Abstract
Angiogenesis, the recruitment of new blood vessels, is an essential component of tumor progression. Malignant brain tumors are highly vascularized and their growth is angiogenesis-dependent. As such, inhibition of the sprouting of new capillaries from pre-existing blood vessels is one of the most promising antiglioma therapeutic approaches. Numerous classes of molecules have been implicated in regulating angiogenesis and, thus, novel agents that target and counteract angiogenesis are now being developed. The therapeutic trials of a number of angiogenesis inhibitors as antiglioma drugs are currently under intense investigation. Preliminary studies of angiogenic blockade in glioblastoma have been promising and several clinical trials are now underway to develop optimum treatment strategies for antiangiogenic agents. This review will cover state-of-the-art antiangiogenic targets for brain tumor treatment and discuss future challenges. An increased understanding of the angiogenic process, the diversity of its inducers and mediators, appropriate drug schedules and the use of these agents with other modalities may lead to radically new treatment regimens to achieve maximal efficacy.
Keywords: angiogenesis, brain tumor, clinical trial, vascular endothelial factor, vascular niche
Angiogenesis, the formation of a vascular network, has been established as an important prerequisite for tumor formation in solid malignancies [1,2]. The tumor mass requires a blood vessel network to grow, and the angiogenic response, which provides oxygen and metabolites to the tumor, appears to parallel the growth of the tumor. The angiogenic process is far from simple as there are a multitude of players involved, and the final result is determined by the local equilibrium of pro- and antiangiogenic factors produced by the tumor cells, infiltrating leukocytes, the stroma and the endothelium itself. It is now widely accepted that the ‘angiogenic switch’ is ‘off’ when the effects of proangiogenic molecules are balanced by that of antiangiogenic molecules, and it is ‘on’ when the net balance is tipped in favor of angiogenesis [3,4]. Given the characteristic high degree of endothelial proliferation, vascular permeability and proangiogenic growth factors, the targeting of blood vessels in brain tumors is a particularly attractive strategy. Two main strategies have been proposed to target the tumor vasculature: antiangiogenic drugs aimed at preventing the process of angiogenesis, the neoformation of blood vessels necessary for the growth and progression of the tumor and metastasis; and antivascular therapy targeted to the already existing tumor vasculature, so-called vascular-targeting agents. In this review, we propose to use the term ‘antiangiogenic therapy’ as a generic term including both antiangiogenic and vascular-targeting therapy according to the definition of Los and Voest [5].
Proangiogenic mechanisms
Glioma angiogenesis is mediated by angiogenic factors that induce an abnormal, inefficient vascular network. Several proangiogenic molecules have been identified in brain tumors (TABLE 1). The overexpression of angiogenic factors is caused by both genetic alterations in tumor cells and induced by hypoxia commonly seen in malignant gliomas.
Table 1.
Angiogenic factors in brain tumors.
| Angiogenic factors | Function |
|---|---|
| Angiogenin | Induces vascularization in malignant tissues |
| FGF-acidic (FGF1) | Possess broad mitogenic and cell survival activities |
| FGF-basic (FGF2) | Mediates the formation of new blood vessels |
| FGF receptor-1 | Interacts with FGFs and acts on cascade of downstream signals |
| FGF receptor-1a and 1b(IIIc)/Fc chimera | Involved in normal development, wound healing and repair and angiogenesis |
| Placenta growth factor (PlGF)/PGF2 | Members of the VEGF subfamily |
| PD-ECGF | Expressed in cells of the subepithelial area with angiogenesis |
| PDGF | Growth of blood vessels from already existing blood vessel tissue |
| Sphingosine-1-phosphate | Responsible for chemotaxis and angiogenesis |
| VEGF-A, -B, -C and -D | Angiogenesis, migration of endothelial cells, mitosis of endothelial cells and creation of blood vessel lumen |
| VEGFR1, 2 and 3 | Stimulates cellular responses by binding to tyrosine kinase receptors on the cell surface |
| Angiopoietin-1 | Promotes angiogenesis, the formation of blood vessels |
| Del-1 | Promotes v3-dependent endothelial cell adhesion and migration |
| Follistatin | Regulates endothelial cell proliferation |
| G-CSF | Activates endothelial proliferation |
| HGF/SF | Induces in vitro expression of VEGF |
| IL-8 | Exerts potent angiogenic properties on endothelial cells through interaction with its cognate receptors CXC receptor 1 and 2 |
| Leptin | Activates leptin receptor (Ob-R) in endothelial cells |
| Midkine | Capable of exerting activities cell proliferation and angiogenesis |
| Pleiotrophin | Directly angiogenic |
| Progranulin | Secreted growth factor, as a strong interacting protein |
| Proliferin | Potent regulator of angiogenesis |
| TGF-α | More potent than EGF in promoting angiogenesis |
| TGF-β | Regulator of proliferation, migration, survival, differentiation and extracellular matrix synthesis in endothelial cells |
| TNF-α | Involved in systemic inflammation and is a member of a group of cytokines that all stimulate the acute-phase reaction |
| SDF-1 | Enhances VEGF expression and VEGF-induced proliferation in endothelial cells |
| BDNF | Survival factor for endothelial cells |
| Eotaxin (CCL11) | Contribute to angiogenesis |
| ENA-78 | ENA-78 production by endothelial cells may be important for the regulation of neutrophil activation in inflammatory reactions |
| FKN | Stimulates angiogenesis by activating the Raf-1/MEK/ERK- and PI3K/Akt/eNOS-dependent signal pathways |
| FLT3 | Member of the type III receptor tyrosine kinase family, which includes c-Kit, PDGFR and M-CSF receptors |
| IGF1 | Regulates angiogenesis |
| SCF | Modulates tumor growth and angiogenesis via the involvement of mast cells |
ENA-78: Epithelial neutrophil-activating peptide-78; FKN: Fractalkine; FLT3: FMS-related tyrosine kinase 3; G-CSF: Granulocyte colony-stimulating factor; PD-ECGF: Platelet-derived endothelial cell growth factor; SCF: Stem cell factor; SDF-1: Stromal cell-derived factor 1; SF: Scatter factor; VEGFR: VEGF receptor.
Role of hypoxia
Glioma cells surround existing brain vasculature to facilitate their own growth [6] and become hypoxic when they are distant (100–200 µm away) from brain vasculature [7]. Hypoxia, both physiologically and in tumors, can induce several DNA-binding complexes (hypoxia-inducible transcription factors [HIFs]) that regulate an extensive panel of genes [8], resulting in the overexpression of VEGF and other proangiogenic factors. The end result of overexpression of the proangiogenic factors results in the formation of new blood vessels that supply tumor tissues. In glioblastoma (GBM), HIF-1 is expressed in hypoxic pseudopallisading cells and in tumor cells at the invasive edge [9]. Hypoxia is the primary stimulus for VEGF and VEGF receptor (VEGFR) expression via the HIF-1 pathway. HIF-1α is the direct effector of hypoxia. Recently, it has been demonstrated that HIF-1α, partly through intratumoral increases in stromal cell-derived factor (SDF)-1α promotes tumor progression by recruiting vascular modulatory bone marrow-derived cells to stimulate angiogenesis [10]. Glioma SDF-1 expression increases with tumor grade and co-localizes with necrosis, angiogenesis and invasive glioma cells [11]. In subcutaneous tumors formed from murine glioma cells, SDF-1/CXCL12 secretion was sufficient to expand the fate of locally recruited bone marrow-derived progenitors that include both endothelial cells (ECs) and pericytes, whereas intracranial glioma SDF-1/CXCL12 secretion led to recruitment of marrow-derived endothelium but not pericytes. The presence of hypoxia in the glioma microenvironment could, however, stimulate the release of angiopoietins that interact with SDF-1/CXCL12 to expand the fate of marrow-derived progenitors to include pericytes. Angiopoietins, which are regulated by hypoxia and influence pericyte biology, appear to recruit angiopoietin 1 (Ang-1) receptor (Tie-2)-expressing marrow-derived proangiogenic monocytes [12].
Growth factors & their cognate receptors
A malignant tumor relies on a variety of factors that promote angiogenesis. These include an array of angiogenic growth factors, including VEGFs (VEGF-B, -C and -D), acidic and basic FGF (aFGF and bFGF), HGF/SF, TGF-α, TGF-β, TNF-α, IL-8 and angiogenin. Other factors involved in angiogenesis include the angiopoietins, which are ligands of the Tie receptor family [13]. Angiogenic factors induce expression and/or activation of proteolytic enzymes, which modify adhesion proteins of vascular ECs, resulting in leaky vessels and, in turn, allowing the ECs to migrate and proliferate [14]. Numerous growth factors, such as VEGF, Ang-1 and -2, and bFGF that stimulate endothelial proliferation, migration and assembly into vascular networks, have been observed in gliomas [15–17]. In addition, a distinct angiogenic switch with an increase of both VEGF and HGF/scatter factor (SF) in malignant gliomas and an independent statistical association of both factors with microvessel density have been reported [18,19]. During angiogenesis, vessels initially dilate and become leaky as an initial response to VEGF, which is secreted by cancer or stromal cells. VEGF-A can stimulate both physiological and pathological angiogenesis. VEGF-A is a ligand for the two receptor tyrosine kinases VEGFR-1 (Flt-1) and VEGFR-2 (KDR/Flk-1). Most biological functions of VEGF-A are mediated via VEGFR-2, whereas the role of VEGFR-1 is largely unknown. Activation of MAPK, stress-activated kinase, PKC and the Akt pathway are implicated in VEGF-A-dependent endothelial function, including cell survival, proliferation, generation of nitric oxide and the induction of angiogenesis. Induction of metalloproteinases and activation of focal adhesion kinase and PI3K are implicated in VEGF-A-induced EC migration. The upregulation of VEGF is frequently observed in grade IV malignant astrocytoma tumor (GBM) biopsies [20–22]. Ang-2 and metalloproteinases mediate the dissolution of the existing basement membrane and interstitial matrix by acting in concert with VEGF. Stem cell factor (SCF) is another protein thought to be involved in activating brain ECs and is usually overexpressed by neurons after brain injury. SCF is expressed in glioma cells and may have a role in tumor-induced angiogenesis in the brain [23]. Many of the multi-targeted tyrosine kinase inhibitors (TKIs) under development for brain tumors also inhibit SCF signaling through its receptor c-Kit, thereby suggesting that it could represent an effective target. PDGF isoforms (PDGFA, B, C and D) and their receptors (PDGFR-α and -β) [24] are not only expressed in perivascular cells but also in brain tumor ECs. The PDGFB isoform can upregulate VEGF [25] and exert autocrine effects on endothelial and perivascular cells [26,26]. However, therapies targeting PDGFR-β (e.g., imatinib mesylate) have not yet shown promising results as single agents in the treatment of GBM [27]. One described mechanism by which VEGF and bFGF promote angiogenesis is by stimulating the activity of integrins αvβ3 and αvβ5 in ECs [28]. Integrin activation and ligand binding results in the propagation of intracellular signals that maintain EC survival and enhance proliferation, motility and capillary sprouting [29]. As a consequence, targeted antagonism of αvβ3 and αvβ5 integrins inhibit brain tumorigenesis in vivo and may represent an important novel adjuvant therapeutic approach to brain tumors [28]. Angiogenin interacts with ECs to induce a wide range of cellular responses necessary for angiogenesis, including endothelial migration [30], proliferation [31] and tube formation [32]. A significant correlation to the malignancy within the gliomas with an increase of angiogenin concentration was reported, suggesting that angiogenin may contribute to the malignant transformation of gliomas [33].
Angiopoietin/Tie-2 signaling, required for normal vascular development by regulating vascular remodeling and maturation, has been shown to have a role on glioma angiogenesis. Increasing Tie-2 expression correlates with higher malignancy grades in glioma [34]. Genetically disrupting Tie-2 inhibits glioma angiogenesis with a decrease in microvascular density, an increase of abnormally dilated vessels and loss of interaction between ECs and perivascular cells [34]. In addition, Tie-2 is also expressed in glial tumor cells [35] and bone marrow-derived monocytes [12]. Ang-1/Tie-2 signaling in tumor cells is associated with activation of integrin-mediated cell adhesion to the extracellular matrix (ECM). Tie-2-expressing monocytes (TEMs) have been implicated in glioma malignancies as they are recruited from bone marrow to orthotopic xenografts [12]. Targeted deletion of TEMs prevented glioma neovascularization and induced significant tumor regression [12]. Targeted therapy against the Ang-1/Tie-2 system is in preclinical development.
PDGF pathways
PDGF is a disulfide-linked dimer of two related polypeptide chains and exerts its biological activity by binding to structurally similar PDGF receptors (PDGFR-α and -β). PDGF has been shown to be essential for the stability of normal blood vessel formation by recruiting pericytes and smooth muscle cells [36]. PDGFs and their cognate receptors, PDGFR-α and -β, were shown to play a role in glioma growth and angiogenesis [37]. Targeted PDGF ligand overexpression in newborn mice can lead to glioma formation, and PDGF expression correlates with malignancy grade of gliomas [37]. PDGFR-α is expressed in adult subventricular zone neural stem cells (NSCs), which can form glioma-like growths in response to PDGF stimulation [38]. PDGFR-β is expressed on glioma-associated ECs [39,40] and on perivascular smooth muscle cells and pericytes, which have important roles in tumor angiogenesis [41].
HGF/SF
HGF/SF and its cognate receptor tyrosine kinase c-met are expressed in glioma cells, and their expression increases with malignant progression and vascularity [42]. Autocrine/paracrine signaling of this pathway in gliomas is associated with tumor proliferation, migration, invasion and angiogenesis [42]. Targeted inhibition of glioma cells by chimeric transgene transfection reduced tumor growth and angiogenesis [43] and showed synergistic anti-tumor effects with γ-radiation [44]. In addition, neutralizing monoclonal antibodies to HGF/SF have demonstrated antitumor activity in both subcutaneous and orthotopic malignant glioma xenograft models [45–47].
Lytic enzymes
Invasion of ECs is an essential event during angiogenesis, as this process involves degradation of the basement membrane and underlying interstitium. ECs must degrade the ECM barriers to permit the cell movement required for new blood vessel formation. To accomplish this, the angiogenic stimulus induces the production of lytic enzymes capable of digesting specific matrix components and favoring cell invasion.
The matrix metalloproteinase (MMP) family of enzymes is considered primarily responsible for ECM degradation. These enzymes are secreted in inactive proenzymatic forms, are zinc-dependent and can be subdivided on the basis of preferential ECM substrate [48]. MMPs are involved in many physiological processes involving matrix remodeling and appear to be essential for angiogenesis, tumor cell invasion and metastasis. In addition to removing physical barriers to migration through degradation of ECM macromolecules [49], MMPs can modulate cell adhesion [50] and generate ECM degradation products that are chemotactic for ECs [51]. MMP-2 and -9 have been shown to play an important role in neoangiogenesis and tumor vascularization in gliomas [52,53]. Therefore, the inactivation of MMPs could be an antiangiogenic treatment option for malignant glioma. Intraperitoneal administration of an anti-MMP agent, SI-27, limited tumor angiogenesis to a level similar to that found in the normal contralateral hemisphere and successfully prolonged survival in a clinically relevant glioma model [54]. We have demonstrated that siRNA-mediated targeting of MMP-9 inhibits glioma angiogenesis in in vitro and in vivo models [55].
Serine proteinases, including urokinase plasminogen activator (uPA), comprise a second family of proteases that promote tumor development. Urokinase converts the zymogen plasminogen to the active enzyme plasmin. uPA is synthesized and secreted by normal cells as well as tumor cells and interacts with a specific cell surface receptor (uPAR), which localizes the enzymatic activity to the cell surface. Expression of antisense uPAR and antisense uPA from a bicistronic adenoviral construct inhibited glioma-induced angiogenesis [56].
Endogenous inhibitors of angiogenesis
All the proangiogenic pathways described above often have natural counterparts. Over the last few years, several novel endogenous inhibitors of angiogenesis have been discovered. Some of these are synthesized by specific cells in different organs and others are created by extracellular proteolytic cleavage of plasma-derived or ECM-localized proteins. There are several studies testing the potential therapeutic efficacy of endogenous inhibitors, such as angiostatin, endostatin, PEX, pigment epithelial-derived factor (PEDF) and thrombospondin (TSP)-1 and -2, in animal models of malignant glioma. Endogenous inhibitors of angiogenesis exert their effects through multiple mechanisms that include the induction of microvascular endothelial cell (MvEC) apoptosis, the inhibition of MvEC proliferation, the inhibition of the function of proangiogenic molecules, and/or the altered regulation of proangiogenic and antiangiogenic molecules [57].
Angiostatin is a major endogenous angiogenesis inhibitor that has the ability to induce tumor regression in various animal models. In vitro studies have suggested that this protein is generated from plasminogen by multiple enzymatic actions [58]. C6 glioma cells were propagated intracerebrally and injected with an adeno-associated virus angiostatin vector; the vector caused markedly smaller tumors with reduced neovascularization and higher apoptotic indices [59].
Angiomotin (Amot), an 80-kDa protein characterized by conserved coiled–coil domains and C-terminal PDZ binding motifs, is expressed in ECs and promotes cell migration and invasion, and stabilizes tube formation in vitro. Amot is a receptor for the angiogenesis inhibitor angiostatin [60]. Amot has been co-localized with focal adhesion kinase (a promigratory, nonreceptor cytoplasmic tyrosine kinase) to lamellipodia at the leading edge of migrating NIH3T3 cells as well as to circular ruffles in spreading human umbilical vein endothelial cells (HUVECs), thereby suggesting a role for Amot in promoting cell motility [60]. Furthermore, DNA vaccination targeting Amot inhibits angiogenesis and tumor growth.
Endostatin, a potent inhibitor of angiogenesis and tumor growth, corresponds to the 184-amino acid C-terminal globular domain of collagen XVIII [61]. Endostatin, as with angiostatin, is believed to be generated following the proteolytic clipping of its precursor and has been detected in the plasma and sera of healthy human donors, the serum and urine of mice and in the lysates of several murine tissues [62]. Two studies reported that endostatin encapsulated within alginate beads was efficacious in treating U87 MG and BT4C glioma tumors propagated in rodents [63,64]. Rat C6 glioma cells stably expressing murine endostatin and implanted subcutaneously into nude mice resulted in a decrease in the tumor growth rate but not a complete inhibition of tumor growth [65]. In BT4C gliosarcoma cells propagated both subcutaneously and intracranially in rats, treatment with recombinant endostatin resulted in decreased tumor size and increased survival [66]. Furthermore, in a xenograft model in which U87 MG human GBM cells were propagated in the nude mouse brain, direct intracerebral microinfusion of endostatin was more effective in decreasing tumor volume and increasing tumor cell apoptosis compared with systemic administration of endostatin [67]. No toxicity was detected with endostatin therapy in these animal models, which suggests recombinant endostatin could be a highly useful new therapeutic tool for patients with malignant gliomas.
PEX is a 210-amino acid fragment of MMP-2 and it corresponds to the hemopexin domain of MMP-2 [68]. PEX binds to integrin αvβ3 and is thought to competitively inhibit the binding of MMP-2 to integrin αvβ3 [68]. PEX binding to integrin αvβ3 inhibits the binding of MMP-2 and inhibits MMP-2 collagenolytic activity in the chick chorioallantoic membrane model of angiogenesis [68]. Purified human PEX fragment inhibits tube formation of ECs isolated from multiple sites and plated on matrigel, and it decreases EC proliferation, as well as the proliferation of several malignant glioma cells (U87 MG, U373 and 118) [69]. PEX has been shown to inhibit tumor growth and angiogenesis in both subcutaneous and intracerebral xenograft models of malignant glioma (U87 MG and U373 cell lines) [69,70]. PEX in combination with carboplatin and etoposide have also been shown to prolong animal survival in an intracranial xenograft model of glioma [70].
TSP-1 and -2 are highly related and belong to the larger family of TSP proteins. Both TSP-1 and -2 contain three type 1 repeat domains, referred to as TSR domains [71]. One mechanism by which TSP-1 and -2 promote their antiangiogenic effect is through CD36 receptor signaling that results in apoptosis of dermal MvEC [72]. In the nude mouse injected subcutaneously with the LN-229 GBM cell line, stable expression of intact TSP-1 resulted in a reduction of mean tumor size and a decrease in mean tumor microvessel number [73]. Kragh et al. also overexpressed intact TSP-1 in the LN-229 human GBM cell line and, when propagating it in a xenograft model, found decreased tumor growth and decreased vascular density but no effect on perfusion [74]. These data suggest that TSP-1 promotes a growth-limiting phenotype via an inhibition of angiogenesis in GBM [74]. A recent study suggests that TSP-1 can also induce apoptosis of primary human brain MvEC [75]. Consistent with the above findings, a report by another group described nude mice that had been injected subcutaneously or intracerebrally with rat C6 glioma cells expressing a fragment of TSP-1 that contained the pro-collagen domain and the three type 1 repeats formed tumors with decreased vascularity, suggesting the possibility that fragments of TSP-1 may promote an antiangiogenic effect [76]. ABT-510, a modified type 1 repeat peptide of TSP, significantly inhibited the growth of intracerebral malignant glioma propagated in a syngeneic mouse model with significantly lower microvessel density and increased MvEC apoptosis [77].
Molecules of the inflammatory cascade
Molecules of the inflammatory cascade act indirectly on angiogenesis via modulation of the expression of direct angiogenic factors.
The COX isoforms (COX-1 and -2) catalyze the synthesis of prostaglandins from arachidonic acid. While COX-1 is ubiquitously expressed in a wide range of tissues, COX-2 is cytokine inducible. COX-2 is expressed in human glioma cells where its expression correlates with malignancy and, as such, is highest in GBM [78]. High COX-2 expression is associated with increasing histological grade and poor survival outcome in patients with gliomas [79].
Cytokines are key immunoregulators, and a wide range of cytokines have been successfully used in experimental brain tumor models. IL-8 is a potent chemoattractant, and recent data suggest that it has a critical role in glial tumor angiogenesis and progression. Levels of IL-8 correlate with histological grade with the highest expression observed in GBM [80]. IL-8 is highly expressed in pseudopallisades around necrotic areas in GBM, reflecting its regulation by hypoxia. The proangiogenic activity of IL-8 is mediated by the CXCR1 and CXCR2 receptors, but further studies are required to define underlying mechanisms [81]. Other relevant chemokines and their receptors include IL-12, CXCR4 and CXCL12. CXCL12 (SDF-1/CXCL12) and its receptor CXCR4, which regulate leukocyte, endothelial and hematopoietic precursor migration, bone-marrow myelopoiesis and angiogenesis, are overexpressed in malignant gliomas [82]. Inhibitors of these chemokines/receptors are under development [83]. IL-12 also possesses antiangiogenic effects, which result from the induction of IFN-γ from helper T lymphocytes and the subsequent stimulation of secondary mediators, including monokine induced by IFN-γ (MIG) and IFN-inducible protein 10 [84]. Intratumoral therapy with IL-12-secreting NSCs prolonged survival compared with treatment with nonsecretory NSCs or saline in intracranial glioma-bearing mice [85].
Cancer stem cell vascular niche
Recently, new concepts have been proposed following the demonstration of highly tumorigenic cancer progenitor cells, also designated as cancer stem cells (CSCs) or cancer-initiating cells, in cancer initiation and progression to metastatic disease states and resistance to conventional therapies. These cancer progenitor cell-based concepts may partially explain the recurrence of the most aggressive cancers after various clinical treatments. Previous studies have indicated that angiogenesis may also be promoted by stem cells that are recruited to a tumor bed and differentiate into ECs or supportive cells [86–88]. An emerging hypothesis states that CSCs drive tumorigenesis by directly inducing an inflammatory phenotype within the CSC-vascular niche complex. This occurs by fostering the infiltration of granulocyte/macrophage progenitors and granulocytes in bone marrow, and promotion of stromal remodeling as seen in aberrant stem cell-vascular niches that contribute to myeloproliferative diseases [89]. CSCs, with the support of the vascular niche, form an intricate microenvironment that empowers CSCs to express their full malignant phenotype and influence surrounding nonmalignant cells. The tumor stroma and supporting cells have been shown to be immunosuppressive and play an important role in inhibiting local responses that regulate tumor rejection [90].
Evidence of the existence of stem cell-vascular niche complexes composed of NSCs, endothelial and mural progenitor cells have been recently elucidated for malignant gliomas [91]. Nestin+/CD133+ CSCs, termed ‘vessel-associated CSCs’ because they are exclusively located in areas of increased microvessel density, were found to directly contact capillary networks, whereas CD133− cancer cells were diffusely distributed with no apparent vascular localization. Li et al. demonstrated that NSCs stimulate neighboring ECs to express increased levels of VEGF-A and BDNF, which induce a robust angiogenic response for stem cell support [92]. It is well established that the glioma angioarchitecture is grossly abnormal as these vessels are of varying caliber and arborization [93]. The glioma vasculature is a result of both angiogenesis and vasculogenesis, with significant contribution from endothelial, progenitor, hematopoietic and possibly mesenchymal cells derived from the bone marrow [91,94]. Given these observations from many groups studying cancers in different tissues, it is conceivable that altered expression of angiogenic factors may translate to an abnormal vascular niche, unregulated stem cell signals and, thus, aberrant stem cells.
The depletion of brain tumor blood vessels was found to eradicate self-renewing CSCs, rendering tumorigenesis unfeasible [91]. DaoyERBB2 medulloblastoma tumor cells engineered to express high levels of VEGF signaling were shown to be susceptible to the antiangiogenic impact of erlotinib (anti-EGFR/ERBB2) and bevacizumab (anti-VEGF) treatment. While the authors note that parenchymal tumor tissue did not show an increase in apoptosis or necrosis, both treatments resulted in a completed loss of self-renewing GFP+/Nestin+/CD133+ CSCs. Single treatment of bevacizumab in a U87 LUC xenograft murine model demonstrated a decrease in the number of vessels associated with Nestin+ and GFP+/Nestin+/CD133+ CSCs, suggesting a role for VEGF signaling in maintaining a vascular microenvironment supportive of CSCs. Similar findings were reported by Folkins et al., who showed that athymic nude mice bearing rat C6 gliomas experienced a significant reduction in tumor sphere-forming units and CSCs when treated with a chemosensitization regimen of antiangiogenic and cytotoxic compounds [95]. Future studies that employ antiangiogenic therapeutics will likely focus on the locus of action as this may be the only path to understanding the functional efficacy of this type of treatment.
Antiangiogenic therapy
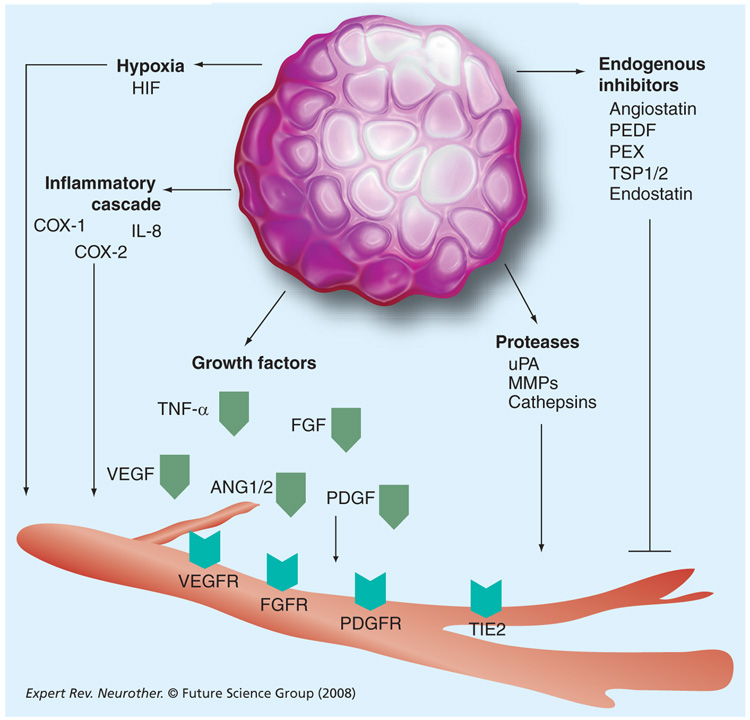
Antiangiogenic therapy has been considered an alternative or complementary paradigm to conventional cancer treatments. FIGURE 1 illustrates the potential targets of antiangiogenic therapy. Targeting blood vessels in brain tumors has been a particularly attractive strategy, given the characteristic high degree of endothelial proliferation, vascular permeability and proangiogenic growth factor expression [96]. The VEGF family and its receptors seem to be the central signaling pathway of glioma angiogenesis. Malignant gliomas with high degrees of VEGF expression and vessel areas are good candidates for antiangiogenic therapy. Inhibition of VEGF expression by a neutralizing anti-VEGF antibody [97], antisense VEGF constructs [98], expression of a dominant-negative mutant form of VEGFR-2 [98,99], specific small molecule inhibitors of the VEGFR-2 tyrosine kinase [100] or neutralizing anti-VEGFR-2 antibody [101] has resulted in a marked suppression of experimental malignant glioma growth. Intraperitoneal injection of IFN-β intramuscular injection and transfection of the endogenous nonspecific angiogenesis inhibitor TSP-1 into glioma cells caused inhibition of VEGF secretion and/or mRNA expression, and resulted in glioma growth inhibition of 70, 84 and 50%, respectively, compared with controls [102].
Figure 1. Potential targets of antiangiogenic therapy.
HIF: Hypoxia-inducible transcription factor; MMP: Matrix metalloproteinase; PDGFR: PDGF receptor; PEDF: Pigment epithelial-derived factor; PEX: Potassium ethyl xanthate; TSP: Throbospondin; uPA: Urokinase plasminogen activator; VEGFR: VEGF receptor.
Most of the antiangiogenic agents currently in Phase I/II trials for brain tumors are blockers of the VEGF pathway (TABLE 2). Using serial, noninvasive MRI techniques, Batchelor and colleagues have shown that cediranib (AZD2171, an oral, pan-VEGF receptor TKI with activity against PDGFR-α, PDGFR-β and c-Kit) temporarily induced a ‘normalization time window’ in tumor vessels in patients with recurrent GBM [103]. This normalization had a rapid onset, was prolonged but reversible and had significant clinical and functional consequences. Cediranib is being studied in a Phase I trial by the Pediatric Brain Tumor Consortium. Other agents (e.g., bevacizumab, sunitinib and sorafenib) are in early-phase clinical trials for children with cancer [104]. Cediranib monotherapy led to an objective radiographic response (i.e., reduction in contrast-enhanced tumor volume on MRI by at least 50%) in more than half of patients and reduced tumor-associated vasogenic edema in most patients. In addition, this study was the first to identify the onset and duration of a vascular normalization time window induced by an antiangiogenic agent. Other studies that support a beneficial effect of anti-VEGF therapy in malignant glioma involved bevacizumab in combination with chemotherapy [105]. Recent clinical trials targeting VEGF signaling with bevacizumab plus irinotecan have shown promising radiographic and clinical responses, while also confirming adequate safety in recurrent GBM patients [106]. Collectively, these results renew the hope that, similar to various extracranial tumors, simultaneous targeting of blood vessels and cancer cells can improve outcomes for this patient population [107]. A Phase I study of escalating doses of atrasentan, an oral selective endothelin-A receptor antagonist that may inhibit cell proliferation and interfere with angiogenesis during glioma growth, was recently completed. Further study of atrasentan with radiation therapy and temozolomide in newly diagnosed GBM is warranted to evaluate the efficacy of this novel agent. A Phase II trial with 60 patients with recurrent malignant astrocytomas indicated that tumor angiogenic profile as determined by VEGF expression and hypoxic profiles predict radiographic response and survival, respectively, in malignant astrocytoma patients treated with bevacizumab and irinotecan. The results need to be validated in a larger clinical trial [108]. A total of 55 patients with recurrent malignant gliomas who received bevacizumab and chemotherapy were reviewed for efficacy, toxicity and patterns of recurrence. The study indicated combination therapy with bevacizumab and chemotherapy is well tolerated and active against recurrent malignant gliomas. At recurrence, continuing bevacizumab and changing the chemotherapy agent provided long-term disease control in only a small subset of patients. It was concluded that bevacizumab may alter the recurrence pattern of malignant gliomas by suppressing enhancing tumor recurrence more effectively than it suppresses nonenhancing, infiltrative tumor growth [109].
Table 2.
Trials of agents targeting the VEGF signaling pathway in brain tumors.
| Drug | Target | Phase | Comments |
|---|---|---|---|
| Bevacizumab | VEGF | Phase I/II/III | Decreases edema in some patients May alter the recurrence pattern of malignant gliomas by suppressing enhancing tumor recurrence more effectively than it suppresses nonenhancing, infiltrative tumor growth |
| Vatalanib | VEGFR1–3, PDGFR-β, c-Kit | Phase I/II | Has a relatively short half-life in circulation (4–6 h) Can potentially improve the effectiveness of additional radiation and chemotherapy |
| Cediranib | VEGFR1–3, PDGFR-β, c-Kit | Phase I/II/III | Induces sustained decreases in edema and has steroid-sparing effects; viable CECs, bFGF and SDF-1 correlate with tumor progression; the targets of cediranib are present on tumor endothelium |
| Sorafenib | VEGFR2,3, BRaf, Raf1, PDGFR-β, c-Kit, Ras, p38 | Phase I/II | May target both malignant and tumor stromal cells Has an ability to slow the growth of the tumor |
| Phase II | In combination with erlotinib | ||
| Phase I/II | In combination with erlotinib, tipifarnib or temsirolimus | ||
| Phase I/II | In combination with temsirolimus | ||
| VEGF Trap | VEGFA, B, PlGF | Phase II | May stop the growth of malignant or anaplastic gliomas by blocking blood flow to the tumor |
| Sunitinib | VEGFR2, PDGFR-β, FLT3, c-Kit | Phase II | May target both malignant and tumor stromal cells Continuous daily basis of the drug reduces the CBFr in a majority of patients with recurrent high-grade gliomas |
| Phase II | Melanoma and renal cell cancer | ||
| Phase II | Non-small-cell lung cancer | ||
| Vandetanib | VEGFR2, EGFR, Ret | Phase I | Combination with radiation therapy Multi-targeted kinase inhibitor exhibiting potent activity against angiogenesis |
| Phase I/II | Combination with radiation therapy and temozolomide | ||
| Phase II | |||
| Pazopanib | VEGFRs, PDGFR-β, c-Kit | Phase II | May stop the growth of tumor cells by blocking some of the enzymes needed for cell growth and by blocking blood flow to the tumor |
| Phase II | With lapanitib | ||
| Phase I | With lapanitib | ||
| Avastin | VEGF, MGMT | Phase II | Combination with radiation and temozolomide and irinotecan Shrinks cancerous tumors by cutting off their blood supply Can slow the growth of the most common and deadly form of brain cancer |
| Aflibercept (VEGF rap) | VEGFA, VEGFB, PlGF | Phase I | Giving aflibercept together with radiation therapy and temozolomide may kill more tumor cells Ongoing trial |
| Thalidomide | bFGF, VEGF | Phase I/II/III | Prevents formation of new blood vessels in tumors No survival benefit for patients with multiple, large or midbrain metastases in combination with radiation |
| AEE788 | ErbB and VEGF receptor family, EGFR | Phase I /II | Maximum-tolerated dose and dose-limiting toxicity of AEE788 |
This information is taken from US NIH clinical trials website.
bFGF: Basic fibroblast growth factor; CBFr: Coronary blood flow reserve; CEC: Circulating endothelial cells; FLT3: FMS-related tyrosine kinase 3; PDGFR: PDGF receptor; PlGF: placental growth factor; VEGFR: VEGF receptor.
Malignant glioma growth also responded to the inhibition of signaling pathways other than VEGF/VEGFR-2. Disruption of FGFR activity or binding to FGFs by a tetracycline-regulated suppression system [110] or suramin [111], respectively, has demonstrated growth delay in orthotopic glioma models. Multi-targeted kinase inhibitors with activity against FGFR are in preclinical development. Although suramin is safe and well tolerated in malignant glioma patients, it failed to demonstrate survival benefit in Phase II clinical trials [112,113]. Imatinib mesylate (Gleevec®, STI571, Novartis Pharmaceuticals, NJ, USA), an inhibitor of PDGFR, c-KIT and Bcr-Ab1 kinases, exhibited antiglioma activity as monotherapy and in combination with radiation in preclinical studies [114,115]. However, imatinib monotherapy has failed to show significant clinical benefits in several Phase I/II trials [27]. Nonetheless, imatinib mesylate in combination with hydroxyurea has demonstrated encouraging anti-tumor efficacy in a patient series [116], which was subsequently confirmed by a Phase II study [117]. In this trial of 33 patients with recurrent GBMs, the radiographic response rate was 9% with a 6-month progression-free survival (PFS-6) of 27% [117]. An additional study confirmed the anti-tumor activity of this regimen in some recurrent grade III malignant glioma patients [118]. The mechanism of combinatorial effects of imatinib and hydroxyurea is unclear. Tandutinib (MLN518, CT53518, Millennium Pharmaceuticals, MA, USA) is a multi-targeted inhibitor with activity against PDGFR, c-Kit and FLT-3 that has demonstrated efficacy in hematologic malignancies [119]. A Phase I/II trial of tandutinib in recurrent GBMs is ongoing. A Phase II trial of AMG-102 (Amgen Inc., CA, USA), a HGF/SF monoclonal antibody, in advanced malignant glioma is ongoing.
Natural angiogenesis inhibitors have also been studied for their ability to inhibit glioma angiogenesis and growth. The efficacy of angiostatin as a therapeutic agent may be increased if administered in conjunction with other gene therapies. For example, in C6 glioma tumors propagated in rats, intratumoral delivery of adeno-associated virus angiostatin vector in combination with an adenovirally expressed suicidal thymidine kinase gene resulted in decreased tumor volume and prolonged animal survival [59]. Furthermore, fragments of angiostatin-containing K1-3 may also be clinically useful, because when such a fragment was expressed in the SHG44 human glioma cells followed by propagation of these cells subcutaneously in the flank of nude mice, significantly reduced tumor growth and angiogenesis were found [120]. In the above studies, angiostatin therapy did not result in observed toxicity, and this suggests that it could be a highly efficacious new therapy for patients with malignant gliomas.
COX-2 has been implicated in angiogenesis by upregulating VEGF expression in several cancers [121]. Celecoxib (Celebrex®, Pfizer, NY, USA) a selective COX-2 inhibitor, has anti-tumor activity as monotherapy and in combination with radiation in murine models of intracranial GBM [122,123]. A recent Phase II study of irinotecan plus celecoxib (400 mg twice daily) in patients with recurrent malignant gliomas demonstrated a 16% radiographic response rate. The median PFS was 11 weeks and the PFS-6 was 25.1% [124]. Another Phase II study employed celecoxib in combination with 13-cis-retinoic acid (Accutane, Roche, NJ, USA) revealed no radiographic response with a PFS-6 rate of 19% [125]. The enthusiasm for the use of celecoxib as a therapeutic agent in malignant glioma may be decreased owing to modest efficacy and cardiovascular risk demonstrated in a colorectal cancer prevention trial [126].
A recent preclinical study revealed anti-tumor benefit of HIF-1α-targeted deletion with temozolomide in a murine subcutaneous GBM xenograft model [127]. A preclinical study of 2-methoxyestradiol (2ME2, Panzem®; EntreMed, Rockville, MD, USA), a microtubule inhibitor with secondary effects on HIF-1, demonstrated dose-dependent inhibition of tumor growth in a rat orthotopic glioma model [128]. Decreased HIF-1 protein level, tissue hypoxia and microtubule destabilization were associated with anti-tumor efficacy [128]. A Phase II trial of 2ME2-nanocrystal colloidal dispersion in patients with recurrent GBM is ongoing. In addition, there is emerging evidence that mTOR inhibitors may exert antiangiogenic property through suppression of HIF-1α expression and transcriptional activation of VEGF [129]. mTOR is a highly conserved serine/threonine kinase that is downstream from AKT, regulating translation and cellular survival. Rapamycin, sirolimus (Rapamune®; Wyeth, PA, USA) and its synthesized analogs, temsirolimus (CCI-779, Wyeth), everolimus (RAD001, Novartis) and AP23573 (Ariad Pharmaceuticals, MA, USA) have been evaluated in clinical trials of malignant gliomas. Two recent Phase II studies of temsirolimus monotherapy in recurrent GBM have demonstrated modest efficacy [130,131]. Combinations of mTOR inhibitors with other targeted agents are ongoing.
Interferon
Interferons were discovered as mediators of antiviral responses and were later found to function in a wider role in inflammation, proliferation and differentiation. Interferons bind to transmembrane glycoprotein receptors, resulting in their oligomerization and subsequent tyrosine phosphorylation. IFN-α and -β have been shown to have antiangiogenic properties both in vitro and in vivo, which are mediated by their inhibitory effects on angiogenic factors [132]. Interferons have demonstrated efficacy in the treatment of pediatric hemangiomas [133]. Based on these clinical and preclinical data, interferons have entered clinical trials against malignancies, including gliomas. A Phase I clinical trial of IFN-β gene therapy for high-grade glioma in five patients was performed. Two patients demonstrated more than 50% reduction while others had stable disease 10 weeks after treatment initiation, and CD34−immunoreactive vessels were notably decreased [134]. A Phase II study with recombinant human IFN-β admimistered after conventional radiation therapy was well tolerated, with a trend toward survival benefit in patients who remained stable after radiation therapy [135]. A Phase II study of a combination of nimustine (ACNU), carboplatin, vincristine and IFN-β with radiotherapy for GBM indicated that this combination is safe and well tolerated, and may prolong survival in patients with GBM [136].
Endothelial cell inhibitors
Thalidomide (THD), which has been in disrepute because of its potent teratogenic effects in humans, was found to inhibit angiogenesis in animal models, providing an insight into the mechanism of the teratogenicity of this agent [137]. The drug was found to be safe in nonpregnant adults, had excellent bioavailability following oral administration and had antiangiogenic effects in animal studies. It is thought to have a therapeutic effect by inhibiting VEGF and bFGF [137,138]. THD monotherapy in patients with recurrent high-grade glioma was associated with a 6% partial response rate and 1-year survival rate of 22% [139]. There are several clinical reports suggesting the efficacy of THD for treating recurrent malignant glioma. A preclinical study demonstrated combinatorial benefit of THD and temozolomide in a rodent model of orthotopic glioma xenografts [140]. However, clinical trials of THD in combination with BCNU, temozolomide or radiation therapy have demonstrated generally limited efficacy [141–143]. THD increases the anti-tumor effect of single high-dose radiation (γ-knife radio surgery) in the rat orthotopic glioma model [144]. THD increased the efficacy of cisplatin by altering the tumor vasculature in a 9L rat gliosarcoma model [145]. The combination of temozolomide, THD and tamoxifen administered as outpatient oral therapy resulted in significantly improved quality of life in patients with malignant astrocytoma without significant toxicity [146]. A Phase II trial of irinotecan and THD in adults with recurrent glioma not taking enzyme-inducing anticonvulsants showed promising activity [147]. A Phase I trial of lenalidomide, a potent analogue of the antiangiogenic agent THD, is well tolerated in patients with recurrent glioma. Among the 36 patients, only 12.5% of patients were progression free at 6 months [148].
Vaccines targeting tumor angiogenesis
Angiogenesis-associated antigens overexpressed in tumor endothelium are specific molecular addresses targeted by antiangiogenic therapy [149,150]. Therapeutic damage of tumor endothelium activates the coagulation cascade and, consequently, results in the obstruction of tumor vasculature, with hypoxia and shrinkage of tumors owing to necrosis, whereas it does not affect blood supply in normal adult tissues [151]. Recently, different vaccine strategies have been reported to inhibit tumor growth and metastasis by induction of specific cellular and/or humoral immunity against angiogenesis-associated antigens in preclinical models, suggesting effective combination of antiangiogenesis and cancer immunotherapy [152]. A pilot study demonstrated partial-to-complete tumor responses in three out of six malignant brain tumor patients, indicating the clinical utility of the antiangiogenic vaccine using fixed whole endothelium. To obtain further insight into the possibilities and limitations of this novel approach, another study employing different dose levels, adjuvants and combination with conventional therapy modalities against various tumor types is now ongoing [153].
Targeting the stem cell vascular niche
Stem cells, whether normal or cancerous, employ similar transduction pathways to recruit ECs and ancillary progenitor cells into their microenvironment. Given the important role of the vascular niche and its components to tumor biology, it is clear why this niche and its associated elements are potential therapeutic targets for brain tumors. Targeting specific cellular components of vessel structure has also proven effective in preclinical studies. The use of SU6668, a kinase inhibitor specific for PDGFRs, induces the detachment of vessel-stabilizing pericytes and disrupts tumor vascularity, revealing PDGFR+ pericytes as complimentary targets to ECs for efficacious antiangiogenic therapy [154]. These studies suggest that antiangiogenic therapeutics may halt tumor growth by targeting the stem cell vascular niche responsible for CSC survival. Multiple studies have explored the value of targeting the CSC vascular niche complex using celldelivered therapeutics. The innate ability of mesenchymal stem cells (MSCs) to engraft into tumor microenvironments forms the basis for their use as cellular vehicles of targeted anticancer agents. Nakamizo and colleagues employed human MSCs as vehicles for the delivery of INF-β to intracranial U87 tumors developed in a murine xenograft model. Their results showed intravascular injection of these therapeutic cells led to the incorporation of MSCs into tumor architecture, prolonged animal survival and suppressed tumor growth [155]. Similarly, GBM-derived MSCs have been used to deliver NK4, an antagonist to HGF, to abolish the effect of HGF on the C-Met signaling pathway, resulting in the inhibition of angiogenesis [156]. Collectively, these studies suggest that, by harnessing the targeting potential of GBM-derived perivascular progenitor cells, one can perturb the stability of the vascular niche and, in turn, target the CSCs that it supports and protects.
However, despite all the promises of antiangiogenic therapy, it is unlikely that it will be able to fully eradicate malignant glioma in the future. While potential benefits are profound, limitations of antiangiogenic therapy have also been identified, suggesting that there is also a need for caution in applying these compounds to the clinical setting. Some studies demonstrate that antiangiogenic therapy can cause excessive vascular regression that can compromise the delivery of drugs and oxygen to tumors [157,158]. Preclinical data have confirmed that tumor hypoxia (known to confer radioresistance) is improved with the delivery of anti-VEGF therapies, and that tumor overexpression of VEGF increases vascular permeability, resulting in increased interstitial fluid pressure and potentially impeding the delivery of cytotoxic therapies [159]. However, careful application of antiangiogenic agents was shown to ‘normalize’ the abnormal tumor vasculature, resulting in more efficient delivery of drugs and oxygen to the targeted cancer cells [160]. Therefore, there is a need for a delicate balance between normalization and excessive vascular regression, emphasizing the requirement for careful selection of the dose and administration schedule for antiangiogenic agents. As experience with antiangiogenic agents accumulates, it is clear that the benefit is only transient, and most tumors eventually progress after a number of months. In a subset of patients receiving antiangiogenic therapy, these tumors recur not as enhancing masses, but with a more infiltrative phenotype resembling gliomatosis. This raises the possibility that, by inhibiting angiogenesis, anti-VEGF and anti-VEGFR agents force tumors cells to co-opt and grow along existing blood vessels, changing their natural history [109]. The blood–brain barrier (BBB) poses a significant problem for drug delivery to brain tumors, especially when antiangiogenic therapy carries a risk of closure of the BBB. Targeting the existent tumor-associated vasculature, instead of preventing the formation of new vasculature, is now considered a potentially powerful approach for tumor therapy. This approach makes use of vascular targeting agents, antibodies or other compounds that recognize tumor vessel-specific proteins, to induce damage to the tumor vasculature, resulting in tumor-specific thrombosis, occlusion of blood vessels and subsequent tumor necrosis. Because the tumor endothelium is targeted, this approach does not suffer from the limitations of tumor cell targeting, such as restricted penetration into tumor tissue of intravenously delivered antibodies. Thus, understanding the characteristics of glioma-associated ECs and the interactions between normal ECs and the tumor microenvironment is essential for the development of more selective antiangiogenic therapies.
Limiting the proliferation of tumor vessels reduces tumor growth, although many tumor cells may remain vital but in a dormant condition. To achieve a maximal growth-reducing effect, several studies have now focused on the combination of antiangiogenesis with chemotherapy or radiotherapy. Malignant glioma cells are inherently chemo- [161] and radioresistant [162]. Also, as radiation of glioma cells may potentiate the expression of proangiogenic molecules [163], combining radiotherapy with antiangiogenic therapy may also prove to be clinically useful and effective. By way of the CSC vascular niche hypothesis, the future of therapeutic strategy will continue to require a multidimensional approach to tumor eradication. If targeted therapy is designed to eliminate CSC populations alone, the remaining tumor parenchyma must also be addressed. Thus, it may prove critical to combine an operative debulking of the tumor with molecular therapeutics designed to eliminate residual CSC populations either by direct CSC or vascular targeting, in addition to radiotherapy. Also, investigators are still concerned about how to achieve the maximum benefit from them and how to monitor patient response [164]. As antiangiogenic strategies for neuro-oncology are explored, it has to be considered that different types of intracranial tumors encounter a different pre-existent angioarchitecture as their source of neovascularization. COX-2, the key enzyme that catalyzes the first steps in the biosynthesis of the prostaglandins from arachidonic acid, appear to be related to vascular pattern in GBM [165]. Currently, there are no markers to identify the net angiogenic activity of a tumor. Since quantification of various aspects of tumor vasculature may provide an indication of angiogenic activity [166,167], it is reasonable to assume that identification of new markers to determine angiogenic activity will help investigators to design specific antiangiogenic treatment strategies.
Expert commentary
Since angiogenesis is virtually absent in normal adults, antiangiogenic therapy is more tumor-specific and yields a low toxicity profile. Different antiangiogenic strategies have been developed: inhibition of proangiogenic factors and/or receptors and/or downstream cell signaling; inactivation of ECs; and inhibition of cellular adhesion molecules and/or ECM remodeling. Inhibitors of angiogenesis are separated into endogenous inhibitors, such as angiostatin, trombospondin or IFN-α, and natural or synthetic inhibitors, such as THD, antibodies against angiogenic growth factors or inhibitors of tyrosine kinase receptors. Several clinical trials of antiangiogenic therapies are being conducted throughout the world, but investigators are still concerned about how to achieve the maximum benefit from them and how to monitor patient response. The side effects of antiangiogenic treatments appear acceptable and moderate. However, this concerns only short-term utilization.
No clear benefits for patients have been reported regarding the use of single antiangiogenic drugs. Interestingly, the levels of angiogenic molecules in circulating blood from patients with tumors have been shown to increase significantly in response to antiangiogenic treatment [103]. The mechanisms involved in this phenomenon are not known. The role of the third compartment (i.e., pericytes, microglia and astrocytes) as well as endothelial and tumor cells remains unclear. Furthermore, several mechanisms are involved in angiogenesis, and the targeting of one molecule or pathway may lead to the increased activity of other pathways, which may then sustain angiogenesis. It seems likely that a combination of these antiangiogenic agents with chemotherapy seems to be more efficient. Glioma cells surround existing vessels to promote their own growth [6], and it is also reasonable to think that these treatments should be applied early, either when the angiogenic switch occurs or to prevent the angiogenic switch. Recent microarray studies have demonstrated differences between tumor grade and type that will help in the design of a more rational approach to treatment. Some evidence from clinical trials has already demonstrated that a better selection of patients, based on individual molecular pathways, should improve the efficacy of antivascular therapies. Clinical experience also demonstrates the failure of conventional imaging to monitor the effect of the antiangiogenic treatments. In order to better monitor the efficacy of these strategies, novel imaging techniques and pharmacodynamic surrogate markers have to be validated and established.
Five-year view
Antiangiogenic therapy has been demonstrated to represent a promising novel approach to the treatment of malignant brain tumors. It seems likely that a combination of antiangiogenic agents with other cytotoxic therapies will be required to achieve maximal efficacy. Antiangiogenic compounds are not expected to reduce the tumor burden but, rather, to exert a cytostatic effect. A crucial issue is, therefore, the search for end points and surrogate markers as indicators of biological response and anti-tumor activity. The need for the development of suitable assays (e.g., circulating markers and analysis on biopsy samples) before entering into clinical studies is clear. Imaging techniques (dynamic enhanced MRI, high frequency ultrasound and PET) to monitor changes in tumor vascular density, blood flow and blood volume are currently being evaluated. Distinction between various angiogenic subtypes of human GBM may prove relevant for antiangiogenic therapy because vascular patterns with variable expression of angiogenic proteins are likely to influence clinical outcome. There is also little information available regarding the temporal evolution of the angiogenic process under treatment. Clinical translation would benefit greatly from improvements in methodologies of tumor evaluation to identify efficacious and safe combinations of antiangiogenic and cytotoxic agents. Not only targeting tumor cells and tumor ECs but also other proangiogenic cell types such as perivascular, stromal and bone marrow-derived proangiogenic cells may augment treatment efficacy and translate into improved patient outcome. Also, new advances in the design of surrogate markers for identification of resistance and therapy response for angiogenesis for both MRI and molecular imaging are likely to be developed. Antiangiogenic therapies have been proposed to avoid the risk of developing resistance to the treatment as they target wild-type vascular ECs that are genetically stable diploid cells (and thus different from genetically unstable neoplastic cells). However, tumor response studies on antiangiogenic therapy by Yu et al. suggest that some tumors can loose responsiveness to antiangiogenic therapy because of cancer cell genetic mutations, as evidenced by a study which demonstrated that mice bearing tumors derived by p53−/− human colorectal cancer were less responsive that mice with p53+/+ tumors [168]. Although the target of antiangiogenic therapy ECs are genetically stable compared with tumor cells, these studies exemplify the importance of other factors, such as the genetics of the tumor cell. Therefore, improving our understanding of the molecular and cellular pathways involved in tumor relapse during or after antiangiogenic and combination therapies will allow future therapeutic strategies to be tailored to each tumor’s profile before, during and after therapy. Furthermore, the CSC theory dictates that any therapeutic intervention unable to eradicate all CSCs may result in regrowth of the residual CSCs, resulting in recurrence and subsequent failure of the therapy. Current strategies of gross tumor resection combined with adjuvant radiotherapy and chemotherapy have shown to be futile against the often radio/chemo-resistant CSC population. Identifying analogous characteristics between normal NSCs and CSCs may provide further insight into how this treatment resistance is mediated and unlock the potential for effective cellular therapeutics.
Key issues
Infiltrative astrocytic neoplasms are by far the most common malignant brain tumors in adults.
Glioblastoma multiforme (GBM; WHO grade IV), the most malignant form of infiltrating astrocytoma, can evolve from a lower-grade precursor tumor (secondary GBM) or can present as a high-grade lesion from the outset (de novo GBM).
Underlying genetic alterations in GBM may tilt the balance in favor of an angiogenic phenotype by upregulation of proangiogenic factors and downregulation of angiogenesis inhibitors.
Increased vascularity and endothelial cell proliferation in GBMs are also driven by hypoxia-induced expression of proangiogenic cytokines, such as VEGF.
Several antiangiogenic agents, such as inhibitors of PDGFs, FGFs, angiopoietins/Tie-2 system, PKC and integrins, are currently in preclinical and clinical development.
Although preliminary studies for these drugs in GBM have been promising, larger prospective trials are required.
Early antiangiogenesis therapy is considered to be a promising tool for treating gliomas, where malignancy is highly related to angiogenesis.
Combination of agents targeting different angiogenic pathways or multimodality combination with radiation, chemotherapy or other targeted therapeutics or immunotherapy will be required to achieve maximal efficacy.
In addition, owing to the angiogenic heterogeneity among individual malignant brain tumors, the antiangiogenic strategies for brain tumor patients will have to be individualized and tailored to the tumor’s angiogenic phenotype using a panel of modern histopathological and imaging techniques.
Acknowledgments
Financial & competing interests disclosure
This research was supported by National Cancer Institute Grant CA75557, CA92393, CA95058, CA116708, NINDS NS47699, NS57529 and NS61835, and Caterpillar Inc., OSF St. Francis Inc. and Peoria, IL (to J Rao). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Contributor Information
Sajani S Lakka, Department of Cancer Biology and Pharmacology, University of Illinois College of Medicine at Peoria, 1 Illini Drive, Peoria, IL 61605, USA.
Jasti S Rao, Department of Neurosurgery, University of Illinois College of Medicine at Peoria, 1 Illini Drive, Peoria, IL 61605, USA and Department of Cancer Biology and Pharmacology, University of Illinois College of Medicine at Peoria, 1 Illini Drive, Peoria, IL 61605, USA, Tel.: +1 309 671 3445, Fax: +1 309 671 3442, jsrao@uic.edu.
References
- 1.Folkman J. Tumor angiogenesis: therapeutic implications. N. Engl. J. Med. 1971;285(21):1182–1126. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 2.Risau W. Mechanisms of angiogenesis. Nature. 1997;386(6626):671–674. doi: 10.1038/386671a0. [DOI] [PubMed] [Google Scholar]
- 3.Carmeliet P. Angiogenesis in life, disease and medicine. Nature. 2005;438(7070):932–936. doi: 10.1038/nature04478. [DOI] [PubMed] [Google Scholar]
- 4.Fidler IJ, Ellis LM. Neoplastic angiogenesis: not all blood vessels are created equal. N. Engl. J. Med. 2004;351(3):215–216. doi: 10.1056/NEJMp048080. [DOI] [PubMed] [Google Scholar]
- 5.Los AM, Voest EE. The potential role of antivascular therapy in the adjuvant and neoadjuvant treatment of cancer. Semin. Oncol. 2001;28(1):93–105. doi: 10.1016/s0093-7754(01)90047-8. [DOI] [PubMed] [Google Scholar]
- 6.Farin A, Suzuki SO, Weiker M, Goldman JE, Bruce JN, Canoll P. Transplanted glioma cells migrate and proliferate on host brain vasculature: a dynamic analysis. Glia. 2006;53(8):799–808. doi: 10.1002/glia.20334. [DOI] [PubMed] [Google Scholar]
- 7.Rijken PF, Bernsen HJ, Peters JP, Hodgkiss RJ, Raleigh JA, van der Kogel AJ. Spatial relationship between hypoxia and the (perfused) vascular network in a human glioma xenograft: a quantitative multi-parameter analysis. Int. J. Radiat. Oncol. Biol. Phys. 2000;48(2):571–582. doi: 10.1016/s0360-3016(00)00686-6. [DOI] [PubMed] [Google Scholar]
- 8.Wenger RH. Mammalian oxygen sensing, signalling and gene regulation. J Exp. Biol. 2000;203(Pt 8):1253–1263. doi: 10.1242/jeb.203.8.1253. [DOI] [PubMed] [Google Scholar]
- 9.Zagzag D, Zhong H, Scalzitti JM, Laughner E, Simons JW, Semenza GL. Expression of hypoxia-inducible factor 1α in brain tumors: association with angiogenesis, invasion, and progression. Cancer. 2000;88(11):2606–2618. [PubMed] [Google Scholar]
- 10.Du R, Lu KV, Petritsch C, et al. HIF1α induces the recruitment of bone marrow-derived vascular modulatory cells to regulate tumor angiogenesis and invasion. Cancer Cell. 2008;13(3):206–220. doi: 10.1016/j.ccr.2008.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rempel SA, Dudas S, Ge S, Gutierrez JA. Identification and localization of the cytokine SDF1 and its receptor, CXC chemokine receptor 4, to regions of necrosis and angiogenesis in human glioblastoma. Clin. Cancer Res. 2000;6(1):102–111. [PubMed] [Google Scholar]
- 12.De Palma M, Venneri MA, Galli R, et al. Tie2 identifies a hematopoietic lineage of proangiogenic monocytes required for tumor vessel formation and a mesenchymal population of pericyte progenitors. Cancer Cell. 2005;8(3):211–226. doi: 10.1016/j.ccr.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 13.Jones N, Iljin K, Dumont DJ, Alitalo K. Tie receptors: new modulators of angiogenic and lymphangiogenic responses. Nat. Rev. Mol. Cell Biol. 2001;2(4):257–267. doi: 10.1038/35067005. [DOI] [PubMed] [Google Scholar]
- 14.Behzadian MA, Windsor LJ, Ghaly N, Liou G, Tsai NT, Caldwell RB. VEGF-induced paracellular permeability in cultured endothelial cells involves urokinase and its receptor. FASEB J. 2003;17(6):752–754. doi: 10.1096/fj.02-0484fje. [DOI] [PubMed] [Google Scholar]
- 15.Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407(6801):249–257. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- 16.Reiss Y, Machein MR, Plate KH. The role of angiopoietins during angiogenesis in gliomas. Brain Pathol. 2005;15(4):311–317. doi: 10.1111/j.1750-3639.2005.tb00116.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yancopoulos GD, Davis S, Gale NW, Rudge JS, Wiegand SJ, Holash J. Vascular-specific growth factors and blood vessel formation. Nature. 2000;407(6801):242–248. doi: 10.1038/35025215. [DOI] [PubMed] [Google Scholar]
- 18.Lamszus K, Schmidt NO, Jin L, et al. Scatter factor promotes motility of human glioma and neuromicrovascular endothelial cells. Int. J. Cancer. 1998;75(1):19–28. doi: 10.1002/(sici)1097-0215(19980105)75:1<19::aid-ijc4>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 19.Schmidt NO, Westphal M, Hagel C, et al. Levels of vascular endothelial growth factor, hepatocyte growth factor/scatter factor and basic fibroblast growth factor in human gliomas and their relation to angiogenesis. Int. J. Cancer. 1999;84(1):10–18. doi: 10.1002/(sici)1097-0215(19990219)84:1<10::aid-ijc3>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 20.Chakravarti A, Dicker A, Mehta M. The contribution of epidermal growth factor receptor (EGFR) signaling pathway to radioresistance in human gliomas: a review of preclinical and correlative clinical data. Int. J. Radiat. Oncol. Biol. Phys. 2004;58(3):927–931. doi: 10.1016/j.ijrobp.2003.09.092. [DOI] [PubMed] [Google Scholar]
- 21.Choe G, Horvath S, Cloughesy TF, et al. Analysis of the phosphatidylinositol 3′-kinase signaling pathway in glioblastoma patients in vivo. Cancer Res. 2003;63(11):2742–2746. [PubMed] [Google Scholar]
- 22.Wang H, Wang H, Zhang W, Huang HJ, Liao WS, Fuller GN. Analysis of the activation status of Akt, NFκB, and Stat3 in human diffuse gliomas. Lab. Invest. 2004;84(8):941–951. doi: 10.1038/labinvest.3700123. [DOI] [PubMed] [Google Scholar]
- 23.Sun L, Hui AM, Su Q, et al. Neuronal and glioma-derived stem cell factor induces angiogenesis within the brain. Cancer Cell. 2006;9(4):287–300. doi: 10.1016/j.ccr.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 24.Dunn IF, Heese O, Black PM. Growth factors in glioma angiogenesis: FGFs, PDGF, EGF, and TGFs. J. Neurooncol. 2000;50(1–2):121–137. doi: 10.1023/a:1006436624862. [DOI] [PubMed] [Google Scholar]
- 25.Fredriksson L, Li H, Eriksson U. The PDGF family: four gene products form five dimeric isoforms. Cytokine Growth Factor Rev. 2004;15(4):197–204. doi: 10.1016/j.cytogfr.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 26.Nystrom HC, Lindblom P, Wickman A, et al. Platelet-derived growth factor B retention is essential for development of normal structure and function of conduit vessels and capillaries. Cardiovasc. Res. 2006;71(3):557–565. doi: 10.1016/j.cardiores.2006.05.019. [DOI] [PubMed] [Google Scholar]
- 27.Wen PY, Yung WK, Lamborn KR, et al. Phase I/II study of imatinib mesylate for recurrent malignant gliomas: North American brain tumor consortium study 99–08. Clin. Cancer Res. 2006;12(16):4899–4907. doi: 10.1158/1078-0432.CCR-06-0773. [DOI] [PubMed] [Google Scholar]
- 28.MacDonald TJ, Taga T, Shimada H, et al. Preferential susceptibility of brain tumors to the antiangiogenic effects of an α(v) integrin antagonist. Neurosurgery. 2001;48(1):151–157. doi: 10.1097/00006123-200101000-00026. [DOI] [PubMed] [Google Scholar]
- 29.Longhurst CM, Jennings LK. Integrin-mediated signal transduction. Cell. Mol. Life Sci. 1998;54(6):514–526. doi: 10.1007/s000180050180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hu G, Riordan JF, Vallee BL. Angiogenin promotes invasiveness of cultured endothelial cells by stimulation of cell-associated proteolytic activities. Proc. Natl Acad. Sci. USA. 1994;91(25):12096–12100. doi: 10.1073/pnas.91.25.12096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hu GF, Riordan JF, Vallee BL. A putative angiogenin receptor in angiogenin-responsive human endothelial cells. Proc. Natl Acad. Sci. USA. 1997;94(6):2204–2209. doi: 10.1073/pnas.94.6.2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jimi S, Ito K, Kohno K, et al. Modulation by bovine angiogenin of tubular morphogenesis and expression of plasminogen activator in bovine endothelial cells. Biochem. Biophys. Res. Commun. 1995;211(2):476–483. doi: 10.1006/bbrc.1995.1838. [DOI] [PubMed] [Google Scholar]
- 33.Eberle K, Oberpichler A, Trantakis C, et al. The expression of angiogenin in tissue samples of different brain tumours and cultured glioma cells. Anticancer Res. 2000;20(3A):1679–1684. [PubMed] [Google Scholar]
- 34.Zadeh G, Qian B, Okhowat A, Sabha N, Kontos CD, Guha A. Targeting the Tie2/Tek receptor in astrocytomas. Am. J. Pathol. 2004;164(2):467–476. doi: 10.1016/S0002-9440(10)63137-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee OH, Xu J, Fueyo J, et al. Expression of the receptor tyrosine kinase Tie2 in neoplastic glial cells is associated with integrin β1-dependent adhesion to the extracellular matrix. Mol. Cancer Res. 2006;4(12):915–926. doi: 10.1158/1541-7786.MCR-06-0184. [DOI] [PubMed] [Google Scholar]
- 36.Lindahl P, Johansson BR, Leveen P, Betsholtz C. Pericyte loss and microaneurysm formation in PDGF-β-deficient mice. Science. 1997;277(5323):242–245. doi: 10.1126/science.277.5323.242. [DOI] [PubMed] [Google Scholar]
- 37.Shih AH, Holland EC. Platelet-derived growth factor (PDGF) and glial tumorigenesis. Cancer Lett. 2006;232(2):139–147. doi: 10.1016/j.canlet.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 38.Jackson EL, Garcia-Verdugo JM, Gil-Perotin S, et al. PDGFR α-positive B cells are neural stem cells in the adult SVZ that form glioma-like growths in response to increased PDGF signaling. Neuron. 2006;51(2):187–199. doi: 10.1016/j.neuron.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 39.Hermansson M, Nister M, Betsholtz C, Heldin CH, Westermark B, Funa K. Endothelial cell hyperplasia in human glioblastoma: coexpression of mRNA for platelet-derived growth factor (PDGF) B chain and PDGF receptor suggests autocrine growth stimulation. Proc. Natl Acad. Sci. USA. 1988;85(20):7748–7752. doi: 10.1073/pnas.85.20.7748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Plate KH, Breier G, Farrell CL, Risau W. Platelet-derived growth factor receptor-β is induced during tumor development and upregulated during tumor progression in endothelial cells in human gliomas. Lab. Invest. 1992;67(4):529–534. [PubMed] [Google Scholar]
- 41.Bergers G, Song S. The role of pericytes in blood-vessel formation and maintenance. Neuro-oncology. 2005;7(4):452–464. doi: 10.1215/S1152851705000232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Abounader R, Laterra J. Scatter factor/hepatocyte growth factor in brain tumor growth and angiogenesis. Neuro-oncology. 2005;7(4):436–451. doi: 10.1215/S1152851705000050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Abounader R, Ranganathan S, Lal B, et al. Reversion of human glioblastoma malignancy by U1 small nuclear RNA/ribozyme targeting of scatter factor/hepatocyte growth factor and c-met expression. J. Natl Cancer Inst. 1999;91(18):1548–1556. doi: 10.1093/jnci/91.18.1548. [DOI] [PubMed] [Google Scholar]
- 44.Lal B, Xia S, Abounader R, Laterra J. Targeting the c-Met pathway potentiates glioblastoma responses to γ-radiation. Clin. Cancer Res. 2005;11(12):4479–4486. doi: 10.1158/1078-0432.CCR-05-0166. [DOI] [PubMed] [Google Scholar]
- 45.Burgess T, Coxon A, Meyer S, et al. Fully human monoclonal antibodies to hepatocyte growth factor with therapeutic potential against hepatocyte growth factor/c-Met-dependent human tumors. Cancer Res. 2006;66(3):1721–1729. doi: 10.1158/0008-5472.CAN-05-3329. [DOI] [PubMed] [Google Scholar]
- 46.Cao B, Su Y, Oskarsson M, et al. Neutralizing monoclonal antibodies to hepatocyte growth factor/scatter factor (HGF/SF) display antitumor activity in animal models. Proc. Natl Acad. Sci. USA. 2001;98(13):7443–7448. doi: 10.1073/pnas.131200498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Martens T, Schmidt NO, Eckerich C, et al. A novel one-armed anti-c-Met antibody inhibits glioblastoma growth in vivo. Clin. Cancer Res. 2006;12(20 Pt 1):6144–6152. doi: 10.1158/1078-0432.CCR-05-1418. [DOI] [PubMed] [Google Scholar]
- 48.Nagase H, Woessner JF., Jr Matrix metalloproteinases. J. Biol. Chem. 1999;274(31):21491–21494. doi: 10.1074/jbc.274.31.21491. [DOI] [PubMed] [Google Scholar]
- 49.Shapiro SD. Matrix metalloproteinase degradation of extracellular matrix: biological consequences. Curr. Opin. Cell Biol. 1998;10(5):602–608. doi: 10.1016/s0955-0674(98)80035-5. [DOI] [PubMed] [Google Scholar]
- 50.Ray JM, Stetler-Stevenson WG. Gelatinase A activity directly modulates melanoma cell adhesion and spreading. EMBO J. 1995;14(5):908–917. doi: 10.1002/j.1460-2075.1995.tb07072.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sternlicht MD, Werb Z. How matrix metalloproteinases regulate cell behavior. Annu. Rev. Cell Dev. Biol. 2001:17463–17516. doi: 10.1146/annurev.cellbio.17.1.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thorns V, Walter GF, Thorns C. Expression of MMP-2, MMP-7, MMP-9, MMP-10 and MMP-11 in human astrocytic and oligodendroglial gliomas. Anticancer Res. 2003;23(5A):3937–3944. [PubMed] [Google Scholar]
- 53.Wang M, Wang T, Liu S, Yoshida D, Teramoto A. The expression of matrix metalloproteinase-2 and -9 in human gliomas of different pathological grades. Brain Tumor Pathol. 2003;20(2):65–72. doi: 10.1007/BF02483449. [DOI] [PubMed] [Google Scholar]
- 54.Yoshida D, Takahashi H, Teramoto A. Inhibition of glioma angiogenesis and invasion by SI-27, an anti-matrix metalloproteinase agent in a rat brain tumor model. Neurosurgery. 2004;54(5):1213–1220. doi: 10.1227/01.neu.0000119237.46690.c6. [DOI] [PubMed] [Google Scholar]
- 55.Lakka SS, Gondi CS, Rao JS. Proteases and glioma angiogenesis. Brain Pathol. 2005:15327–15341. doi: 10.1111/j.1750-3639.2005.tb00118.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gondi CS, Lakka SS, Yanamandra N, et al. Expression of antisense uPAR and antisense uPA from a bicistronic adenoviral construct inhibits glioma cell invasion, tumor growth, and angiogenesis. Oncogene. 2003;22(38):5967–5975. doi: 10.1038/sj.onc.1206535. [DOI] [PubMed] [Google Scholar]
- 57.Rege TA, Fears CY, Gladson CL. Endogenous inhibitors of angiogenesis in malignant gliomas: nature’s antiangiogenic therapy. Neuro-oncology. 2005;7(2):106–121. doi: 10.1215/S115285170400119X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.O’Reilly MS, Holmgren L, Shing Y, et al. Angiostatin: a novel angiogenesis inhibitor that mediates the suppression of metastases by a Lewis lung carcinoma. Cell. 1994;79(2):315–328. doi: 10.1016/0092-8674(94)90200-3. [DOI] [PubMed] [Google Scholar]
- 59.Ma HI, Lin SZ, Chiang YH, et al. Intratumoral gene therapy of malignant brain tumor in a rat model with angiostatin delivered by adeno-associated viral (AAV) vector. Gene Ther. 2002;9(1):2–11. doi: 10.1038/sj.gt.3301616. [DOI] [PubMed] [Google Scholar]
- 60.Troyanovsky B, Levchenko T, Mansson G, Matvijenko O, Holmgren L. Angiomotin: an angiostatin binding protein that regulates endothelial cell migration and tube formation. J. Cell Biol. 2001;152(6):1247–1254. doi: 10.1083/jcb.152.6.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.O’Reilly MS, Boehm T, Shing Y, et al. Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell. 1997;88(2):277–285. doi: 10.1016/s0092-8674(00)81848-6. [DOI] [PubMed] [Google Scholar]
- 62.Sasaki T, Fukai N, Mann K, Gohring W, Olsen BR, Timpl R. Structure, function and tissue forms of the C terminal globular domain of collagen XVIII containing the angiogenesis inhibitor endostatin. EMBO J. 1998;17(15):4249–4256. doi: 10.1093/emboj/17.15.4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Joki T, Machluf M, Atala A, et al. Continuous release of endostatin from microencapsulated engineered cells for tumor therapy. Nat. Biotechnol. 2001;19(1):35–39. doi: 10.1038/83481. [DOI] [PubMed] [Google Scholar]
- 64.Read TA, Farhadi M, Bjerkvig R, et al. Intravital microscopy reveals novel antivascular and antitumor effects of endostatin delivered locally by alginate-encapsulated cells. Cancer Res. 2001;61(18):6830–6837. [PubMed] [Google Scholar]
- 65.Peroulis I, Jonas N, Saleh M. Antiangiogenic activity of endostatin inhibits C6 glioma growth. Int. J. Cancer. 2002;97(6):839–845. doi: 10.1002/ijc.10115. [DOI] [PubMed] [Google Scholar]
- 66.Sorensen DR, Leirdal M, Iversen PO, Sioud M. Combination of endostatin and a protein kinase Cα DNA enzyme improves the survival of rats with malignant glioma. Neoplasia. 2002;4(6):474–479. doi: 10.1038/sj.neo.7900271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schmidt A, Wenzel D, Ferring I, et al. Influence of endostatin on embryonic vasculo- and angiogenesis. Dev. Dyn. 2004;230(3):468–480. doi: 10.1002/dvdy.20072. [DOI] [PubMed] [Google Scholar]
- 68.Brooks PC, Silletti S, von Schalscha TL, Friedlander M, Cheresh DA. Disruption of angiogenesis by PEX, a noncatalytic metalloproteinase fragment with integrin binding activity. Cell. 1998;92(3):391–400. doi: 10.1016/s0092-8674(00)80931-9. [DOI] [PubMed] [Google Scholar]
- 69.Bello L, Lucini V, Carrabba G, et al. Simultaneous inhibition of glioma angiogenesis, cell proliferation, and invasion by a naturally occurring fragment of human metalloproteinase-2. Cancer Res. 2001;61(24):8730–8736. [PubMed] [Google Scholar]
- 70.Bello L, Francolini M, Marthyn P, et al. α(v)β3 and α(v)β5 integrin expression in glioma periphery. Neurosurgery. 2001;49(2):380–389. doi: 10.1097/00006123-200108000-00022. [DOI] [PubMed] [Google Scholar]
- 71.Adams JC, Lawler J. The thrombospondins. Int. J. Biochem. Cell Biol. 2004;36(6):961–968. doi: 10.1016/j.biocel.2004.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Volpert OV, Zaichuk T, Zhou W, et al. Inducer-stimulated Fas targets activated endothelium for destruction by anti-angiogenic thrombospondin-1 and pigment epithelium-derived factor. Nat. Med. 2002;8(4):349–357. doi: 10.1038/nm0402-349. [DOI] [PubMed] [Google Scholar]
- 73.Tenan M, Fulci G, Albertoni M, et al. Thrombospondin-1 is downregulated by anoxia and suppresses tumorigenicity of human glioblastoma cells. J. Exp. Med. 2000;191(10):1789–1798. doi: 10.1084/jem.191.10.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kragh M, Quistorff B, Tenan M, Van Meir EG, Kristjansen PE. Overexpression of thrombospondin-1 reduces growth and vascular index but not perfusion in glioblastoma. Cancer Res. 2002;62(4):1191–1195. [PubMed] [Google Scholar]
- 75.Rege TA, Stewart JE, Jr, Henkin J, Silverstein RL, Gladson CL. Presented at: Second National Meeting of the American Society for Matrix Biology. San Diego CA, USA: 2004. Nov 10–13, TSP-1 and type 1 repeat domain peptides induce apoptosis of human brain microvascular endothelial cells. Abstract. [Google Scholar]
- 76.de Fraipont F, Keramidas M, El AM, Chambaz EM, Berger F, Feige JJ. Expression of the thrombospondin 1 fragment 167–569 in C6 glioma cells stimulates tumorigenicity despite reduced neovascularization. Oncogene. 2004;23(20):3642–3649. doi: 10.1038/sj.onc.1207438. [DOI] [PubMed] [Google Scholar]
- 77.Anderson JC, Grammer JR, Wang W, et al. ABT-510, a modified type 1 repeat peptide of thrombospondin, inhibits malignant glioma growth in vivo by inhibiting angiogenesis. Cancer Biol. Ther. 2007;6(3):454–462. doi: 10.4161/cbt.6.3.3630. [DOI] [PubMed] [Google Scholar]
- 78.Deininger MH, Weller M, Streffer J, Mittelbronn M, Meyermann R. Patterns of cyclooxygenase-1 and -2 expression in human gliomas in vivo. Acta Neuropathol. 1999;98(3):240–244. doi: 10.1007/s004010051075. [DOI] [PubMed] [Google Scholar]
- 79.Shono T, Tofilon PJ, Bruner JM, Owolabi O, Lang FF. Cyclooxygenase-2 expression in human gliomas: prognostic significance and molecular correlations. Cancer Res. 2001;61(11):4375–4381. [PubMed] [Google Scholar]
- 80.Brat DJ, Bellail AC, Van Meir EG. The role of interleukin-8 and its receptors in gliomagenesis and tumoral angiogenesis. Neuro-oncology. 2005;7(2):122–133. doi: 10.1215/S1152851704001061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Charalambous C, Pen LB, Su YS, Milan J, Chen TC, Hofman FM. Interleukin-8 differentially regulates migration of tumor-associated and normal human brain endothelial cells. Cancer Res. 2005;65(22):10347–10354. doi: 10.1158/0008-5472.CAN-05-0949. [DOI] [PubMed] [Google Scholar]
- 82.Salmaggi A, Gelati M, Pollo B, et al. CXCL12 in malignant glial tumors: a possible role in angiogenesis and cross-talk between endothelial and tumoral cells. J. Neurooncol. 2004;67(3):305–317. doi: 10.1023/b:neon.0000024241.05346.24. [DOI] [PubMed] [Google Scholar]
- 83.Rubin JB, Kung AL, Klein RS, et al. A small-molecule antagonist of CXCR4 inhibits intracranial growth of primary brain tumors. Proc. Natl Acad. Sci. USA. 2003;100(23):13513–13518. doi: 10.1073/pnas.2235846100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sgadari C, Angiolillo AL, Tosato G. Inhibition of angiogenesis by interleukin-12 is mediated by the interferon-inducible protein 10. Blood. 1996;87(9):3877–3882. [PubMed] [Google Scholar]
- 85.Ehtesham M, Kabos P, Kabosova A, Neuman T, Black KL, Yu JS. The use of interleukin 12-secreting neural stem cells for the treatment of intracranial glioma. Cancer Res. 2002;62(20):5657–5663. [PubMed] [Google Scholar]
- 86.Allport JR, Shinde Patil VR, Weissleder R. Murine neuronal progenitor cells are preferentially recruited to tumor vasculature via α4-integrin and SDF-1α-dependent mechanisms. Cancer Biol. Ther. 2004;3(9):838–844. doi: 10.4161/cbt.3.9.1036. [DOI] [PubMed] [Google Scholar]
- 87.Annabi B, Naud E, Lee YT, Eliopoulos N, Galipeau J. Vascular progenitors derived from murine bone marrow stromal cells are regulated by fibroblast growth factor and are avidly recruited by vascularizing tumors. J. Cell Biochem. 2004;91(6):1146–1158. doi: 10.1002/jcb.10763. [DOI] [PubMed] [Google Scholar]
- 88.Fears CY, Sontheimer HW, Bullard DC, Gladson CL. Could labeled neuronal progenitor cells be used to target glioma tumor endothelium? Cancer Biol. Ther. 2004;3(9):845–846. doi: 10.4161/cbt.3.9.1123. [DOI] [PubMed] [Google Scholar]
- 89.Walkley CR, Olsen GH, Dworkin S, et al. A microenvironment-induced myeloproliferative syndrome caused by retinoic acid receptor γ deficiency. Cell. 2007;129(6):1097–1110. doi: 10.1016/j.cell.2007.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pollard JW. Tumour-educated macrophages promote tumour progression and metastasis. Nat. Rev. Cancer. 2004;4(1):71–78. doi: 10.1038/nrc1256. [DOI] [PubMed] [Google Scholar]
- 91.Calabrese C, Poppleton H, Kocak M, et al. A perivascular niche for brain tumor stem cells. Cancer Cell. 2007;11(1):69–82. doi: 10.1016/j.ccr.2006.11.020. [DOI] [PubMed] [Google Scholar]
- 92.Li Q, Ford MC, Lavik EB, Madri JA. Modeling the neurovascular niche. J. Neurosci. Res. 2006;84(8):1656–1668. doi: 10.1002/jnr.21087. [DOI] [PubMed] [Google Scholar]
- 93.Santarelli JG, Sarkissian V, Hou LC, Veeravagu A, Tse V. Molecular events of brain metastasis. Neurosurg. Focus. 2007;22(3):E1. doi: 10.3171/foc.2007.22.3.2. [DOI] [PubMed] [Google Scholar]
- 94.Bao S, Wu Q, Sathornsumetee S, et al. Stem cell-like glioma cells promote tumor angiogenesis through vascular endothelial growth factor. Cancer Res. 2006;66(16):7843–7848. doi: 10.1158/0008-5472.CAN-06-1010. [DOI] [PubMed] [Google Scholar]
- 95.Folkins C, Man S, Xu P, Shaked Y, Hicklin DJ, Kerbel RS. Anticancer therapies combining antiangiogenic and tumor cell cytotoxic effects reduce the tumor stem-like cell fraction in glioma xenograft tumors. Cancer Res. 2007;67(8):3560–3564. doi: 10.1158/0008-5472.CAN-06-4238. [DOI] [PubMed] [Google Scholar]
- 96.Sundberg C, Nagy JA, Brown LF, et al. Glomeruloid microvascular proliferation follows adenoviral vascular permeability factor/vascular endothelial growth factor-164 gene delivery. Am. J. Pathol. 2001;158(3):1145–1160. doi: 10.1016/S0002-9440(10)64062-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kim KJ, Li B, Winer J, et al. Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumour growth in vivo. Nature. 1993;362(6423):841–844. doi: 10.1038/362841a0. [DOI] [PubMed] [Google Scholar]
- 98.Cheng SY, Huang HJ, Nagane M, et al. Suppression of glioblastoma angiogenicity and tumorigenicity by inhibition of endogenous expression of vascular endothelial growth factor. Proc. Natl Acad. Sci. USA. 1996;93(16):8502–8507. doi: 10.1073/pnas.93.16.8502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Millauer B, Shawver LK, Plate KH, Risau W, Ullrich A. Glioblastoma growth inhibited in vivo by a dominant-negative Flk-1 mutant. Nature. 1994;367(6463):576–579. doi: 10.1038/367576a0. [DOI] [PubMed] [Google Scholar]
- 100.Vajkoczy P, Menger MD, Vollmar B, et al. Inhibition of tumor growth, angiogenesis, and microcirculation by the novel Flk-1 inhibitor SU5416 as assessed by intravital multi-fluorescence videomicroscopy. Neoplasia. 1999;1(1):31–41. doi: 10.1038/sj.neo.7900006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kunkel P, Ulbricht U, Bohlen P, et al. Inhibition of glioma angiogenesis and growth in vivo by systemic treatment with a monoclonal antibody against vascular endothelial growth factor receptor-2. Cancer Res. 2001;61(18):6624–6628. [PubMed] [Google Scholar]
- 102.Takano S, Matsumura A. Anti-angiogenesis treatment for brain tumors: present and future. No Shinkei Geka. 2006;34(7):657–678. [PubMed] [Google Scholar]
- 103.Batchelor TT, Sorensen AG, di TE, et al. AZD2171, a pan-VEGF receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patients. Cancer Cell. 2007;11(1):83–95. doi: 10.1016/j.ccr.2006.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Maris JM, Courtright J, Houghton PJ, et al. Initial testing of the VEGFR inhibitor AZD2171 by the pediatric preclinical testing program. Pediatr. Blood Cancer. 2008;50(3):581–587. doi: 10.1002/pbc.21232. [DOI] [PubMed] [Google Scholar]
- 105.Pope WB, Lai A, Nghiemphu P, Mischel P, Cloughesy TF. MRI in patients with high-grade gliomas treated with bevacizumab and chemotherapy. Neurology. 2006;66(8):1258–1260. doi: 10.1212/01.wnl.0000208958.29600.87. [DOI] [PubMed] [Google Scholar]
- 106.Vredenburgh JJ, Desjardins A, Herndon JE, et al. Bevacizumab plus irinotecan in recurrent glioblastoma multiforme. J. Clin. Oncol. 2007;25(30):4722–4729. doi: 10.1200/JCO.2007.12.2440. [DOI] [PubMed] [Google Scholar]
- 107.Jain RK, Duda DG, Clark JW, Loeffler JS. Lessons from Phase III clinical trials on anti-VEGF therapy for cancer. Nat. Clin. Pract. Oncol. 2006;3(1):24–40. doi: 10.1038/ncponc0403. [DOI] [PubMed] [Google Scholar]
- 108.Sathornsumetee S, Cao Y, Marcello JE, et al. Tumor angiogenic and hypoxic profiles predict radiographic response and survival in malignant astrocytoma patients treated with bevacizumab and irinotecan. J. Clin. Oncol. 2008;26(2):271–278. doi: 10.1200/JCO.2007.13.3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Norden AD, Young GS, Setayesh K, et al. Bevacizumab for recurrent malignant gliomas: efficacy, toxicity, and patterns of recurrence. Neurology. 2008;70(10):779–787. doi: 10.1212/01.wnl.0000304121.57857.38. [DOI] [PubMed] [Google Scholar]
- 110.Miraux S, Lemiere S, Pineau R, et al. Inhibition of FGF receptor activity in glioma implanted into the mouse brain using the tetracyclin-regulated expression system. Angiogenesis. 2004;7(2):105–113. doi: 10.1007/s10456-004-1037-0. [DOI] [PubMed] [Google Scholar]
- 111.Takano S, Gately S, Engelhard H, Tsanaclis AM, Brem S. Suramin inhibits glioma cell proliferation in vitro and in the brain. J. Neurooncol. 1994;21(3):189–201. doi: 10.1007/BF01063768. [DOI] [PubMed] [Google Scholar]
- 112.Grossman SA, Phuphanich S, Lesser G, et al. Toxicity, efficacy, and pharmacology of suramin in adults with recurrent high-grade gliomas. J. Clin. Oncol. 2001;19(13):3260–3266. doi: 10.1200/JCO.2001.19.13.3260. [DOI] [PubMed] [Google Scholar]
- 113.Laterra JJ, Grossman SA, Carson KA, Lesser GJ, Hochberg FH, Gilbert MR. Suramin and radiotherapy in newly diagnosed glioblastoma: Phase 2 NABTT CNS Consortium study. Neuro-oncology. 2004;6(1):15–20. doi: 10.1215/S1152851703000127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Geng L, Shinohara ET, Kim D, et al. STI571 (Gleevec) improves tumor growth delay and survival in irradiated mouse models of glioblastoma. Int. J. Radiat. Oncol. Biol. Phys. 2006;64(1):263–271. doi: 10.1016/j.ijrobp.2005.08.025. [DOI] [PubMed] [Google Scholar]
- 115.Kilic T, Alberta JA, Zdunek PR, et al. Intracranial inhibition of platelet-derived growth factor-mediated glioblastoma cell growth by an orally active kinase inhibitor of the 2-phenylaminopyrimidine class. Cancer Res. 2000;60(18):5143–5150. [PubMed] [Google Scholar]
- 116.Dresemann G. Imatinib and hydroxyurea in pretreated progressive glioblastoma multiforme: a patient series. Ann. Oncol. 2005;16(10):1702–1708. doi: 10.1093/annonc/mdi317. [DOI] [PubMed] [Google Scholar]
- 117.Reardon DA, Egorin MJ, Quinn JA, et al. Phase II study of imatinib mesylate plus hydroxyurea in adults with recurrent glioblastoma multiforme. J. Clin. Oncol. 2005;23(36):9359–9368. doi: 10.1200/JCO.2005.03.2185. [DOI] [PubMed] [Google Scholar]
- 118.Desjardins A, Quinn JA, Vredenburgh JJ, et al. Phase II study of imatinib mesylate and hydroxyurea for recurrent grade III malignant gliomas. J. Neurooncol. 2007;83(1):53–60. doi: 10.1007/s11060-006-9302-2. [DOI] [PubMed] [Google Scholar]
- 119.DeAngelo DJ, Stone RM, Heaney ML, et al. Phase 1 clinical results with tandutinib (MLN518), a novel FLT3 antagonist, in patients with acute myelogenous leukemia or high-risk myelodysplastic syndrome: safety, pharmacokinetics, and pharmacodynamics. Blood. 2006;108(12):3674–3681. doi: 10.1182/blood-2006-02-005702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zhang X, Bu XY, Zhen HN, Fei Z, Zhang JN, Fu LA. Expression and localisation of urokinase-type plasminogen activator gene in gliomas. J. Clin. Neurosci. 2000;7(2):116–119. doi: 10.1054/jocn.1999.0161. [DOI] [PubMed] [Google Scholar]
- 121.Williams CS, Mann M, DuBois RN. The role of cyclooxygenases in inflammation, cancer, and development. Oncogene. 1999;18(55):7908–7916. doi: 10.1038/sj.onc.1203286. [DOI] [PubMed] [Google Scholar]
- 122.Kang KB, Wang TT, Woon CT, et al. Enhancement of glioblastoma radioresponse by a selective COX-2 inhibitor celecoxib: inhibition of tumor angiogenesis with extensive tumor necrosis. Int. J. Radiat. Oncol. Biol. Phys. 2007;67(3):888–896. doi: 10.1016/j.ijrobp.2006.09.055. [DOI] [PubMed] [Google Scholar]
- 123.Nam DH, Park K, Park C, et al. Intracranial inhibition of glioma cell growth by cyclooxygenase-2 inhibitor celecoxib. Oncol. Rep. 2004;11(2):263–268. [PubMed] [Google Scholar]
- 124.Reardon DA, Quinn JA, Vredenburgh J, et al. Phase II trial of irinotecan plus celecoxib in adults with recurrent malignant glioma. Cancer. 2005;103(2):329–338. doi: 10.1002/cncr.20776. [DOI] [PubMed] [Google Scholar]
- 125.Levin VA, Giglio P, Puduvalli VK, et al. Combination chemotherapy with 13-cis-retinoic acid and celecoxib in the treatment of glioblastoma multiforme. J. Neurooncol. 2006;78(1):85–90. doi: 10.1007/s11060-005-9062-4. [DOI] [PubMed] [Google Scholar]
- 126.Solomon SD, McMurray JJ, Pfeffer MA, et al. Cardiovascular risk associated with celecoxib in a clinical trial for colorectal adenoma prevention. N. Engl. J. Med. 2005;352(11):1071–1080. doi: 10.1056/NEJMoa050405. [DOI] [PubMed] [Google Scholar]
- 127.Li L, Lin X, Shoemaker AR, Albert DH, Fesik SW, Shen Y. Hypoxia-inducible factor-1 inhibition in combination with temozolomide treatment exhibits robust antitumor efficacy in vivo. Clin. Cancer Res. 2006;12(15):4747–4754. doi: 10.1158/1078-0432.CCR-05-2842. [DOI] [PubMed] [Google Scholar]
- 128.Kang SH, Cho HT, Devi S, et al. Antitumor effect of 2-methoxyestradiol in a rat orthotopic brain tumor model. Cancer Res. 2006;66(24):11991–11997. doi: 10.1158/0008-5472.CAN-06-1320. [DOI] [PubMed] [Google Scholar]
- 129.Del Bufalo D, Ciuffreda L, Trisciuoglio D, et al. Antiangiogenic potential of the Mammalian target of rapamycin inhibitor temsirolimus. Cancer Res. 2006;66(11):5549–5554. doi: 10.1158/0008-5472.CAN-05-2825. [DOI] [PubMed] [Google Scholar]
- 130.Chang SM, Wen P, Cloughesy T, et al. Phase II study of CCI-779 in patients with recurrent glioblastoma multiforme. Invest. New Drugs. 2005;23(4):357–361. doi: 10.1007/s10637-005-1444-0. [DOI] [PubMed] [Google Scholar]
- 131.Galanis E, Buckner JC, Maurer MJ, et al. Phase II trial of temsirolimus (CCI-779) in recurrent glioblastoma multiforme: a north central cancer treatment group study. J. Clin. Oncol. 2005;23(23):5294–5304. doi: 10.1200/JCO.2005.23.622. [DOI] [PubMed] [Google Scholar]
- 132.Singh RK, Gutman M, Bucana CD, Sanchez R, Llansa N, Fidler IJ. Interferons α and β down-regulate the expression of basic fibroblast growth factor in human carcinomas. Proc. Natl Acad. Sci. USA. 1995;92(10):4562–4566. doi: 10.1073/pnas.92.10.4562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Chang E, Boyd A, Nelson CC, et al. Successful treatment of infantile hemangiomas with interferon-α-2b. J. Pediatr. Hematol. Oncol. 1997;19(3):237–244. doi: 10.1097/00043426-199705000-00011. [DOI] [PubMed] [Google Scholar]
- 134.Wakabayashi T, Natsume A, Hashizume Y, Fujii M, Mizuno M, Yoshida J. A Phase I clinical trial of interferon-β gene therapy for high-grade glioma: novel findings from gene expression profiling and autopsy. J. Gene Med. 2008;10(4):329–339. doi: 10.1002/jgm.1160. [DOI] [PubMed] [Google Scholar]
- 135.Colman H, Berkey BA, Maor MH, et al. Phase II Radiation Therapy Oncology Group trial of conventional radiation therapy followed by treatment with recombinant interferon-β for supratentorial glioblastoma: results of RTOG 9710. Int. J. Radiat. Oncol. Biol. Phys. 2006;66(3):818–824. doi: 10.1016/j.ijrobp.2006.05.021. [DOI] [PubMed] [Google Scholar]
- 136.Aoki T, Takahashi JA, Ueba T, et al. Phase II study of nimustine, carboplatin, vincristine, and interferon-β with radiotherapy for glioblastoma multiforme: experience of the Kyoto Neuro-Oncology Group. J. Neurosurg. 2006;105(3):385–391. doi: 10.3171/jns.2006.105.3.385. [DOI] [PubMed] [Google Scholar]
- 137.D’Amato RJ, Loughnan MS, Flynn E, Folkman J. Thalidomide is an inhibitor of angiogenesis. Proc. Natl Acad. Sci. USA. 1994;91(9):4082–4085. doi: 10.1073/pnas.91.9.4082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Volcker HE. Thalidomide inhibits corneal angiogenesis induced by vascular endothelial growth factor. Graefes Arch. Clin. Exp. Ophthalmol. 1998:236461–236466. doi: 10.1007/s004170050106. [DOI] [PubMed] [Google Scholar]
- 139.Fine HA, Figg WD, Jaeckle K, et al. Phase II trial of the antiangiogenic agent thalidomide in patients with recurrent high-grade gliomas. J. Clin. Oncol. 2000;18(4):708–715. doi: 10.1200/JCO.2000.18.4.708. [DOI] [PubMed] [Google Scholar]
- 140.Son MJ, Kim JS, Kim MH, et al. Combination treatment with temozolomide and thalidomide inhibits tumor growth and angiogenesis in an orthotopic glioma model. Int. J. Oncol. 2006;28(1):53–59. [PubMed] [Google Scholar]
- 141.Baumann F, Bjeljac M, Kollias SS, et al. Combined thalidomide and temozolomide treatment in patients with glioblastoma multiforme. J. Neurooncol. 2004;67(1–2):191–200. doi: 10.1023/b:neon.0000021803.01170.03. [DOI] [PubMed] [Google Scholar]
- 142.Chang SM, Lamborn KR, Malec M, et al. Phase II study of temozolomide and thalidomide with radiation therapy for newly diagnosed glioblastoma multiforme. Int. J. Radiat. Oncol. Biol. Phys. 2004;60(2):353–357. doi: 10.1016/j.ijrobp.2004.04.023. [DOI] [PubMed] [Google Scholar]
- 143.Fine HA, Wen PY, Maher EA, et al. Phase II trial of thalidomide and carmustine for patients with recurrent high-grade gliomas. J. Clin. Oncol. 2003;21(12):2299–2304. doi: 10.1200/JCO.2003.08.045. [DOI] [PubMed] [Google Scholar]
- 144.Lee JI, Itasaka S, Kim JT, Nam DH. Antiangiogenic agent, thalidomide increases the antitumor effect of single high dose irradiation (gamma knife radiosurgery) in the rat orthotopic glioma model. Oncol. Rep. 2006;15(5):1163–1168. [PubMed] [Google Scholar]
- 145.Murphy S, Davey RA, Gu XQ, et al. Enhancement of cisplatin efficacy by thalidomide in a 9L rat gliosarcoma model. J. Neurooncol. 2007;85(2):181–189. doi: 10.1007/s11060-007-9406-3. [DOI] [PubMed] [Google Scholar]
- 146.Rabbani G, Benzil D, Wallam MN, et al. Combination therapy with thalidomide, temozolomide and tamoxifen improves quality of life in patients with malignant astrocytomas. Anticancer Res. 2007;27(4C):2729–2736. [PubMed] [Google Scholar]
- 147.Puduvalli VK, Giglio P, Groves MD, et al. Phase II trial of irinotecan and thalidomide in adults with recurrent glioblastoma multiforme. Neuro-oncology. 2008;10(2):216–222. doi: 10.1215/15228517-2007-060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Fine HA, Kim L, Albert PS, et al. A Phase I trial of lenalidomide in patients with recurrent primary central nervous system tumors. Clin. Cancer Res. 2007;13(23):7101–7106. doi: 10.1158/1078-0432.CCR-07-1546. [DOI] [PubMed] [Google Scholar]
- 149.Liao F, Doody JF, Overholser J, et al. Selective targeting of angiogenic tumor vasculature by vascular endothelial–cadherin antibody inhibits tumor growth without affecting vascular permeability. Cancer Res. 2002;62(9):2567–2575. [PubMed] [Google Scholar]
- 150.Shaheen RM, Ahmad SA, Liu W, et al. Inhibited growth of colon cancer carcinomatosis by antibodies to vascular endothelial and epidermal growth factor receptors. Br. J. Cancer. 2001;85(4):584–589. doi: 10.1054/bjoc.2001.1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Burrows FJ, Thorpe PE. Eradication of large solid tumors in mice with an immunotoxin directed against tumor vasculature. Proc. Natl Acad. Sci. USA. 1993;90(19):8996–9000. doi: 10.1073/pnas.90.19.8996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Okaji Y, Tsuno NH, Saito S, et al. Vaccines targeting tumour angiogenesis – a novel strategy for cancer immunotherapy. Eur. J. Surg. Oncol. 2006;32(4):363–370. doi: 10.1016/j.ejso.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 153.Okaji Y, Tsuno NH, Tanaka M, et al. Pilot study of anti-angiogenic vaccine using fixed whole endothelium in patients with progressive malignancy after failure of conventional therapy. Eur. J. Cancer. 2008;44(3):383–390. doi: 10.1016/j.ejca.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 154.Bergers G, Song S, Meyer-Morse N, Bergsland E, Hanahan D. Benefits of targeting both pericytes and endothelial cells in the tumor vasculature with kinase inhibitors. J. Clin. Invest. 2003;111(9):1287–1295. doi: 10.1172/JCI17929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Nakamizo A, Marini F, Amano T, et al. Human bone marrow-derived mesenchymal stem cells in the treatment of gliomas. Cancer Res. 2005;65(8):3307–3318. doi: 10.1158/0008-5472.CAN-04-1874. [DOI] [PubMed] [Google Scholar]
- 156.Kanehira M, Xin H, Hoshino K, et al. Targeted delivery of NK4 to multiple lung tumors by bone marrow-derived mesenchymal stem cells. Cancer Gene Ther. 2007;14(11):894–903. doi: 10.1038/sj.cgt.7701079. [DOI] [PubMed] [Google Scholar]
- 157.Fenton BM, Paoni SF, Ding I. Effect of VEGF receptor-2 antibody on vascular function and oxygenation in spontaneous and transplanted tumors. Radiother. Oncol. 2004;72(2):221–230. doi: 10.1016/j.radonc.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 158.Ma J, Pulfer S, Li S, Chu J, Reed K, Gallo JM. Pharmacodynamic-mediated reduction of temozolomide tumor concentrations by the angiogenesis inhibitor TNP-470. Cancer Res. 2001;61(14):5491–5498. [PubMed] [Google Scholar]
- 159.Jain RK. Normalizing tumor vasculature with anti-angiogenic therapy: a new paradigm for combination therapy. Nat. Med. 2001;7(9):987–989. doi: 10.1038/nm0901-987. [DOI] [PubMed] [Google Scholar]
- 160.Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307(5706):58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- 161.Kang MK, Kang SK. Tumorigenesis of chemotherapeutic drug-resistant cancer stem-like cells in brain glioma. Stem Cells Dev. 2007;16(5):837–847. doi: 10.1089/scd.2007.0006. [DOI] [PubMed] [Google Scholar]
- 162.Bao S, Wu Q, McLendon RE, et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444(7120):756–760. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- 163.Parthymou A, Kardamakis D, Pavlopoulos I, Papadimitriou E. Irradiated C6 glioma cells induce angiogenesis in vivo and activate endothelial cells in vitro. Int. J. Cancer. 2004;110(6):807–814. doi: 10.1002/ijc.20188. [DOI] [PubMed] [Google Scholar]
- 164.Bertolini F, Mancuso P, Shaked Y, Kerbel RS. Molecular and cellular biomarkers for angiogenesis in clinical oncology. Drug Discov. Today. 2007;12(19–20):806–812. doi: 10.1016/j.drudis.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 165.Onguru O, Gamsizkan M, Ulutin C, Gunhan O. Cyclooxygenase-2 (Cox-2) expression and angiogenesis in glioblastoma. Neuropathology. 2008;28(1):29–34. doi: 10.1111/j.1440-1789.2007.00828.x. [DOI] [PubMed] [Google Scholar]
- 166.Di IA, Grizzi F, Ceva-Grimaldi G, et al. Fractal dimension as a quantitator of the microvasculature of normal and adenomatous pituitary tissue. J. Anat. 2007;211(5):673–680. doi: 10.1111/j.1469-7580.2007.00804.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Grizzi F, Colombo P, Taverna G, et al. Geometry of human vascular system: is it an obstacle for quantifying antiangiogenic therapies? Appl. Immunohistochem. Mol. Morphol. 2007;15(2):134–139. doi: 10.1097/01.pai.0000213105.18569.fa. [DOI] [PubMed] [Google Scholar]
- 168.Yu JL, Rak JW, Coomber BL, Hicklin DJ, Kerbel RS. Effect of p53 status on tumor response to antiangiogenic therapy. Science. 2002;295(5559):1526–1528. doi: 10.1126/science.1068327. [DOI] [PubMed] [Google Scholar]