Abstract
Prostaglandin E2 (PGE2) has been identified as a PG necessary for ovulation, but the ovulatory gonadotropin surge also increases PGF2α levels in primate periovulatory follicles. To better understand the role of PGF2α in ovulation, pathways utilized for PGF2α synthesis by the primate follicle were examined. Monkeys were treated with gonadotropins to stimulate multiple follicular development; follicular aspirates and whole ovaries were removed before and at specific times after administration of an ovulatory dose of hCG to span the 40-hour periovulatory interval. Human granulosa cells were also obtained (typically 34-36 hours after hCG) from in vitro fertilization patients. PGF2α can be synthesized from PGH2 via the aldo-keto reductase (AKR) 1C3. AKR1C3 mRNA and protein levels in monkey granulosa cells were low before hCG and peaked 24-36 hours after hCG administration. Human granulosa cells converted PGD2 into 11β-PGF2α, confirming that these cells possess AKR1C3 activity. PGF2α can also be synthesized from PGE2 via the enzymes AKR1C1 and AKR1C2. Monkey granulosa cell levels of AKR1C1/AKR1C2 mRNA was low 0-12 hours, peaked at 24 hours, and returned to low levels by 36 hours after hCG administration. Human granulosa cell conversion of 3H-PGE2 into 3H-PGF2α was reduced by a AKR1C2-selective inhibitor, supporting the concept that granulosa cells preferentially express AKR1C2 over AKR1C1. In summary, the ovulatory gonadotropin surge increases granulosa cell expression of AKR1C1/AKR1C2 and AKR1C3. Both of these enzyme activities are present in periovulatory granulosa cells. These data support the concept that follicular PGF2α can be synthesized via two pathways during the periovulatory interval.
Keywords: ovulation, ovary, prostaglandin, granulosa cell, monkey, primate
INTRODUCTION
Prostaglandin (PG) production by the follicle in response to the midcycle luteinizing hormone (LH) surge is necessary for ovulation to occur. Follicular fluid concentrations of both PGE2 and PGF2α peak just before the expected time of ovulation in primates as in many mammalian species (Duffy & Stouffer, 2001, Sirois, 1994, Sirois & Dore, 1997). While studies with PGF2α receptor knockout mice suggest that PGF2α is not required for ovulation (Sugimoto et al., 1997), subfertility in mice lacking expression of the PGE2 receptor EP2 focused attention on PGE2 as the key ovulatory PG (Hizaki et al., 1999). Blockade of PG production within the follicle prevented cumulus expansion, follicle rupture and oocyte release; restoration of periovulatory events with coadministration of the PG synthesis inhibitor and PGE2 identified PGE2 as a key mediator of ovulation in mammals (Peters et al., 2004, Sogn et al., 1987) including primates (Duffy & Stouffer, 2002). However, additional studies have demonstrated restoration of ovulation with PGF2α administration (Janson et al., 1988, Murdoch et al., 1986, Sogn et al., 1987, Wallach et al., 1975), suggesting that PGF2α may also play a role in ovulatory processes.
Synthesis of PGF2α begins with conversion of arachidonic acid into the unstable intermediate PGH2 through the activity of cyclooxygenase (COX) (Vane et al., 1998) (Figure 1). PGH2 can then be converted to bioactive PGs by specific PG synthases. 11α-PGF2α (traditionally referred to as PGF2α) can be produced from either PGH2 or PGE2; a third pathway produces 11β-PGF2α from PGH2. All enzymes which produce PGF2α are members of the aldo-keto reductase (AKR) family (Nishizawa et al., 2000). PGH2 can be directly converted to PGF2α by the action of AKR1C3, also known as PGF synthase (Suzuki-Yamamoto et al., 1999); this same enzyme converts PGD2 into 11β-PGF2α. 11β-PGF2α is equipotent with PGF2α in the stimulation of PGF2α receptors (Sugimoto et al., 1994). Finally, two closely-related enzymes, AKR1C1 and AKR1C2, function as PGE-F isomerases, which interconvert PGE2 and PGF2α (Nishizawa et al., 2000).
Figure 1.
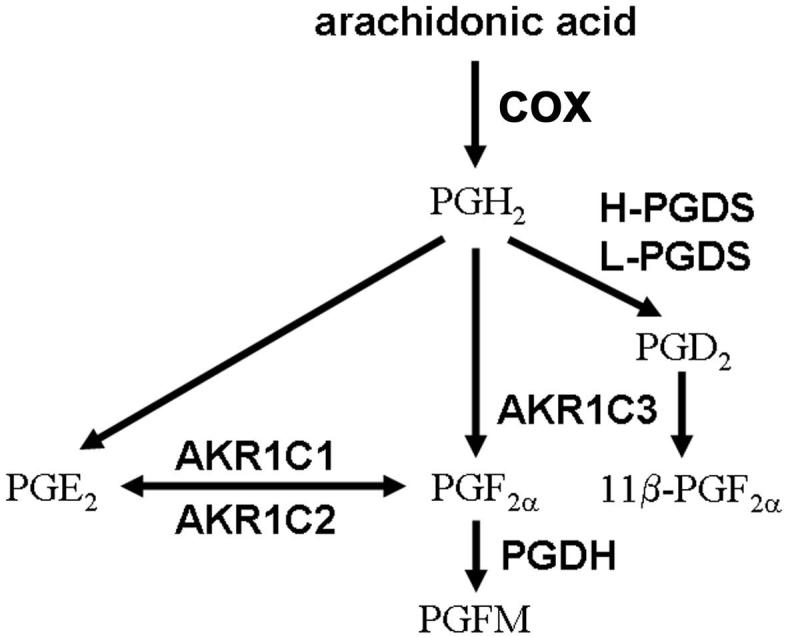
Three candidate pathways for the synthesis of PGF2α. A cyclooxygenase (COX) enzyme produces PGH2 from arachidonic acid. PGF2α can be synthesized either directly from PGH2 via AKR1C3 or indirectly from PGE2 via AKR1C1 and AKR1C2. 11β-PGF2α can be produced from PGH2 via PGD synthase (PGDS) and AKR1C3. PGF2α is converted to inactive PGF metabolites (PGFM) via the 15-hydroxy PG dehydrogenase (PGDH). Adapted from (Watanabe, 2002).
To better understand the pathways utilized for PGF2α synthesis by the primate follicle, follicular expression and activity of enzymes which can produce PGF2α were assessed. Previous studies by our laboratory demonstrated that the ovulatory gonadotropin surge increases granulosa cell expression of key enzymes required for PGE2 synthesis; increased enzyme levels parallel the rising follicular concentration of PGE2 just before ovulation (Duffy & Stouffer, 2001, Duffy et al., 2005a, 2005b). We hypothesize that the ovulatory gonadotropin surge also stimulates granulosa cell expression of enzymes necessary for the synthesis of PGF2α. Similarities in periovulatory processes in humans and non-human primates are well-established (Zeleznik et al., 1994). The majority of the studies presented below were conducted using a non-human primate, the cynomolgus macaque. Additional experiments used human luteinizing granulosa cells to extend findings to the human periovulatory follicle.
METHODS
Animals
Granulosa cells, follicular fluid, and whole ovaries were obtained from adult female cynomolgus macaques (Macaca fascicularis) at Eastern Virginia Medical School (EVMS). All animal protocols and experiments were approved by the EVMS Animal Care and Use Committee and were conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals. Adult females with regular menstrual cycles were maintained as previously described and checked daily for menstruation; the first day of menstruation was designated day 1 of the menstrual cycle (Duffy et al., 2005c). Blood samples were obtained under ketamine chemical restraint (10 mg/kg body weight) by femoral or saphenous venipuncture, and serum was stored at -20C. Aseptic surgical procedures (either midline laparotomy or laparoscopy) were performed under isofluorane anesthesia in a dedicated surgical suite.
A controlled ovarian stimulation model developed for the collection of multiple oocytes for in vitro fertilization was used as previously described (Duffy et al., 2005c) to obtain monkey granulosa cells and follicular fluid. Recombinant human FSH (r-hFSH, Serono Reproductive Biology Institute, Rockland, MA (90 IU/day) or Organon, a part of Schering Plough Corporation, Roseland, NJ (60 IU/day)) was administered for 6-8 days, followed by administration of r-hFSH plus r-hLH (Serono, 60 IU/day) for 2-3 days to stimulate the growth of multiple follicles. The GnRH antagonist Antide (0.5 mg/kg body weight; Serono) was also administered daily to prevent an endogenous ovulatory LH surge. Adequate follicular development was monitored by serum estradiol levels and ultrasonography (Wolf et al., 1996). Aspiration of follicles at least 4 mm in diameter or ovariectomy was performed before (0 hour) or 12, 24, and 36 hours after administration of 1000 IU r-hCG (Serono) to induce ovulatory events. To obtain undiluted follicular fluid as well as granulosa cells, each follicle was pierced with a 22-gauge needle, and the aspirated contents of all follicles were pooled.
Whole ovaries were bisected to maintain at least 2 follicles of at least 4 mm in diameter on each piece. One piece of ovarian tissue was fixed in 4% paraformaldehyde and embedded in paraffin; another was coated in OCT compound (Sakura, Torrance, CA), flash frozen in liquid propane, and stored at -80C until sectioned. Additional monkey tissues such as seminal vesicle were obtained at necropsy and processed as described above.
Monkey Granulosa Cells
Monkey granulosa cells and follicular fluid were obtained from follicular aspirates as described previously (Chaffin et al., 1999). Briefly, aspirates were subjected to centrifugation to pellet the oocytes and granulosa cells; the resulting supernatant (i.e., follicular fluid) was removed and stored at -80C. Oocytes were mechanically removed, and a granulosa cell-enriched population of the remaining cells was obtained by Percoll gradient centrifugation. Viability of granulosa cell-enriched preparations averaged 80% as assessed by trypan blue exclusion. Cells were either used immediately for cell culture or were frozen in liquid nitrogen and stored at -80C for preparation of total RNA or cell lysates.
Human Luteinizing Granulosa Cells
Human luteinizing granulosa cells were obtained after oocyte removal from follicular aspirates from patients experiencing ovarian stimulation at The Jones Institute for Reproductive Medicine, EVMS. This use of discarded human granulosa cells does not constitute human subjects research as determined by the EVMS Institutional Review Board. No information is obtained regarding an individual patient’s diagnosis or treatment protocol. However, most patients receive hCG 34-36 hours prior to follicle aspiration, so these human cells are very similar to monkey granulosa cells obtained 36 hours after hCG administration. Human granulosa cells were enriched by Percoll gradient centrifugation as described above for monkey granulosa cells.
Prostaglandin Assays
Follicular fluid obtained from aspirates was acidified and extracted with ethyl acetate prior to assay as previously described (Duffy et al., 2005c). [3H]PGE2 was added to each sample prior to extraction to correct for procedural losses; the mass of [3H]PGE2 added was <0.1% of the total PGE2 content of each follicular fluid sample. Samples were resuspended in assay buffer (see below), and an aliquot was subjected to scintillation counting to calculate PG recovery, which averaged 84%. Follicular fluid concentrations of PGF2α, 11β-PGF2α, and the primary PGF2α metabolite 13,14-dihydroketo PGF2α (PGFM) were determined by enzyme immunoassay (EIA, Cayman Chemical, Ann Arbor, MI); the PG content of each sample was corrected based on [3H]PGE2 recovery calculated for each sample. Culture media were assayed without extraction. The intra- and inter-assay coefficients of variation for the PGF2α EIA were 12.1% and 18.6%, respectively. The intra- and inter-assay coefficients of variation for the PGFM EIA were 23.9% and 6.3%, respectively. The intra- and inter-assay coefficients of variation for the 11β-PGF2α EIA were 23.0% and 7.0%, respectively. The lower limit of detection for 11β-PGF2α averaged 1.2 ng/ml after correction for extraction recovery.
Real Time Reverse Transcription PCR (RT-PCR)
Multiple mRNAs were analyzed in each granulosa cell sample by real time RT-PCR using a Roche LightCycler (Indianapolis, IN). Total RNA was obtained from granulosa cells using Trizol reagent (Invitrogen, Rockville, MD) and was stored at -80C. Total RNA was incubated with DNase, and reverse transcription was performed as described previously (Chaffin & Stouffer, 1999). PCR was performed using the FastStart DNA Master SYBR Green I kit (Roche) with the exception of L-PGDS, which was amplified using the QuantiTect SYBR Green PCR kit (Qiagen, Valencia, CA). PCR primers were designed based on the human or monkey sequences using LightCycler Probe Design software (Roche), and nucleotide sequences of monkey PCR products were determined (Microchemical Core Facility, San Diego State University, CA) (Table). Whenever possible, primers span an intron to prevent undetected amplification of genomic DNA. Due to the significant (98%) nucleic acid identity between human AKR1C1 and AKR1C2 cDNA, PCR primers were designed which shared 100% identity with both these human cDNAs as well as reported cynomolgus monkey cDNA sequences for AKR1C1 (a monkey AKR1C2 cDNA sequence was not available). At least 5 log dilutions of the sequenced PCR product was included in each assay and used to generate a standard curve. All data were expressed as the ratio of enzyme mRNA to β-actin mRNA for each sample. Intra- and inter-assay coefficients of variation were less than 10%.
Table.
Reaction Conditions for Real Time RT-PCR
| Target (primer concentration) | Primer Sequences (5′→3′) | [MgCl2] (mM) | amplified cDNA (bp) | % identity between amplified monkey sequence and sequence used for primer design | accession number and species of sequence used for primer design | monkey amplified fragment accession number |
|---|---|---|---|---|---|---|
| AKR1C1/AKR1C2 (0.25 μM) | up: GCAATTCCCATCGACC dn: GCAGAAATCCAGCAGTT |
3 | 359 | 95.4 | NM_001353 NM_001354 human | DQ124199 |
| AKR1C3 (0.25 μM) | up: AGGAACTTTCACCAACAG dn: CCACCCATCGTTTGTC |
3 | 309 | 96.4 | AB018580 human | DQ104393 |
| H-PGDS (0.25 μM) | up:CTGCTCACGTATAATGCG dn: CTTGAAGGCAACATGG |
4 | 267 | 95.8 | NM_014485 human | DQ093856 |
| L-PGDS (0.5 μM) | up: CTACTCATCACACGCTG dn:GGGTCTCACACTGGTT |
4 | 270 | 97.7 | AB032480 M. fuscata | DQ093857 |
| β-actin (0.5 μM) | up: ATCCGCAAAGACCTGT dn: GTCCGCCTAGAAGCAT |
4 | 270 | 97.4 | NM_001101 human | AY765990 |
Western Blotting for AKR1C3
Granulosa cells were thoroughly lysed on ice in PBS containing 0.5% sodium dodecyl sulfate and 0.1% Triton X-100, mixed with denaturing sample buffer, heated to 95C for 10 minutes, and loaded onto 4-20% gradient polyacrylamide Tris-HCl gels (BioRad, Hercules, CA). Proteins were transferred to PDVF membranes (Immobilon, Millipore, Billerica, MA), and western blotting proceeded as previously reported (Duffy et al., 2005c). The anti-AKR1C3 primary antibody was a rabbit polyclonal generated against the human AKR1C3 protein (Suzuki-Yamamoto et al., 1999) and was used at a 1:2000 dilution; an anti-rabbit IgG-horseradish peroxidase conjugate secondary antibody (Amersham, Piscataway, NJ) was used at a dilution of 1:20,000. A single band of 36 kD was consistently detected by chemiluminescence (ECL, Amersham); no bands were detected when the primary antibody was omitted (not shown). Blots were then stripped of all antibodies following instructions provided by the membrane manufacturer, and western blotting was performed on the stripped membranes using a mouse anti-tubulin primary antibody (1:1000 dilution, Sigma) and an anti-mouse IgG-horseradish peroxidase conjugate secondary antibody (1:20,000 dilution, Amersham). Molecular size of bands representing AKR1C3 and tubulin proteins were determined by comparison to prestained standards (BioRad). Each experiment included at least 4 lanes of serial-diluted granulosa cell lysate; detection and densitometric analysis of the protein of interest and tubulin in these samples was used to generate standard curves for semi-quantitative analysis of granulosa cell lysates. Films were scanned and analyzed densitometrically using SigmaGel software (Jandel Scientific, San Rafael, CA). Tubulin levels in granulosa cell lysates were not different between treatment groups. All data are expressed as a ratio of AKR1C3/tubulin content for each granulosa cell sample.
Immunohistochemical Detection of AKR1C3
Immunohistochemical detection of AKR1C3 in ovarian tissues was performed with 5 μm sections of paraffin-embedded tissues as previously described (Duffy et al., 2005c) using the anti-AKR1C3 antibody described above for western blotting (1:500 dilution), a biotinylated bovine anti-rabbit IgG secondary antibody, and peroxide-conjugated avidin solution (ABC kit, Vector Laboratories, Burlingame, CA); peroxidase activity was visualized with Nova Red chromagen (Vector). Omission of the primary antibody served as a negative control.
In Situ Hybridization
Primers used for in situ detection of a region common to AKR1C1 and AKR1C2 mRNA (antisense 5′-3′ GGGATCACTTCCTCACC; sense 5′-3′ GGTGAGGAAGTGATCCC) shared 100% sequence identity to human AKR1C1 and AKR1C2. Digoxgenin-labeled oligoprobes were produced using the DIG Oligonucleotide Tailing Kit, 2nd Generation (Roche) according to the manufacturer’s protocol. Labeling efficiency was determined by dot blot assay using the DIG Nucleic Acid Detection kit (Roche) according to the manufacturer’s protocol. In situ hybridization was performed essentially as described by Dijkman et al. (Dijkman et al., 1995) with prehybridization in a buffer containing 0.3 M sodium chloride, 0.03 M sodium citrate, pH 7.0 (2X SSC), 500 μg/ml sheared salmon sperm DNA (Eppendorf, Westbury, NY) denatured for 5 min at 95C, 10 μg/ml poly(A) solution (Roche), and 25% deionized formamide (Sigma). Hybridization of probes with slide-mounted frozen ovarian tissue sections was performed using 50 ng/ml oligoprobe for 2 hours at 55C, followed by washing at 55C for 5 min in 2X SSC, 1X SSC, and twice in 0.25X SSC. Sections were then washed twice for 5 min in 2X SSC at room temperature. Immunological detection was performed using the DIG Nucleic Acid Detection kit. Sections were then counterstained with Nuclear Fast Red (Vector) and mounted in glycerol.
Granulosa Cell Culture
Granulosa cells were cultured on tissue culture plates coated with fibronectin and maintained at 37C in 5% CO2 in serum-free DMEM-Ham’s F12 medium containing insulin (2 μg/ml), transferrin (5 μg/ml), selenium (0.25 nM), aprotinin (25 mg/ml), and human low density lipoprotein (25 μg/ml) as previously described (Duffy & Stouffer, 2003).
For analysis of RNA, monkey granulosa cells were obtained after ovarian stimulation before (0 h) hCG administration; 100,000 cells/400 μl media were treated with hCG (100 ng/ml, Serono) or no treatment (control) for up to 48 hours. Cells were lysed in situ with Trizol reagent, and total RNA was prepared with the addition of glycogen (10 μg) to improve recovery; RT-PCR was then performed as described above.
AKR1C3 Activity
While PGH2 is unstable in solution, PGD2 is a stable substrate for assay of AKR1C3 activity. Preliminary studies demonstrated that human luteinizing granulosa cells produced very low levels of 11β-PGF2α in culture (3.4 ± 0.7 pg/ml media). Therefore, AKR1C3 activity was assessed by measuring the conversion of PGD2 to 11β-PGF2α. Granulosa cells were plated (3×105 cells/ml media) and cultured for 4 hours with vehicles (final concentrations 0.03% DMSO + 0.04% ethanol), PGD2 (0.5 μM, Cayman), PGD2 + the AKR1C3 inhibitor medroxyprogesterone acetate (MPA, Sigma (Higaki et al., 2003)), PGD2 + MPA (10 μM) + the progesterone receptor antagonist mifepristone (10 μM, Sigma (El-Ashry et al., 1989)), or PGD2 + the AKR1C3 inhibitor bimatoprost (Cayman (Koda et al., 2004)); media were collected and stored at -20C until assayed for 11β-PGF2α concentration by EIA (Cayman). Preliminary experiments (not shown) confirmed that 1) 11β-PGF2α production increased linearly with increased PGD2 concentration (0.01-10 μM), cell number (1-5.5 ×105), and incubation time (1-8 hours). The concentrations of PGD2, MPA, mifepristone, and bimatoprost used in this study did not alter detection of 11β-PGF2α by EIA (not shown).
Activity of AKR1C1/AKR1C2
Human luteinizing granulosa cells were suspended in Hanks buffered salt solution, pH 7.4 (Sigma) + 0.1% BSA at a concentration of 4×106 cells/ml. Either [3H]PGE2 or [3H]PGF2α was added (0.2 μCu/ml, PerkinElmer, Wellesley, MA), and cells were incubated for 4 hours at 37C in a shaking water bath (Murdoch & Farris, 1988). In each experiment, media+[3H]PG was incubated in the absence of cells to determine the dpms of [3H] which comigrated with PGE2 and PGF2α in the absence of enzymatic conversion. The AKR1C2-selective inhibitor taurodeoxycholanic acid (Steraloids, Newport, RI (Bauman et al., 2005)) was added to some cells before incubation; vehicle (ethanol, 0.04%) did not compromise PG synthesis (not shown). After incubation, HCl was added to achieve a final concentration of 0.1 N, and cells + media were extracted with ethyl acetate (Duffy & Stouffer, 2001). The PG-containing organic layer was dried under nitrogen and resuspended in chloroform: methanol (95:5). Radioinert PGE2 and PGF2α (10 μg each) were added to each sample before loading onto silica gel 60 plates (E. Merck, Darmstadt, Germany) and separated in ethyl acetate: isooctane: glacial acetic acid (100:50:20) (Campbell & Ojeda, 1987). The locations of PGE2 and PGF2α in each lane were identified after exposure to iodine vapor. Silica scraped from these portions of the sample lanes was counted in a scintillation counter (LS 6500, Beckman Coulter, Fullerton, CA). To assess conversion of [3H]PGE2 to [3H]PGF2α, dpms comigrating with PGF2α were expressed as a percentage of the total dpms recovered minus the percentage of total dpms comigrating with PGF2α when cells were excluded from the reaction. For each patient, percent of dpms representing PGF2α in the presence of taurodeoxycholanic acid was expressed relative to percent of dpms present as PGF2α in absence of the inhibitor. Similar experiments were performed to assess conversion of [3H]PGF2α to [3H]PGE2.
Data Analysis
All data were assessed for heterogeneity of variance using Bartlett’s test and log transformed when Bartlett’s test yielded a significance of <0.05; data presented in Figures 2, 3, 5B, and 6 were log transformed before further analysis. Data in Figures 2 and 3 were analyzed by ANOVA, followed by Newman-Keuls test. Data in Figure 5 were analyzed by 2-tailed paired t-test. Data in Figure 6 were analyzed by ANOVA with one repeated measure, followed by Newman-Keuls test. Statistical analyses above were performed using StatPak v4.12 software (Northwest Analytical, Portland, OR). Data are presented as mean ± standard error of the mean (SEM), and significance was assumed at p<0.05.
Figure 2.
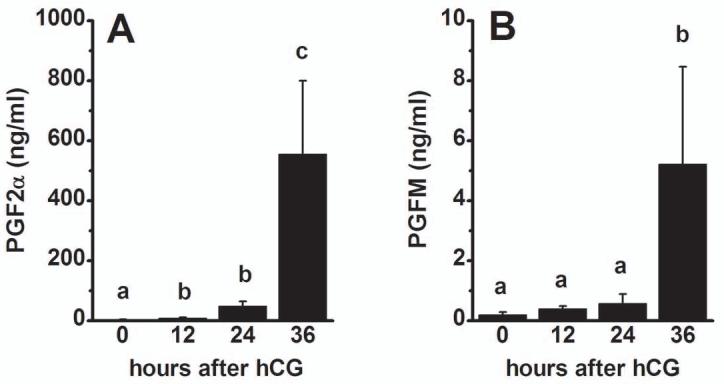
PGF2α (A) and PGFM (B) levels in monkey follicular fluid. Follicular fluid was obtained from monkeys experiencing controlled ovarian stimulation before (0 hour) and 12, 24, and 36 hours after administration of an ovulatory dose of hCG. PG levels were determined by EIA. Within each panel, groups with different superscripts are different by ANOVA and Newman Keuls test, p<0.05. Data are presented as mean±SEM, n=5-6/group.
Figure 3.
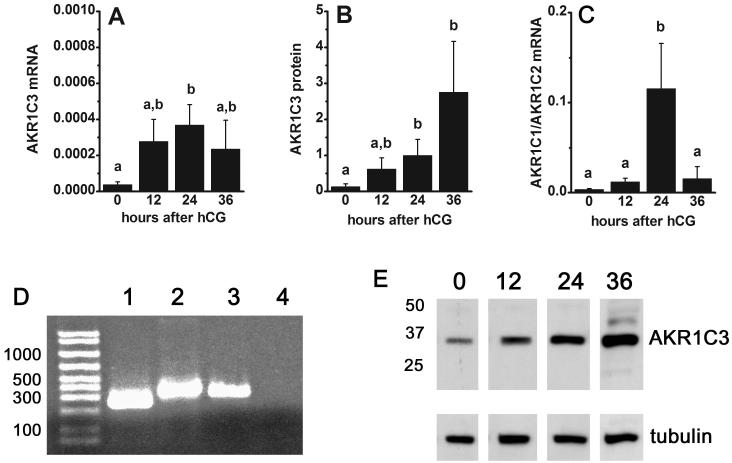
Granulosa cell levels of PGF2α synthesis enzyme mRNA and protein. Granulosa cells obtained from monkeys experiencing controlled ovarian stimulation before (0 hour) and 12, 24, and 36 hours after hCG were assessed for AKR1C3 mRNA (A) and protein (B) levels by real time RT-PCR and western blotting, respectively. Panel C. Granulosa cell level of mRNA for AKR1C1/AKR1C2 was determined by real time RT-PCR. Panel D. Representative gel shows cDNA amplified by real time RT-PCR representing detection of mRNA for β-actin (lane 1), AKR1C1/AKR1C2 (lane 2), and AKR1C3 (lane 3) in monkey granulosa cells; negative control is also shown (RT omitted, lane 4). Panel E. A representative western blot shows detection of AKR1C3 at 36 kD in lysates of monkey granulosa cells obtained 0, 12, 24, and 36 hours after hCG; detection of tubulin in each sample is also shown. All enzyme mRNA levels were normalized to the level of β-actin mRNA in the same sample. Western blotting data were normalized to tubulin content of each sample. Within each panel, groups with no common superscripts are different by ANOVA and Newman Keuls test, p<0.05. Data are presented as mean±SEM, n=4-5/group.
Figure 5.
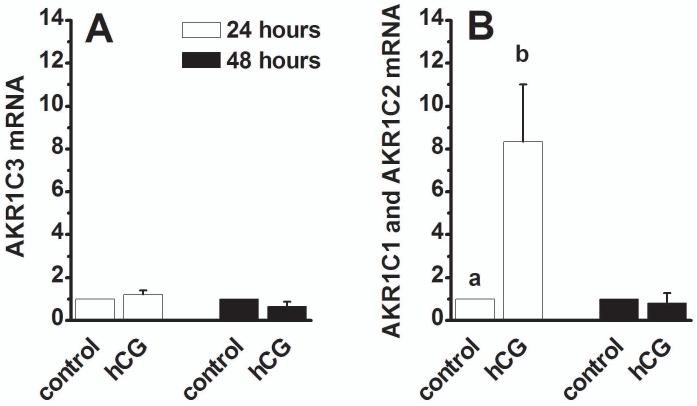
Granulosa cell expression of PGF2α synthesis enzyme mRNAs in vitro. Granulosa cells obtained from monkeys experiencing ovarian stimulation before administration of hCG were cultured in the absence (control) and presence of hCG (100 ng/ml) for 24 (white bars) or 48 (black bars) hours. Total RNA was assessed for AKR1C3 mRNA (Panel A) and AKR1C1/AKR1C2 mRNA (Panel B) by real time RT-PCR. All enzyme mRNA levels were normalized to the level of β-actin mRNA in the same sample. Then, mRNA level after hCG treatment was expressed as the fold increase over control cells collected after the same number of hours in vitro. Groups with no common superscripts are different by paired t-test, p<0.05. Data are presented as mean±SEM, n=4-6/group.
Figure 6.
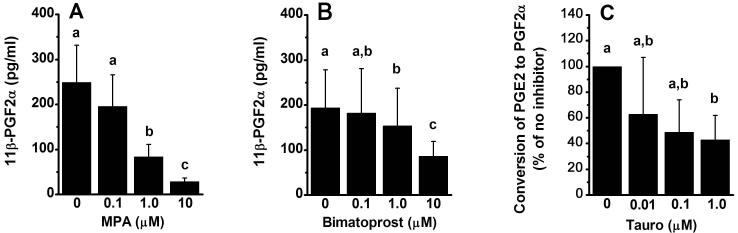
Activity of PGF2α synthesis enzymes in human luteinizing granulosa cells. A. Granulosa cells were incubated with the precursor PGD2 alone (0 μM) or with the AKR1C3 inhibitor medroxyprogesterone acetate (MPA); media 11β-PGF2α concentrations were determined by EIA (n=4/group). B. Granulosa cells were incubated with the precursor PGD2 alone (0 μM) or with the AKR1C3 inhibitor bimatoprost; media 11β-PGF2α concentrations were determined by EIA (n=4-5/group). C. To assess AKR1C1 and AKR1C2 activity, human luteinizing granulosa cells were incubated with [3H]PGE2 in the absence (0 μM) or presence of the AKR1C2-selective inhibitor taurodeoxycholanic acid (Tauro); PGs were separated by thin layer chromatography. Data are expressed as the percentage of total dpms recovered which comigrated with PGF2α after inhibitor treatment relative to the percentage of total dpms comigrating with PGF2α in untreated cells (n=3-9/group). Within each panel, groups with no common superscripts are different by ANOVA and Newman-Keuls test, p<0.05; data are presented as mean±SEM.
RESULTS
Follicular Fluid PGF2α, PGFM, and 11β-PGF2α Concentrations
Follicular fluid contained measurable concentrations of PGF2α throughout the periovulatory interval (Figure 2). Similar to our previous report of PGF2α levels in rhesus monkey follicular fluid (Duffy & Stouffer, 2001) and PGE2 levels in cynomolgus monkey follicular fluid (Duffy et al., 2005c), levels of PGF2α in follicular fluid of cynomolgus macaques were lowest before (0 hour) hCG administration, rose slightly by 12-24 hours after hCG, and reached peak levels 36 hours after hCG administration, just before the expected time of ovulation (n=5-6/group). Levels of the primary PGF2α metabolite (PGFM) were also low at 0 hour hCG. Mean PGFM levels were higher (though not significantly elevated) 12-24 hours after hCG and were highest 36 hours after hCG administration (n=5/group). In contrast, 11β-PGF2α was not detected in the majority of follicular fluid samples assayed (not shown). No samples obtained 0-12 h after hCG (n=4/time point) and only 1 of 4 samples obtained 24 hours after hCG administration contained detectable 11β-PGF2α. In follicular fluid samples obtained 36 hours after hCG, 3 of 5 contained detectable 11β-PGF2α, which in these samples was 6.9 ± 3.2 ng/ml.
Expression of PGF2α Synthesis Enzymes
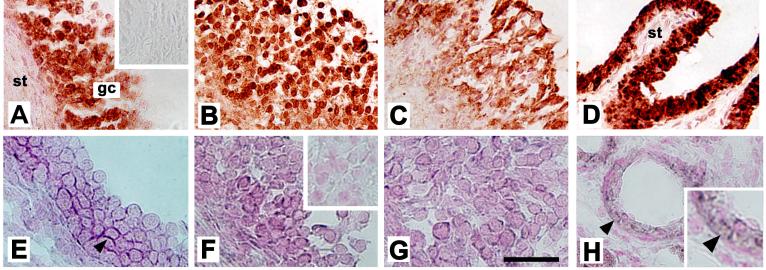
AKR1C3 converts PGH2 directly into PGF2α (Figure 1). Granulosa cell mRNA levels of AKR1C3 were low at 0 hours hCG, intermediate 12 hours after hCG, peaked 24 hours after hCG, and fell to intermediate levels 36 hours after hCG administration (Figure 3A, n=4-5/group). AKR1C3 protein levels in granulosa cell lysates were assessed by western blotting. In all monkey granulosa cell lysates examined, AKR1C3 was detected as a single band of 36 kD (Figure 3E), consistent with previous detection of a single 36.8 kD protein in human lung (Suzuki-Yamamoto et al., 1999). Granulosa cell levels of AKR1C3 protein were low at 0 hour, rose to intermediate levels 12-24 hours after hCG, and peaked 36 hours after hCG administration (Figure 3B, n=4/group). AKR1C3 protein was detected in granulosa cells of periovulatory follicles obtained before (0 hour) and at all times after hCG administration by immunohistochemistry (Figure 4A-C). Stromal cells consistent with identification as theca cells of monkey periovulatory follicles were consistently AKR1C3-negative. AKR1C3 detection in luminal epithelial cells of the monkey seminal vesicle served as a positive control (Figure 4D).
Figure 4.
Localization of AKR1C3 protein as well as PGE-F isomerase (AKR1C1/AKR1C2) mRNA to cells of monkey follicles obtained 0 (A, E), 12 (not shown), 24 (B, F), and 36 (C, G) hours after hCG administration. All follicles are shown with stroma (st) in lower left, granulosa cells (gc) central, and the follicle antrum in the upper right of the image. AKR1C3 was detected by immunocytochemistry (red/brown) in granulosa cells of monkey follicles (A-C); stromal cells were consistently devoid of staining. No staining was observed when the primary antibody was omitted (inset, A). Monkey seminal vesicle showed intense immunostaining in the luminal epithelium, but not stroma (st), which served as a positive control (D). Sections in Panels A-D were not counterstained. AKR1C1/AKR1C2 mRNA was detected by in situ hybridization in granulosa cells of monkey follicles (E-G); staining appears dark purple (arrowhead). Inset in Panel F shows absence of dark purple staining in granulosa cells when sense probe was used; only nuclear counterstain (pink) is visible. Small vessels in the monkey ovarian stroma stained for AKR1C1/AKR1C2 mRNA (Panel H, arrows) and served as a positive control; ovarian stroma located away from follicles did not stain. An enlarged view of image in panel H (inset) shows staining in cells near the vessel lumen. For Panels A-D, bar in Panel G=50 μm. For Panels E-H, bar in Panel G=25 μm. Data shown are representative of n=3-4 animals/group.
PGE2 and PGF2α can be interconverted via the activity of AKR1C1 and AKR1C2 (Figure 1). Levels of AKR1C1/AKR1C2 mRNA were detectable at 0-12 hours after hCG, elevated by 24 hours after hCG, then returned to 0 hour levels by 36 hours after hCG administration (Figure 3D, n=4/group). An antibody recognizing monkey AKR1C1 and/or AKR1C2 protein could not be identified, so in situ hybridization with an antisense oligonucleotide probe common to both AKR1C1 and AKR1C2 mRNA was used to localize these mRNAs to the cells of the monkey periovulatory follicle (Figure 4E-G). Dark purple precipitate representing staining for AKR1C1/AKR1C2 mRNA was observed in granulosa cells of monkey ovarian follicles obtained 0, 12, 24, and 36 hours after hCG administration. Staining was occasionally noted in the stroma immediately surrounding monkey periovulatory follicles, consistent with the location of theca cells (not shown). However, ovarian stroma located at a distance from follicles was generally devoid of staining for AKR1C1/AKR1C2 mRNA (Figure 4H). AKR1C1/AKR1C2 mRNA was also detected in small blood vessels (Eyster et al., 2007) within the ovarian stroma, which served as a positive control (Figure 4H).
Expression of 11β-PGF2α Synthesis Enzymes
Production of 11β-PGF2α requires the activity of PGDS to convert PGH2 into PGD2, then AKR1C3 converts PGD2 into 11β-PGF2α (Figure 1). Granulosa cells expressed H-PGDS; mRNA levels did not change in response to hCG administration (n=4-6/group, data not shown). Similarly, granulosa cells expressed L-PGDS mRNA, but levels were not altered by exposure to an ovulatory dose of hCG (n=4/group, data not shown).
These data support the identification of 2 pathways which may produce PGF2α in the primate periovulatory follicle. AKR1C3 mRNA and protein are expressed in monkey granulosa cells in a manner consistent with the rise in PGF2α measured in follicular fluid late in the periovulatory interval; previous studies demonstrated that the inducible form of cyclooxygenase (COX-2) is expressed by primate granulosa cells to provide PGH2 as substrate for PGF2α production via AKR1C3 (Duffy & Stouffer, 2001, Duffy et al., 2005b). Granulosa cell expression of AKR1C1/AKR1C2 mRNA also increased late in the periovulatory interval, and high follicular fluid levels of PGE2 produced by the PGE synthase mPGES-1 (Duffy et al., 2005a) would provide adequate substrate for PGF2α production via this enzyme. In contrast, very low levels of 11β-PGF2α measured in follicular fluid (above) and granulosa cell cultures (below) argue against a key role for this PG in mediating periovulatory events. Therefore, the following studies focused on PGF2α production via AKR1C3 and AKR1C1/AKR1C2.
Gonadotropin Regulation of PGF2α Synthesis Enzyme Expression In Vitro
The ovulatory gonadotropin surge increases granulosa cell levels of mRNA for AKR1C3 and AKR1C1/AKR1C2 in vivo (Figure 3). To determine if hCG acts directly at granulosa cells to modulate expression of these mRNAs, granulosa cells obtained from large periovulatory follicles before (0 h) administration of hCG were placed in vitro and treated with an ovulatory dose of hCG (Zelinski-Wooten et al., 1997). AKR1C3 mRNA levels were not different between control and hCG-treated granulosa cells after 24 and 48 hours in vitro (Figure 5A, n=4-6/group). In contrast, hCG treatment for 24 hours increased expression of mRNA for AKR1C1/AKR1C2 8-fold above mRNA levels measured in control cells (Figure 5B, n=5/group). After 48 hours in vitro, AKR1C1/AKR1C2 mRNA levels in control and hCG-treated granulosa cells were not different. This pattern of gonadotropin-regulated expression of mRNA for AKR1C1/AKR1C2 was very similar to that seen in granulosa cells exposed to hCG in vivo (Figure 3C).
PGF2α Synthesis Enzyme Activity in Human Luteinizing Granulosa Cells
The PGF2α precursor PGH2 is highly unstable in solution, so AKR1C3 activity was examined by assessing the conversion of PGD2 to 11β-PGF2α. Human granulosa cells produce very little 11β-PGF2α in vitro in the absence of added PGD2 (3.4 ± 0.7 pg/ml media, n=9); media levels of 11β-PGF2α were 60-fold higher in the presence of added PGD2 (Figure 6A-B). Synthesis of 11β-PGF2α from PGD2 was reduced by the AKR1C3 inhibitor MPA in a dose-dependent manner (Figure 6A), consistent with the reported IC50 of 0.28 μM (Higaki et al., 2003), while the structurally-similar molecule cholesterol had no effect on 11β-PGF2α synthesis and served as a negative control (data not shown). To ensure that MPA inhibited AKR1C3 activity independent of the ability of MPA to regulate transcription via the progesterone receptor, additional cells were cultured with MPA and the progesterone receptor antagonist mifepristone (El-Ashry et al., 1989); AKR1C3 activity in the presence of MPA and mifepristone was similar to AKR1C3 activity in the presence of MPA alone (data not shown). As an additional approach, the prostaglandin analog bimatoprost was also used to inhibit AKR1C3 activity. Bimatoprost reduced 11β-PGF2α production by human luteinizing granulosa cells in a dose-dependent manner (Figure 6B), consistent with the reported IC50 of 5 μM (Koda et al., 2004).
AKR1C1 and AKR1C2 possess bidirectional PGE-F isomerase activity and are capable of converting PGE2 to PGF2α as well as PGF2α to PGE2 (Nishizawa et al., 2000). To determine if human luteinizing granulosa cells convert PGE2 to PGF2α, cells were incubated with 3H-PGE2, and production of 3H-PGF2α was assessed (Figure 6C). Granulosa cells converted 9.2 ± 1.3% of 3H-PGE2 to 3H-PGF2α during the 4 hour incubation period. This conversion was inhibited by taurodeoxycholanic acid in a dose-sensitive manner, consistent with the reported IC50 for inhibition of AKR1C2 (0.56 μM) but not AKR1C1 (61 μM) (Bauman et al., 2005). Human luteinizing granulosa cells incubated with 3H-PGF2α did not produce 3H-PGE2 (n=3, not shown), so the PGE-F isomerase activity in primate granulosa cells is predominantly or exclusively in the direction of PGE2 to PGF2α and is catalyzed by AKR1C2.
DISCUSSION
This report is the first to demonstrate that the primate follicle expresses the enzymes and possesses the enzymatic activities required for two pathways of PGF2α synthesis. Follicular PGF2α levels peak just before ovulation. PGFM levels in follicular fluid increase in parallel with PGF2α, supporting the concept that the rate of PGF2α synthesis peaks late in the periovulatory interval. Granulosa cells of the periovulatory follicle contain enzymatic activities capable of PGF2α production via AKR1C3 (from PGH2) and via AKR1C2 (from PGE2). In contrast, 11β-PGF2α production via AKR1C3 (from PGD2) is unlikely to contribute to bioactive PGF2α levels within the follicle. AKR1C3 mRNA and protein reach maximal levels in monkey granulosa cells late in the periovulatory interval, and human granulosa cells obtained just before ovulation possess AKR1C3 activity. AKR1C1/AKR1C2 mRNA peaks just before follicular fluid PGF2α levels reach maximum levels in monkey follicles, and human granulosa cells obtained just before ovulation can convert PGE2 to PGF2α via AKR1C2. Taken together, these data support the conclusion that PGF2α can be produced by the primate periovulatory follicle via two pathways.
Primate granulosa cells likely convert PGH2 to PGF2α via AKR1C3, the traditional PGF synthase (Watanabe, 2002). AKR1C3 mRNA and protein were detected in periovulatory monkey granulosa cells, and peak AKR1C3 protein levels coincided with peak follicular fluid PGF2α concentrations. Interestingly, hCG increased granulosa cell AKR1C3 mRNA level in vivo, but not in vitro, suggesting that elevated AKR1C3 expression in vivo may require participation of a non-granulosa cell type, either as the site of gonadotropin action or for the production of a necessary permissive factor. Human luteinizing granulosa cells possess AKR1C3 activity just before ovulation; these cells converted PGD2 to 11β-PGF2α, and both MPA and bimatoprost reduced 11β-PGF2α production. The use of two chemically-disparate inhibitors supports the conclusion that these agents act directly at the enzyme to reduce AKR1C3 activity as previously reported (Higaki et al., 2003, Koda et al., 2004), not via transcriptional regulation of the AKR1C3 gene via the progesterone receptor (in the case of MPA) or the PGF2α receptor (in the case of bimatoprost). These data represent the first demonstration of gonadotropin-regulated AKR1C3 expression and activity in the mammalian periovulatory follicle and identify granulosa cell AKR1C3 as a possible enzyme involved in follicular PGF2α synthesis.
AKR1C1 and AKR1C2 are closely-related enzymes with PGE-F isomerase activity. Monkey granulosa cell AKR1C1/AKR1C2 mRNA levels increased in response to an ovulatory dose of hCG both in vivo and in vitro, reaching peak levels 24 hours after hCG administration and consistent with the measurement of maximal PGF2α levels in follicular fluid just before ovulation. While PGE-F isomerase activity can be bidirectional (Nishizawa et al., 2000), only conversion of PGE2 to PGF2α was detected in human granulosa cells in the present study. The AKR1C2-selective inhibitor taurodeoxycholanic acid inhibited PGE-F isomerase activity, supporting the conclusion that AKR1C2, but not AKR1C1, is the primary PGE-F isomerase in the primate follicle. Taurodeoxycholanic acid (0.1-1.0 μM) most likely reduced PGF2α synthesis through its ability to inhibit PGE-F isomerase activity (Higaki et al., 2003) and not regulation of gene transcription, as similar bile acids regulate transcription with EC50 values of 10-100 μM (Makishima et al., 1999, Parks et al., 1999). Expression and activity of PGE-F isomerase has been reported in whole ovaries and periovulatory follicles from rodents and domestic animals (Iwata et al., 1990, Murdoch & Farris, 1988). In sheep follicles, PGE-F isomerase activity increased in response to an ovulatory gonadotropin surge to peak just before ovulation (Murdoch & Farris, 1988). These previous reports, in combination with data from the present study, support a role for the AKR1C2 form of the PGE-F isomerase in the gonadotropin-stimulated conversion of PGE2 to PGF2α by granulosa cells of the periovulatory follicle.
Despite the presence of two forms of PGDS mRNA as well as AKR1C3 mRNA in monkey granulosa cells, 11β-PGF2α levels in follicular fluid were low/nondetectable, suggesting that conversion of PGH2 to PGD2 and then 11β-PGF2α within the periovulatory follicle is inefficient. Therefore, it is unlikely that 11β-PGF2α acts via the PGF2α (FP) receptor to play a major role in ovulation. PGD2 produced within the ovulatory follicle may contribute to periovulatory events by stimulating PGD2 (DP) receptors (Saito et al., 2002). PGD2 is also a precursor for the production of the PPARγ ligand PGJ2. PPARγ has been implicated in the regulation of periovulatory events such as steroidogenesis (Komar et al., 2001) and granulosa cell apoptosis (Lovekamp-Swan & Chaffin, 2005). While further study regarding the ovulatory role of PGD2 in the primate follicle is warranted, PGD2 does not represent a likely substrate for PGF2α synthesis.
Many members of AKR enzyme group are multifunctional, capable of both prostaglandin and steroid synthesis (Nishizawa et al., 2000). AKR1C3 possesses primarily PG synthesis activity as this enzyme clearly prefers PG substrates over steroidogenic substrates (Nishizawa et al., 2000, Suzuki-Yamamoto et al., 1999). AKR1C1 and AKR1C2 both possess PG reductase activity, but AKR1C1 possesses 20α-hydroxysteroid dehydrogenase activity while AKR1C2 possesses 3α-hydroxysteroid dehydrogenase activity (Nishizawa et al., 2000). Steroid hormones present in the ovarian follicle may reduce the PG reductase activity of AKR1C1 and AKR1C2 during the periovulatory interval. Similarly, PGs may reduce steroid hormone synthesis via members of the AKR enzyme family. The primate periovulatory follicle contains micromolar levels of both prostaglandins and steroid hormones just before ovulation (Chaffin et al., 1999, Duffy & Stouffer, 2001), so determining if the PG synthase activity or the steroid dehydrogenase activity of any individual enzyme predominates in granulosa cells may be exceedingly complex.
While granulosa cells have been established as the primary site of PG synthesis by the periovulatory follicle, a role for theca cells in PG production remains equivocal. AKR1C3 mRNA was expressed by isolated human theca cells (Nelson et al., 2001), but AKR1C3 protein was not detected in monkey stroma consistent with theca cells (present study). In situ hybridization localized a sequence common to AKR1C1 and AKR1C2 mRNA to the stroma of monkey periovulatory follicles in the present study. AKR1C1 mRNA and 20α-hydroxysteroid dehydrogenase activity have been reported in human and bovine theca cells (Brown et al., 2006, Nelson et al., 2001), so AKR1C1 is likely the predominant AKR1C in monkey theca. Theca cells produce little PGF2α in vitro, and theca cell PGF2α synthesis is relatively insensitive to gonadotropin stimulation (Duffy et al., 2005a, Patwardhan & Lanthier, 1981, Wong & Richards, 1991), so theca AKR1C1 may have predominantly steroidogenic activity in periovulatory follicles. While a minor contribution to follicular fluid PGF2α may be made by theca cells, the gonadotropin-stimulated periovulatory rise in follicular fluid PGF2α is likely due to production primarily, if not exclusively, by granulosa cells.
The specific contribution made by PGF2α to periovulatory events remains unclear. Administration of PGF2α to PG-synthesis inhibitor-treated animals can restore ovulation in rodents, domestic animals, and primates (Janson et al., 1988, Murdoch et al., 1986, Sogn et al., 1987, Wallach et al., 1975). However, disruption of FP receptor expression did not prevent ovulation in mice (Sugimoto et al., 1997), arguing against receptor-mediated action of PGF2α to initiate ovulatory events in this species. FP receptor mRNA has been detected in follicles of domestic animals and women (Bridges & Fortune, 2007, Ristimaki et al., 1997, Sayasith et al., 2006). FP receptors capable of signal transduction have been detected in luteal cells of many species (Davis et al., 1988, Stocco et al., 2003), including monkeys (Houmard et al., 1992); functional FP receptors have also been studied in long-term cultures of human luteinized granulosa cells, which serve as an in vitro model for the corpus luteum (Carrasco et al., 1997, Tai et al., 2001). However, it is important to note that the presence of functional FP receptors has never been reported for follicular granulosa cells of any mammalian species. Disruption of the PGE2 receptor EP2 reduces the efficiency of ovulation in mice (Hizaki et al., 1999), and previous studies support a role for PGE2 to regulate periovulatory events in mammals (Peters et al., 2004, Sogn et al., 1987) including primates (Duffy & Stouffer, 2002). It remains possible that either or both PGE2 and PGF2α may be able to initiate ovulatory events in the follicles of primates and domestic animals. Treatment of PG synthesis inhibitor-treated animals with nonmetabolizable EP and FP receptor-selective agonists will likely be required to determine which PG receptors and, therefore, which PG(s), are needed to restore ovulation in these species.
The present study identifies two pathways for the synthesis of PGF2α by the primate periovulatory follicle. As discussed above, a direct role for PGF2α in periovulatory events will require demonstration of functional FP receptors in the cells of the follicle. As an alternative, PGF2α may serve as a substrate for the production of ovulatory PGE2 via the PGE-F isomerase activity present in granulosa cells. Finally, PGF2α synthesis by the periovulatory follicle may function primarily as a metabolic pathway for removal of follicular PGE2 through conversion into PGF2α via this PGE-F isomerase activity. In this scenario, PGF2α synthesis may represent a gonadotropin-stimulated mechanism which acts in concert with PGE2 metabolism via PGDH activity (Duffy et al., 2005c) to modulate the levels of periovulatory PGE2 within the primate follicle.
Acknowledgements
The authors would like to thank Ms. Carrie Seachord for technical assistance and Ms. Kim Hester for her role in animal training and animal protocols. Recombinant human gonadotropins and Antide used for these studies were generously provided by Serono Reproductive Biology Institute, Rockland, MA and Organon, a part of Schering Plough Corporation, Roseland, NJ. These studies were supported by NIH grants HD39872 and HD54691 (DMD) and Eastern Virginia Medical School.
Footnotes
Publisher's Disclaimer: Disclaimer. This is not the definitive version of record of this article. This manuscript has been accepted for publication in Reproduction, but the version presented here has not yet been copy edited, formatted or proofed. Consequently, the journal accepts no responsibility for any errors or omissions it may contain.
References
- Bauman DR, Rudnick SI, Szewczuk LM, Gophishetty S, Penning TM. Development of nonsteroidal anti-inflammatory drug analogs and steroid carboxylates selective for human aldo-keto reductase isoforms: potential antineoplastic agents that work independently of cyclooxygenase isozymes. Molecular Pharmacology. 2005;67:60–68. doi: 10.1124/mol.104.006569. [DOI] [PubMed] [Google Scholar]
- Bridges PJ, Fortune JE. Regulation, action and transport of prostaglandins during the periovulatory period in cattle. Molecular and Cellular Endocrinology. 2007;263:1–9. doi: 10.1016/j.mce.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Brown KA, Boerboom D, Bouchard N, Dore M, Lussier JG, Sirois J. Human chorionic gonadotropin-dependent induction of an equine aldo-keto reductase (AKR1C23) with 20α-hydroxysteroid dehydrogenase activity during follicular luteinization in vivo. Journal of Molecular Endocrinology. 2006;36:449–461. doi: 10.1677/jme.1.01987. [DOI] [PubMed] [Google Scholar]
- Campbell WB, Ojeda SR. Measurement of prostaglandins by radioimmunoassay. Methods in Enzymology. 1987;141:323–341. doi: 10.1016/0076-6879(87)41080-x. [DOI] [PubMed] [Google Scholar]
- Carrasco MP, Asboth G, Phaneuf S, Lopez Bernal A. Activation of the prostaglandin FP receptor in human granulosa cell. Journal of Reproduction and Fertility. 1997;111:309–317. doi: 10.1530/jrf.0.1110309. [DOI] [PubMed] [Google Scholar]
- Chaffin CL, Stouffer RL. Expression of matrix metalloproteinases and their tissue inhibitor messenger ribonucleic acids in macaque periovulatory granulosa cells: Time course and steroid regulation. Biology of Reproduction. 1999;61:14–21. doi: 10.1095/biolreprod61.1.14. [DOI] [PubMed] [Google Scholar]
- Chaffin CL, Hess DL, Stouffer RL. Dynamics of periovulatory steroidogenesis in the rhesus monkey follicle after controlled ovarian stimulation. Human Reproduction. 1999;14:642–649. doi: 10.1093/humrep/14.3.642. [DOI] [PubMed] [Google Scholar]
- Davis JS, Alila HW, West LA, Corradino RA, Hansel W. Acute effects of prostaglandin F2 alpha on inositol phospholipid hydrolysis in the large and small cells of the bovine corpus luteum. Molecular and Cellular Endocrinology. 1988;58:43–50. doi: 10.1016/0303-7207(88)90052-4. [DOI] [PubMed] [Google Scholar]
- Dean RB, Dixon WJ. Simplified statistics for small numbers of observations. Anal.Chem. 1951;23:636–638. [Google Scholar]
- Dijkman HBPM, Mentzel S, deJong AS, Assmann KJM. RNA in situ hybridization using digoxigenin-labeled cRNA probes. Biochemica. 1995;2:21–25. [Google Scholar]
- Duffy DM, Stouffer RL. The ovulatory gonadotrophin surge stimulates cyclooxygenase expression and prostaglandin production by the monkey follicle. Molecular Human Reproduction. 2001;7:731–739. doi: 10.1093/molehr/7.8.731. [DOI] [PubMed] [Google Scholar]
- Duffy DM, Stouffer RL. Follicular administration of a cyclooxygenase inhibitor can prevent oocyte release without alteration of normal luteal function in rhesus monkeys. Human Reproduction. 2002;17:2825–2831. doi: 10.1093/humrep/17.11.2825. [DOI] [PubMed] [Google Scholar]
- Duffy DM, Stouffer RL. Luteinizing hormone acts directly at granulosa cells to stimulate periovulatory processes: modulation of luteinizing hormone effects by prostaglandins. Endocrine. 2003;22:249–256. doi: 10.1385/ENDO:22:3:249. [DOI] [PubMed] [Google Scholar]
- Duffy DM, Seachord CL, Dozier BL. Microsomal prostaglandin E synthase-1 (mPGES-1) is the primary form of PGES expressed by the primate periovulatory follicle. Human Reproduction. 2005a;20:1485–1492. doi: 10.1093/humrep/deh784. [DOI] [PubMed] [Google Scholar]
- Duffy DM, Seachord CL, Dozier BL. An ovulatory gonadotropin stimulus increases cytosolic phospholipase A2 (cPLA2) expression and activity in granulosa cells of primate periovulatory follicles. Journal of Clinical Endocrinology and Metabolism. 2005b;90:5858–5865. doi: 10.1210/jc.2005-0980. [DOI] [PubMed] [Google Scholar]
- Duffy DM, Dozier BL, Seachord CL. Prostaglandin dehydrogenase (PGDH) and prostaglandin levels in periovulatory follicles: Implications for control of primate ovulation by PGE2. Journal of Clinical Endocrinology and Metabolism. 2005c;90:1021–1027. doi: 10.1210/jc.2004-1229. [DOI] [PubMed] [Google Scholar]
- El-Ashry D, Onate SA, Nordeen SK, Edwards DP. Human progesterone receptor complexed with the antiprogestin RU 486 binds to hormone response element in a structurally altered form. Molecular Endocrinology. 1989;3:1545–1558. doi: 10.1210/mend-3-10-1545. [DOI] [PubMed] [Google Scholar]
- Eyster KM, Mark CJ, Gayle R, Martin DS. The effects of estrogen and testosterone on gene expression in the rat mesenteric arteries. Vascular Pharmacology. 2007;47:238–247. doi: 10.1016/j.vph.2007.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higaki Y, Usami N, Shintani S, Ishikura S, El-Kabbani O, Hara A. Selective and potent inhibitors of human 20α-hydroxysteroid dehydrogenase (AKR1C1) that metabolizes neurosteroids derived from progesterone. Chemico-Biological Interactions. 2003;134-144:503–513. doi: 10.1016/s0009-2797(02)00206-5. [DOI] [PubMed] [Google Scholar]
- Hizaki H, Segi E, Sugimoto Y, Hirose M, Saji T, Ushikubi F, Matsuoka T, Noda Y, Tanaka T, Yoshida N, et al. Abortive expansion of the cumulus and impaired fertility in mice lacking the prostaglandin E receptor subtype EP 2. Proceedings of the National Academy of Sciences, USA. 1999;96:10501–10506. doi: 10.1073/pnas.96.18.10501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houmard BS, Guan Z, Stokes BT, Ottobre JS. Activation of the phosphatidylinositol pathway in the primate corpus luteum by prostaglandin F2 alpha. Endocrinology. 1992;131:743–748. doi: 10.1210/endo.131.2.1639020. [DOI] [PubMed] [Google Scholar]
- Iwata N, Inazu N, Satoh T. Changes in rat ovarian carbonyl reductase activity and content during the estrous cycle, and localization. Biology of Reproduction. 1990;42:161–166. doi: 10.1095/biolreprod42.1.161. [DOI] [PubMed] [Google Scholar]
- Janson PO, Brannstrom M, Holmes PV, Sogn J. Studies on the mechansim of ovulation using the model of the isolated ovary. Annals of the New York Academy of Sciences. 1988;541:22–29. doi: 10.1111/j.1749-6632.1988.tb22238.x. [DOI] [PubMed] [Google Scholar]
- Koda N, Tsutsui Y, Niwa H, Ito S, Woodward DF, Watanabe K. Synthesis of prostaglandin F ethanolamide by prostaglandin F synthase and identification of Bimatoprost as a potent inhibitor of the enzyme: new enzyme assay method using LC/ESI/MS. Archives of Biochemistry and Biophysics. 2004;424:128–136. doi: 10.1016/j.abb.2004.02.009. [DOI] [PubMed] [Google Scholar]
- Komar CM, Braissant O, Wahli W, Curry TE., Jr. Expression and localization of PPARs in the rat ovary during follicular development and the periovulatory period. Endocrinology. 2001;142:4831–4838. doi: 10.1210/endo.142.11.8429. [DOI] [PubMed] [Google Scholar]
- Lovekamp-Swan T, Chaffin CL. The peroxisome proliferator-activated receptor gamma ligand troglitazone induces apoptosis and p53 in rat granulosa cells. Molecular and Cellular Endocrinology. 2005;233:15–24. doi: 10.1016/j.mce.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Makishima M, Okamoto AY, Repa JJ, Tu H, Learned RM, Luk A, Hull MV, Lustig KD, Mangelsdorf DJ, Shan B. Identification of a nuclear receptor for bile acids. Science. 1999;284:1362–1365. doi: 10.1126/science.284.5418.1362. [DOI] [PubMed] [Google Scholar]
- Murdoch WJ, Farris ML. Prostaglandin E2-9-ketoreductase activity of preovulatory ovine follicles. Journal of Animal Science. 1988;66:2924–2929. doi: 10.2527/jas1988.66112924x. [DOI] [PubMed] [Google Scholar]
- Murdoch WJ, Peterson TA, Van Kirk EA, Vincent DL, Inskeep EK. Interactive roles of progesterone, prostaglandins, and collagenase in the ovulatory mechanism of the ewe. Biology of Reproduction. 1986;35:1187–1194. doi: 10.1095/biolreprod35.5.1187. [DOI] [PubMed] [Google Scholar]
- Nelson VL, Qin K-N, Rosenfield RL, Wood JR, Penning TM, Legro RS, Strauss JFI, McAllister JM. The biochemical basis for increased testosterone production in theca cells propagated from patients with polycystic ovary syndrome. Journal of Clinical Endocrinology and Metabolism. 2001;86:5925–5933. doi: 10.1210/jcem.86.12.8088. [DOI] [PubMed] [Google Scholar]
- Nishizawa M, Nakajima T, Yasuda K, Kanzaki H, Sasaguri Y, Watanabe K, Ito S. Close kinship of human 20α-hydroxysteroid dehydrogenase gene with three aldo-keto reductase genes. Genes to Cells. 2000;5:111–125. doi: 10.1046/j.1365-2443.2000.00310.x. [DOI] [PubMed] [Google Scholar]
- Parks DJ, Blanchard SG, Bledsoe RK, Chandra G, Consler TG, Kliewer SA, Stimmel JB, Willson TM, Zavacki AM, Moore DD, et al. Bile acids: natural ligands for an orphan nuclear receptor. Science. 1999;284:1365–1368. doi: 10.1126/science.284.5418.1365. [DOI] [PubMed] [Google Scholar]
- Patwardhan VV, Lanthier A. Prostaglandins PGE and PGF in human ovarian follicles: Endogenous contents and in vitro formation by theca and granulosa cells. Acta Endocrinologica. 1981;97:543–550. doi: 10.1530/acta.0.0970543. [DOI] [PubMed] [Google Scholar]
- Peters MW, Pursley JR, Smith GW. Inhibition of intrafollicular PGE2 synthesis and ovulation following ultrasound-mediated intrafollicular injection of the selective cyclooxygenase-2 inhibitor NS-398 in cattle. Journal of Animal Science. 2004;82:1656–1662. doi: 10.2527/2004.8261656x. [DOI] [PubMed] [Google Scholar]
- Ristimaki A, Jaatinen R, Ritvos O. Regulation of prostaglandin F2α receptor expression in cultured human granulosa-luteal cells. Endocrinology. 1997;138:191–195. doi: 10.1210/endo.138.1.4891. [DOI] [PubMed] [Google Scholar]
- Saito S, Tsuda H, Michimata T. Prostaglandin D2 and reproduction. American Journal of Reproductive Immunology. 2002;47:295–302. doi: 10.1034/j.1600-0897.2002.01113.x. [DOI] [PubMed] [Google Scholar]
- Sayasith K, Bouchard N, Dore M, Sirois J. Molecular cloning and gonadotropin-dependent regulation of equine prostaglandin F2α receptor in ovarian follicles during the ovulatory process in vivo. Prostaglandins and other Lipid Mediators. 2006;80:81–92. doi: 10.1016/j.prostaglandins.2006.05.020. [DOI] [PubMed] [Google Scholar]
- Sirois J. Induction of prostaglandin endoperoxide synthase-2 by human chorionic gonadotropin in bovine preovulatory follicles in vivo. Endocrinology. 1994;135:841–848. doi: 10.1210/endo.135.3.8070377. [DOI] [PubMed] [Google Scholar]
- Sirois J, Dore M. The late induction of prostaglandin G/H synthase-2 in equine preovulatory follicles supports its role as a determinant of the ovulatory process. Endocrinology. 1997;138:4427–4434. doi: 10.1210/endo.138.10.5462. [DOI] [PubMed] [Google Scholar]
- Sogn JH, Curry TE, Jr., Brannstrom M, Lemaire WJ, Koos RD, Papkoff H, Janson PO. Inhibition of follicle-stimulating hormone-induced ovulation by indomethacin in the perfused rat ovary. Biology of Reproduction. 1987;36:536–542. doi: 10.1095/biolreprod36.3.536. [DOI] [PubMed] [Google Scholar]
- Stocco C, Djiane J, Gibori G. Prostaglandin F2α (PGF2α) and prolactin signaling: PGF2α-mediated inhibition of prolactin receptor expression in the corpus luteum. Endocrinology. 2003;144:3301–3305. doi: 10.1210/en.2003-0420. [DOI] [PubMed] [Google Scholar]
- Sugimoto Y, Hasumoto K, Namba T, Irie A, Katsuyama M, Negishi M, Kakizuka A, Narumiya S, Ichikawa A. Cloning and expression of a cDNA for mouse prostaglandin F receptor. The Journal of Biological Chemistry. 1994;269:1356–1360. [PubMed] [Google Scholar]
- Sugimoto Y, Yamasaki A, Segi E, Tsuboi K, Aze Y, Nishimura T, Oida H, Yoshida N, Tanaka T, Katsuyama M, et al. Failure of parturition in mice lacking the prostaglandin F receptor. Science. 1997;277:681–683. doi: 10.1126/science.277.5326.681. [DOI] [PubMed] [Google Scholar]
- Suzuki-Yamamoto T, Nishizawa M, Fukui M, Okuda-Ashitaka E, Nakajima T, Ito S, Watanabe K. cDNA cloning, expression and characterization of human prostaglandin F synthase. FEBS Letters. 1999;462:335–340. doi: 10.1016/s0014-5793(99)01551-3. [DOI] [PubMed] [Google Scholar]
- Tai CJ, Kang SK, Choi KC, Tzeng CR, Leung PCK. Role of mitogen-activated protein kinase in prostaglandin F2α action in human granulosa-luteal cells. Journal of Clinical Endocrinology and Metabolism. 2001;86:375–380. doi: 10.1210/jcem.86.1.7159. [DOI] [PubMed] [Google Scholar]
- Vane JR, Bakhle YS, Botting RM. Cyclooxygenases 1 and 2. Annual Review of Pharmacology and Toxicology. 1998;38:97–120. doi: 10.1146/annurev.pharmtox.38.1.97. [DOI] [PubMed] [Google Scholar]
- Wallach EE, Bronson R, Hamada Y, Wright KH, Stevens VC. Effectiveness of prostaglandin F2α in restoration of hMG-hCG induced ovulation in indomethacin-treated rhesus monkeys. Prostaglandins. 1975;10:129–138. doi: 10.1016/0090-6980(75)90099-4. [DOI] [PubMed] [Google Scholar]
- Watanabe K. Prostaglandin F synthase. Prostaglandins and other Lipid Mediators. 2002;68-69:401–407. doi: 10.1016/s0090-6980(02)00044-8. [DOI] [PubMed] [Google Scholar]
- Wolf DP, Alexander M, Zelinski-Wooten MB, Stouffer RL. Maturity and fertility of rhesus monkey oocytes collected at different intervals after an ovulatory stimulus (human chorionic gonadotropin) in in vitro fertilization cycles. Molecular Reproduction and Development. 1996;43:76–81. doi: 10.1002/(SICI)1098-2795(199601)43:1<76::AID-MRD10>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Wong WYL, Richards JS. Evidence for two antigenically distinct molecular weight variants of prostaglandin H synthase in the rat ovary. Molecular Endocrinology. 1991;5:1269–1279. doi: 10.1210/mend-5-9-1269. [DOI] [PubMed] [Google Scholar]
- Zeleznik AJ, Benyo DF, Knobil E, Neill JD. The physiology of reproduction. Raven Press, Ltd.; New York: 1994. Control of follicular development, corpus luteum function, and the recognition of pregnancy in higher primates; pp. 751–782. [Google Scholar]
- Zelinski-Wooten MB, Hutchison JS, Trinchard-Lugan I, Hess DL, Wolf DP, Stouffer RL. Initiation of periovulatory events in gonadotrophin-stimulated macaques with varying doses of recombinant human chorionic gonadotrophin. Human Reproduction. 1997;12:1877–1885. doi: 10.1093/humrep/12.9.1877. [DOI] [PubMed] [Google Scholar]