesvirus PCR, antibodies against Treponema pallidum, antibodies against Borrelia burgdorferi, antibodies against Toxoplasma gondii, LCM virus RNA, echinococcus spp. DNA. |
We hereby excluded other differential diagnosis such as herpes simplex encephalitis, Hashimoto encephalitis, and limbic encephalitis. Assuming a postvaccinal demyelinating encephalitis we started intravenous treatment with methylprednisolone for a period of 5-days, 1000 mg a day, followed by oral methylprednisolone with decreasing dosages for three weeks. This treatment with methylprednisolone was initiated 11-weeks after symptom onset. Methylprednisolone treatment resulted in a temporary slight improvement of the amnestic impairment. During the methylprednisolone treatment, the patient’s condition deteriorated again with short-term and long-term memory disturbances and progressive reduction of alertness and concentration. Furthermore, the patient revealed reduced vigilance. We decided to initiate plasma exchange 13-weeks after symptom onset, five treatments within 10-days. A few days after initiation of this therapy neurological improvement subsequently commenced. Afterwards the patient was alert following common and more complex requests. The patient did not receive other immunosuppressive drugs or intravenous preparations of immune globulin (IVIG).
Four months later, follow-up examination revealed the curing of the amnestic impairment. The patient had not suffered from any further epileptic seizures. Cranial MRI scans revealed a reduction of the white matter lesion size. However, dysphagia persisted, therefore, tracheostoma was still mandatory.
The risk of postvaccinal autoimmune inflammatory CNS demyelination is unknown but certainly rare. The probable pathogenetic mechanism is a molecular mimicry between vaccine proteins and myelin components. Cross-reactivity of HbsAg with a myelin peptide derived from a proteolipid protein (PLP) has been demonstrated (Gran et al 2000).
In our case, we suggest that the patient acquired an acute demyelinating encephalitis in consequence of vaccination against HAV, HBV, diphtheria, tetanus and poliovirus. The multiple temporal white matter lesions in combination with seizures and amnestic disorder could also be consistent with a diagnosis of limbic encephalitis. Further relevant differential diagnoses in our patient could be herpes simplex encephalitis and Hashimoto encephalitis. But the medical history, the diagnostic findings (Table 1) including normal levels of thyroid antibodies and negative herpes-virus PCR-screening, and the repeated exclusion of malignancy make it most likely that the encephalitis was caused by the vaccination.
We could not prove the causative antigen which could appear after all five antigens. But we consider that the combination of these multiple vaccinations given on one day are probably sufficient to trigger this severe autoimmune inflammatory CNS demyelination. In the early 1990s, several cases of patients with CNS demyelination events within eight weeks after recombinant hepatitis B (HB) vaccine injection have been described (Kaplanski et al 1995; Pirmohamed and Winstanley 1997; Tourbah et al 1999; Cabrera-Gomez et al 2002). Thus, we suspect that the HB vaccine essentially contributed to the postvaccinal reaction. To our knowledge, it had never been reported a case with postvaccinal demyelinating encephalitis triggered by multiple vaccinations given at one day.
Methylprednisolone treatment initially resulted in a slight improvement. But the patient’s condition deteriorated already during this therapy. The time course of the improvement starting a few days after the beginning of plasma exchange suggest that the improvement can be ascribed to plasma exchange therapy. The observed time course reduced the assumption that a delayed effect of steroid treatment or the natural course of disease have crucially contributed to the recovery of our patient.
Accepted treatment methods of acute CNS demyelination include intravenous methylprednisolone, immunoglobulines or a combination of both (Tselis 2001). Few reports highlight the importance of plasmapheresis in ADEM (Kanter et al 1995; Lin et al 2004). The preferential treatment effect of methylprednisolone is a suppression of T-cell mediated immune response. After non-response to steroid treatment, the removal of B-cell mediated immunoglobulines and circulating antibodies from the serum using plasma exchange seems to be obvious. Moreover, plasmapheresis also affects the balance of T helper type-1 and T helper type-2 cells of circulating peripheral lymphocytes in patients with neuroimmunological diseases (Goto et al 2001). In cases of acute CNS demyelination, treatment with plasmapheresis was suggested to be beneficial (Kanter et al 1995; RamachandranNair et al 2005). In a retrospective analysis of Keegan (2002), 59 consecutive patients had been treated with plasmapheresis for acute CNS demyelination, including 10 patients with ADEM (Keegan et al 2002). Beneficial therapeutic response had been obtained, even when plasmapheresis had been initiated 60-days after onset of neurological symptoms. However, it remains unclear if the improved patients had ADEM or other acute severe demyelinating diseases. It was also not reported if one of the patients acquired ADEM after vaccination. Others report a clear improvement in five of six patients with acute steroid-insensitive inflammatory CNS demyelinations due to treatment with plasma exchange (Bennetto et al 2004). However, in this article four patients had MS, one patient had acute transverse myelitis, one had clinical isolated optico-spinal syndrome, and none of the patients suffered from ADEM. There are only a few reports which address the effect of plasma exchange in ADEM depending on an advanced illness stage (Lin et al 2004; RamachandranNair et al 2005). Lin and colleagues analyzed 14 patients treated with plasma exchange for ADEM. Plasmapheresis was initiated with a range of 3–18 days after symptom onset and improvement began three days after initiation (Lin et al 2004). RamachandranNair reported two cases which demonstrated that plasma exchange could be useful even in the fifth week of severe ADEM not responding to steroid therapy (RamachandranNair et al 2005). However, to our knowledge there are no reported cases of postvaccinal ADEM where the benefits of plasma exchange were seen in such a late disease course more than 13-weeks after symptom onset.
Standard therapy for acute severe demyelinating encephalitis is high dose intravenous corticosteroids. For patients who fail to improve with this regime, a plasma exchange should be considered. Here we report a case of postvaccinal demyelination with rapidly repeated progression after treatment with methylprednisolone. In our case, we show an improvement due to plasmapheresis more than 3-months after symptom onset. This resulted in a remarkable amelioration of neurological outcome.
References
- Bennetto L, Totham A, Healy P, et al. Plasma exchange in episodes of severe inflammatory demyelination of the central nervous system. A report of six cases. J Neurol. 2004;251:1515–21. doi: 10.1007/s00415-004-0588-8. [DOI] [PubMed] [Google Scholar]
- Cabrera-Gomez JA, Echazabal-Santana N, Garcia GL, et al. A severe episode in a patient with recurrent disseminated acute encephalitis due to vaccination against hepatitis B. For or against vaccination? Rev Neurol. 2002;34:358–63. [PubMed] [Google Scholar]
- Gamboa ET, Cowen D, Eggers A, et al. Delayed onset of post-rabies vaccination encephalitis. Ann Neurol. 1983;13:676–8. doi: 10.1002/ana.410130619. [DOI] [PubMed] [Google Scholar]
- Goto H, Matsuo H, Nakane S, et al. Plasmapheresis affects T helper type-1/T helper type-2 balance of circulating peripheral lymphocytes. Ther Apher. 2001;5:494–6. doi: 10.1046/j.1526-0968.2001.00386.x. [DOI] [PubMed] [Google Scholar]
- Gout O. Vaccinations and multiple sclerosis. Neurol Sci. 2001;22:151–4. [PubMed] [Google Scholar]
- Gran B, Bielekova B, McFarland HF, et al. Development of multiple sclerosis after hepatitis B vaccination: an immunologic case report. Neurology. 2000;54:A164. [Google Scholar]
- Kanter DS, Horensky D, Sperling RA, et al. Plasmapheresis in fulminant acute disseminated encephalomyelitis. Neurology. 1995;45:824–7. doi: 10.1212/wnl.45.4.824. [DOI] [PubMed] [Google Scholar]
- Kaplanski G, Retornaz F, Durand J, et al. Central nervous system demyelination after vaccination against hepatitis B and HLA haplotype. J Neurol Neurosurg Psychiatry. 1995;58:758–9. doi: 10.1136/jnnp.58.6.758-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keegan M, Pineda AA, McClelland RL, et al. Plasma exchange for severe attacks of CNS demyelination: predictors of response. Neurology. 2002;58:143–6. doi: 10.1212/wnl.58.1.143. [DOI] [PubMed] [Google Scholar]
- Lin CH, Jeng JS, Yip PK. Plasmapheresis in acute disseminated encephalomyelitis. J Clin Apher. 2004;19:154–9. doi: 10.1002/jca.20022. [DOI] [PubMed] [Google Scholar]
- Mancini J, Chabrol B, Moulene E, et al. Relapsing acute encephalopathy: a complication of diphtheria-tetanus-poliomyelitis immunization in a young boy. Eur J Pediatr. 1996;155:136–8. doi: 10.1007/BF02075768. [DOI] [PubMed] [Google Scholar]
- Miller H, Cendrowski W, Shapira K. Multiple sclerosis and vaccination. Br Med J. 1967;2:210–13. doi: 10.1136/bmj.2.5546.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirmohamed M, Winstanley P. Hepatitis B vaccine and neurotoxicity. Postgrad Med J. 1997;73:462–3. doi: 10.1136/pgmj.73.861.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RamachandranNair R, Parameswaran M, Girija AS. Acute disseminated encephalomyelitis treated with plasmapheresis. Singapore Med J. 2005;46:561–3. [PubMed] [Google Scholar]
- Schattner A. Consequence or coincidence? The occurrence, pathogenesis and significance of autoimmune manifestations after viral vaccines. Vaccine. 2005;23:3876–86. doi: 10.1016/j.vaccine.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Sriram S, Steinman L. Postinfectious and postvaccinial encephalomyelitis. Neurol Clin. 1984;2:341–53. [PubMed] [Google Scholar]
- Tourbah A, Gout O, Liblau R, et al. Encephalitis after hepatitis B vaccination: recurrent disseminated encephalitis or MS? Neurology. 1999;53:396–401. doi: 10.1212/wnl.53.2.396. [DOI] [PubMed] [Google Scholar]
- Tselis A. Acute Disseminated Encephalomyelitis. Curr Treat Options Neurol. 2001;3:537–42. doi: 10.1007/s11940-001-0016-7. [DOI] [PubMed] [Google Scholar]