Abstract
Adoptive cell transfer of tumor-infiltrating lymphocytes (TILs) after lymphodepletion mediates regression in 50% of patients with metastatic melanoma. In vivo persistence and telomere length of the transferred cells correlate with antitumor response. In an attempt to prolong the in vivo survival of the transferred cells, TILs were genetically engineered to produce interleukin (IL)-2. In vitro, these transduced TILs secreted IL-2 while retaining tumor specificity and exhibited prolonged survival after IL-2 withdrawal. In a phase I/II clinical trial, seven evaluable patients received transduced TILs and one patient experienced a partial response associated with in vivo persistence of IL-2-transduced TILs in circulating lymphocytes. An additional five patients received transduced TILs in conjunction with IL-2 administration. Persistence of IL-2-transduced TILs was observed in three patients, including one partial responder. The transgene DNA as well as vector-derived IL-2 mRNA could be detected for 4 months in responding patients. The low response rate in this trial was possibly due to a reduction in telomere length in cells as a result of prolonged in vitro culture. In this study, insertion of the IL-2 gene into antitumor TILs increased their ability to survive after IL-2 withdrawal in vitro but did not increase their in vivo persistence or clinical effectiveness.
INTRODUCTION
Cancer treatment using adoptive immunotherapy requires the isolation of tumor-reactive T lymphocytes ex vivo followed by expansion of these cells to generate high numbers for infusion. The adoptive transfer of tumor-infiltrating lymphocytes (TILs) after a lymphodepleting preparative regimen mediates objective cancer regression in 50% of patients with metastatic melanoma (Dudley et al., 2002a, 2005). Reduction in endogenous lymphocytes that act as competitors for homeostatic cytokines interleukin (IL)-7 and IL-15, as well as a reduction in regulatory T cells, appears to be critical for mediating this high response rate (Antony et al., 2005; Gattinoni et al., 2005a).
Studies of patients who received this adoptive cell therapy showed a significant correlation between persistence of the infused TILs and the likelihood of experiencing an objective response (Robbins et al., 2004). Telomere length of TIL populations was also correlated with prolonged cell persistence, implying that longer telomeres and hence increased proliferative potential was associated with the likelihood of a tumor response (Zhou et al., 2005).
The TILs used in these studies were generated from tumor fragments, using high doses of IL-2 (Dudley et al., 2003). After extensive culture these cells become highly dependent on IL-2 and undergo apoptotic cell death after IL-2 withdrawal (Liu and Rosenberg, 2001). To sustain survival of the transferred TILs after adoptive transfer, exogenous high-dose IL-2 is administered to patients; however, the number of doses that can be tolerated by patients is limited by the toxicity of IL-2 (Lotze et al., 1985; Rosenberg et al., 1989).
Insertion of retrovirus carrying the human IL-2 gene into TILs, with subsequent expression of IL-2, has been shown to prolong their survival in vitro after IL-2 withdrawal while maintaining their tumor specificity and function (Liu and Rosenberg, 2001, 2003). We therefore conducted a clinical trial to determine whether adoptive transfer of IL-2-transduced TILs resulted in increased TIL persistence and more durable responses in patients with metastatic melanoma.
MATERIALS AND METHODS
Patient treatments and clinical assessment
The Surgery Branch IL-2 (SBIL2) human gene therapy protocol was reviewed and approved by the National Institutes of Health institutional biosafety committee (Bethesda, MD); the National Cancer Institute institutional review board (Bethesda, MD); the Recombinant DNA Advisory Committee of the Office of Biotechnology Activities, Office of the Director, National Institutes of Health (Bethesda, MD); and the Center for Biologics Evaluation and Research of the U.S. Food and Drug Administration (Bethesda, MD). Eligibility of treatment has been described previously (Dudley et al., 2005; Morgan et al., 2006) and all patients signed an institutional review board-approved informed consent form. Patient characteristics are shown in Table 1.
Table 1.
Patient Characteristics
| Cohort | Patient no. | Age/sex | Sites of metastasis | Prior therapies |
Clinical outcome |
|---|---|---|---|---|---|
| I | 1 | 42/F | Subcut, LN, intramuscular | IFN, HD-IL2 | NR |
| 2 | 52/F | Subcut, liver, lung | HD-IL2 | NR | |
| 3 | 40/F | Subcut, skin | IFN, GM-CSF, cispl, HD-IL2 | NR | |
| II | 4 | 45/M | Intramuscular, LN | IFN, MDX, HD-IL2, NMA TILs | NR |
| 5a | 56/M | Subcut, lung, LN | IFN, GM-CSF, cispl, HD-IL2 | PR (4 mo) | |
| 6 | 56/M | Liver, LN | MDX, HD-IL2 | NR | |
| 7b | 34/F | Subcut, LN, lung | IFN, HD-IL2 | NR | |
| 8 | 53/M | Lung, LN, stomach | MDX, MART-TCR | TRM | |
| III | 9 | 45/M | Subcut, lung, pelvis, brain | IFN, HD-IL2 | PR (4 mo) |
| 10 | 30/M | Axilla, lung, brain, pelvis, subcut | HD-IL2, radiation | NR/Mixed | |
| 11 | 53/F | Lung, mediastinum | HD-IL2, gp100-TCR | NR/Mixed | |
| 12 | 56/F | Subcut, lung, brain | HD-IL2 | NR | |
| 13 | 64/M | Skin, lung, liver | Radiation, HD-IL2 | NR |
Abbreviations: M indicates male; F, female; subcut, subcutaneous metastasis; LN, lymph node involvement; IFN, interferon treatment; HD-IL2, high-dose IL-2 treatment; cispl, cisplatinum treatment; MDX, anti-CTLA4 mAb treatment; NMA, nonmyeloablation; NR, non-responder; PR, partial responder (duration in months), and TRM, treatment related mortality.
Patient received hematopoietic stem cell transplant at day 17 due to absence of reconstitution.
Patient received second treatment with IL-2 transduced TIL and IL-2 injections.
Patients received nonmyeloablative lymphodepleting chemotherapy as previously described (Dudley et al., 2005), consisting of 2 days of cyclophosphamide (60 mg/kg) followed by 5 days of fludarabine (25 mg/m2). One day after the final dose, patients received IL-2-transduced TILs via intravenous infusion over 30 min.
Hematologic parameters were monitored daily by complete and differential blood counts, and peripheral blood mononuclear cells (PBMCs) and serum were collected. Patient tumor response to treatment was assessed on the basis of the Response Evaluation Criteria in Solid Tumors (RECIST) (Therasse et al., 2000).
Patient TILs and cell lines
TIL cultures for treatment were generated as previously described (Dudley et al., 2003). Briefly, multiple independent cultures were initiated from an excised tumor specimen in a 24-well plate in 2 ml of complete medium [CM: RPMI 1640 (Invitrogen, Carlsbad, CA) supplemented with 10% heat-inactivated human AB serum (Gemini Bio-Products, West Sacramento, CA), 2 mM glutamine, 25 mM HEPES buffer, penicillin (100 U/ml), streptomycin (100 μg/ml) (Biofluids, Rockville, MD), and 0.05 mM 2-mercaptoethanol (Invitrogen) with the addition of IL-2 (6000 IU/ml; Chiron, Emeryville, CA)].
The cell lines used in this study were autologous melanoma tumor lines (when available) as well as melanoma lines 938mel and 888mel (both HLA-A2 negative), and 624mel and 526mel (both HLA-A2 positive). These cell lines as well as Jurkat cells were cultured in RPMI 1640 containing 10% fetal calf serum (FCS) (Gemini Bio-Products) and antibiotics.
SBIL2 retroviral transduction and expansion of TILs
Generation of the SBIL2 retrovirus has been described previously (Liu and Rosenberg, 2003). In brief, the SBIL2 vector, containing the MFG backbone derived from Moloney murine leukemia virus (MMLV) with a cDNA copy of the human IL-2 gene under the control of the 5′ long terminal repeat (LTR) promoter, was pseudotyped in the PG13 packaging cell line, which provides the gibbon ape leukemia virus (GaLV) envelope protein. A stable producer clone (PG13SBIL2#3) was generated that contained three copies of the integrated retroviral IL-2 DNA. Clinical GMP-grade SBIL2 retroviral supernatant was produced by the National Gene Vector Laboratory at Indiana University (Indianapolis, IN). For TIL transduction, 6-well non-tissue-culture plates (Becton Dickinson, Franklin Lakes, NJ) were coated with Retronectin (CH-296, 25 μg/ml in phosphate-buffered saline [PBS], GMP grade; Takara Bio, Otsu, Japan), blocked with PBS–2% human serum albumin (HSA), and preloaded for 4 hr with thawed SBIL2 viral supernatant (5 ml/well) at 32°C and 10% CO2. TILs were added at 3 ml/well for 18–24 hr at 37°C and 5% CO2, transferred to a second set of SBIL2-loaded plates, and cultured for an additional 18–24 hr, after which TILs were harvested and resuspended in fresh medium.
Three cohorts of patients were treated (Table 2). In cohort I, TILs from 3 patients were transduced twice (using 3 × 106 cells per well) during the culture of TILs in CM, followed by one rapid expansion as previously described (Dudley et al., 2003) with OKT3 antibody (30 ng/ml; Ortho Biotech, Bridgewater, NJ), a 200-fold excess of irradiated (4000 rad) allogeneic feeder PBMCs, and IL-2 (6000 IU/ml) in 50/50 medium [50% CM mixed with 50% AIM-V (Invitrogen)], which typically resulted in an ∼1000-fold expansion in 14 days. In cohort II (patients 4–8), patient TILs were transduced during days 7 and 8 of the first rapid expansion, using 3 × 106 cells per well, followed by a second expansion to obtain sufficient cell numbers for treatment. Patients in cohorts I and II did not receive exogenous IL-2 administration. In cohort III (patients 9–13), patient TILs were transduced on days 3 and 4 of the first expansion, using 1 × 106 cells per well, followed by a second expansion, and patients then received concomitant high-dose IL-2 injections (720,000 IU/kg intravenously) every 8 hr until tolerance developed.
Table 2.
Treatment Characteristics
|
Patient no. |
Cells (×1010) |
CD8 (%) |
CD4 (%) |
IL-2 dosesa |
Percent transduction |
IL-2 (pg/ml)b |
IFNγ (pg/ml)c |
Telom (kb) |
||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cohort | PCR | TaqMan | Medium | OKT-3 | Mismatch | Match | ||||||
| I | 1e | 1 | 99 | <1 | — | <20 | ND | 23 | 417 | 47 | 4,080 | 3.8 |
| 2e | 0.3 | 26 | 74 | — | <10 | 7 | 277 | 394 | 636 | 2,390 | ND | |
| 3e | 1 | 98 | <1 | — | 34 | 15 | 47 | 2,171 | 28 | 3,235 | ND | |
| II | 4e | 1 | 99 | <1 | — | 50 | ND | 58 | 144 | 44 | 518 | 4.3 |
| 5e | 4.1 | 58 | 41 | — | 80 | 250 | 4,030d | 10,591d | 46 | 1,815 | ND | |
| 6e | 4.6 | 87 | 12 | — | 70 | 15 | 142 | 3,066 | 91 | 5,265 | 5.5 | |
| 7e | 10 | 99 | <1 | — | 191 | 125 | 127 | 1,346 | 55 | 4,420 | ND | |
| 8e | 4.9 | 97 | 3 | — | 90 | ND | 552 | 3,117 | 227 | 606 | 4.1 | |
| III | 9e | 5 | 96 | 4 | 11 | 173 | 250 | >1,500 | 13,890 | 1,260 | 12,115 | 4.5 |
| 10e | 5.1 | 93 | 7 | 8 | 69 | 45 | 70 | 921 | 685 | 7,550 | 3.2 | |
| 11e | 5.8 | 79 | 21 | 9 | 169 | 190 | 874 | 7,978 | 169 | 9,110 | 3.0 | |
| 12e | 3 | 69 | 31 | 9 | 70 | 49 | 425 | 5,423 | 486 | 3,250 | 5.0 | |
| 13e | 6.4 | 20 | 80 | 4 | ND | 74 | 226 | 3,965 | 60 | 925 | 4.3 | |
Abbreviations: ND, not determined; PCR, polymerase chain reaction; Telom, telomere length in kilobases.
IL-2 administered at 720,000 IU/kg every 8 hr.
In the presence of anti-Tac for 4 days.
IFN-γ production was determined 7 days before infusion; the highest IFN-γ concentration, relative to autologous or HLA-matched tumor, is shown.
These data were obtained from second expension day 8 samples; all others were obtained on day 14.
Patient received second treatment with SBIL2 TILs with IL-2 injections. Clinical outcome, NR.
Cytokine secretion assays
TILs were screened for tumor reactivity by interferon (IFN)-γ secretion in a coculture assay of 1 × 105 TILs with 1 × 105 tumor cells, either autologous if available or HLA-matched tumor cell lines. After 18–24 hr of incubation, supernatants were tested by IFN-γ enzyme-linked immunosorbent assay (ELISA) (Endogen, Cambridge, MA) according to the manufacturer's recommendations. To determine the IL-2 production of TILs, cells were washed three times with CM and plated (2 × 105) in 96-well round-bottom plates (Corning, Corning, NY) in either medium alone, on OKT3-precoated wells, or in coculture with 1 × 105 tumor cells in 50/50 medium. OKT3 (1 μg/ml in PBS, 200 μl/well) was coated for 2 hr at 37°C, followed by a wash with PBS. An anti-CD25 antibody (anti-Tac, which blocks the IL-2Rα chain; a gift from Y. Tagaya, Metabolism Branch, National Cancer Institute) or an IgG2a isotype control antibody (BD Biosciences, San Jose, CA) at 10 μg/ml was added to the cultures to block uptake of IL-2 by the TILs. Supernatants were harvested after 4 days of culture and assayed for IL-2 by ELISA (Endogen). IL-2 release was considered tumor specific when autologous/matched tumor stimulation induced at least twice the amount of IL-2 produced against mismatched tumors and >100 pg/ml.
Proliferation assays
Cells were washed three times and 1 × 105 TILs were cocultured with 1 × 105 irradiated (18,000 rad) tumor cells in 50/50 medium without IL-2 in 96-well round-bottom plates. After 48 hr of incubation, cells were pulsed with 1 μCi of [3H]thymidine (PerkinElmer Life and Analytical Sciences, Waltham, MA) for an additional 18 hr and incorporation was assessed with a Wallac MicroBeta Trilux counter (PerkinElmer Life and Analytical Sciences).
Analysis of gene-marked cells
Patient PBMCs were cryopreserved after Ficoll-Hypaque isolation and genomic DNA was isolated from 1–5 × 106 cells, using a QIAamp DNA blood midi kit (Qiagen, Valencia, CA) or a Maxwell instrument (Promega, Madison, WI) according to the manufacturer's guidelines. Genomic DNA from autopsy samples obtained from patient 8 was isolated with a DNeasy tissue kit (Qiagen).
For semiquantitative detection of the inserted SBIL2 gene, 500 ng of DNA was subjected to a 28-cycle polymerase chain reaction (PCR) as described previously (Liu and Rosenberg, 2003). This PCR method distinguishes the genomic endogenous IL-2 gene from the vector-derived exogenous IL-2 gene on the basis of an intron sequence, resulting in a 311-bp PCR product from the endogenous IL-2 and a 221-bp PCR product from the vector IL-2 (Fig. 1A). PCR products were fractionated on a 4% NuSieve agarose gel (Cambrex Bio Science, Rockland, ME) containing SYBR Green (Cambrex Bio Science). Results were quantified on an LAS-1000 luminescent image analyzer system using ImageGauge software (FUJIfilm, Valhalla, NY) according to the following calculation: % transduced = (signal exogenous IL-2/signal endogenous IL-2) × 100 × 2 (two endogenous IL-2 alleles).
FIG. 1.
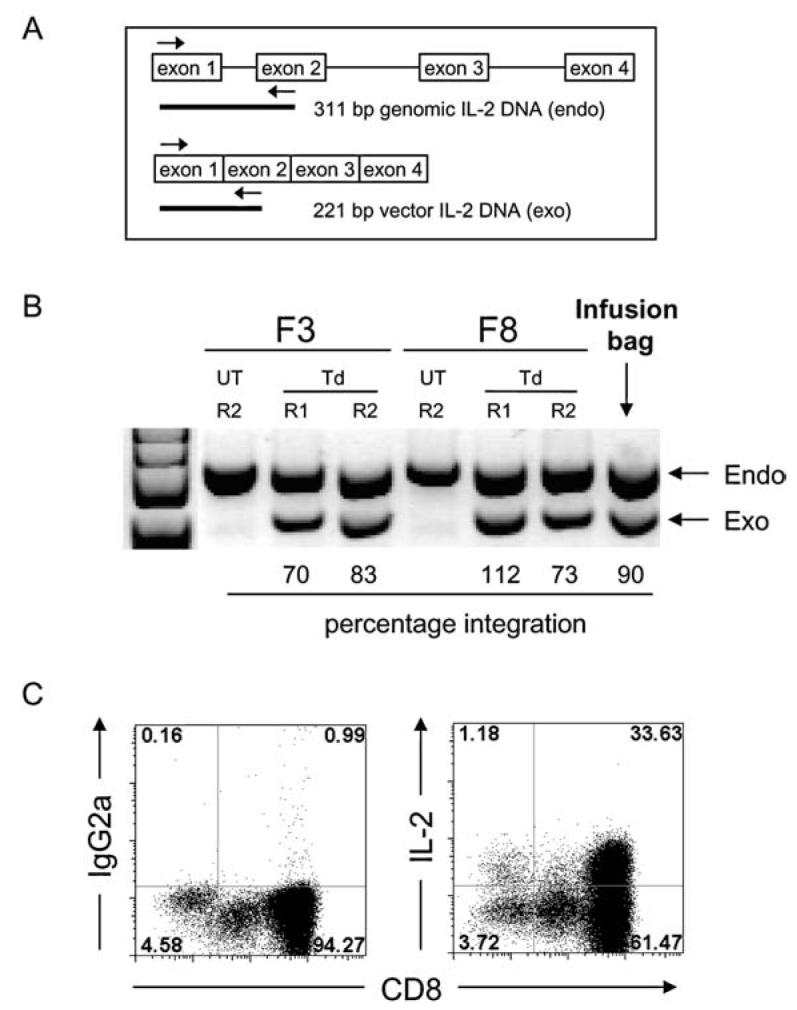
Transduction of patient TILs with a retroviral vector encoding human IL-2 cDNA. (A) The introduced IL-2 gene was confirmed by semiquantitative PCR, in which the primers flank exon 1 in the genomic DNA. The resulting PCR product from the endogenous gene is 311 bp, whereas the exogenous vector-derived IL-2 gene results in a smaller fragment of 221 bp. (B) Percentages of transduced TILs were assessed by quantifying the intensities of the endogenous (endo) IL-2 and the exogenous (exo) IL-2 signals and calculated as described in Materials and Methods. An example of two TIL fragments (F3 and F8) of patient 8 on day 9 of the first rapid expansion (R1) and day 14 of the second rapid expansion (R2) is shown as well as the infusion TIL sample of the combined fragments. (C) IL-2-transduced TILs secrete IL-2 in vitro as determined by intracellular cytokine staining. An infusion sample of transduced TILs from patient 9 is shown after a 6-hr culture in the absence of stimulation and in the presence of an exocytosis inhibitor. Left: Isotype control staining. Right: Percentage of IL-2-producing cells within the CD8+ and CD8− TIL population. UT, untransduced; Td, transduced.
For real-time quantitative PCR analysis (TaqMan; Applied Biosystems, Foster City, CA) 100 ng of DNA was analyzed in an ABI 7500 fast real-time PCR instrument (Applied Biosystems), using a gene-specific assay designed by ABI Assays-by-Design software (Applied Biosystems). This assay detects only the vector-derived IL-2 gene, as the forward primer (SBIL2TQF, 5′-GCCCGAGGCCTGGAT) binds in the vector and the reverse primer (SBIL2TQR, 5′-CAAGACTTAGTGCAATGCAAGACA) binds in the IL-2 gene (FAM-labeled probe, 5′-CATGTACAGGATGCAACTC). An IL-2-transduced Jurkat clone was generated to obtain a standard curve by mixing known ratios of transduced Jurkat cells into untransduced Jurkat cells. To normalize for DNA input, TaqMan β-actin control reagents (Applied Biosystems) were used. For the assessment of IL-2-transduced TILs within autopsy samples of patient 8, a patient-specific standard curve was generated by mixing known ratios of infusion TILs of patient 8 into untransduced peripheral blood lymphocytes (PBLs).
RNA was isolated from 2–5 × 106 PBMCs, using the Stratagene Absolutely RNA miniprep kit (Stratagene, La Jolla, CA). RNA (500 ng) was reverse transcribed and assayed for vector-derived IL-2 mRNA, using the one-step cMasterRTplusPCR kit (Eppendorf North America, Westbury, NY) in a PCR as previously described (Liu and Rosenberg, 2003). The vector-derived IL-2 transcript directed the synthesis of an 885-bp fragment, whereas the β-actin control transcript resulted in a 621-bp fragment.
Flow cytometric analysis
Infusion TILs (1 × 106, IL-2 transduced) were cultured in the presence of BD GolgiPlug (BD Biosciences) for 6 hr in CM without IL-2. Staining for intracellular IL-2 was performed with BD CytoFix/CytoPerm reagents (BD Biosciences), using an anti-phycoerythrin (PE)-conjugated IL-2 monoclonal antibody (mAb) and an isotype control PE-conjugated IgG2a mAb in conjunction with fluorescein isothiocyanate (FITC)-conjugated anti-CD8 and peridinin chlorophyll protein (PerCP)-conjugated anti-CD3 mAb (all from BD Biosciences).
To determine cell subsets in PBMC samples, cells were stained with FITC-conjugated anti-CD4 mAb, PE-conjugated anti-CD8 mAb, and allophycocyanin (APC)-conjugated anti-CD3, as well as anti-CD56–FITC, anti-CD20–PE, and anti-CD3–APC. FoxP3-positive T cells were detected with a FoxP3 staining kit (Ebioscience, San Diego, CA).
Statistical analysis
Significance of variance between groups was evaluated by Wilcoxon rank sum test, with p ≤ 0.05 considered significant.
RESULTS
Characteristics of patients and TIL transductions
Thirteen patients were enrolled in the SBIL2 clinical trial (Table 1). In cohort I (patients 1–3), TILs were transduced during culture before rapidly expanding them for treatment. Analysis of transduction efficiencies in these patients, using a semiquantitative PCR technique (Liu and Rosenberg, 2003) (Fig. 1A), showed that TILs were transduced at a low frequency (mean ± SEM, 21.3 ± 7.0%; Table 2). In cohorts II (patients 4–8) and III (patients 9–13), TILs were therefore transduced during a first rapid expansion when cells were actively dividing, followed by a second stimulation to expand the cells to sufficient numbers for treatment, resulting in an increased transduction efficiency (mean ± SEM, 96.2 ± 24.6% and 120.3 ± 29.3% for cohorts II and III, respectively). In cohort III, patients also received administration of exogenous IL-2 after adoptive cell transfer. Figure 1B shows an example (patient 8) in which two separate TIL fragments (F3 and F8) were transduced and assayed for the IL-2 insert on day 9 of the first rapid expansion (R1) and on day 14 of the second rapid expansion (R2). The additional round of stimulation and expansion had little impact on transduction efficiency. The final infusion bag contained the combined transduced TIL fragments F3 and F8 with an estimated transduction efficiency of 90%. Of note, however, is that these frequencies are calculated on the assumption of a single gene insertion per cell, whereas some cells might contain more than one copy of the IL-2 gene. This explains the occurrence of >100% transduction efficiency as observed in Fig. 1B, fragment F8 R1, as well as in Table 2. Intracellular cytokine staining of transduced TILs on the day of infusion (14 days after the last restimulation) showed that these cells were producing IL-2 in ∼25–35% of TILs (example, patient 9; see Fig. 1C).
Characteristics of IL-2-transduced TILs in vitro
The characteristics of all patient TILs transduced with the IL-2 gene are shown in Table 2. To determine the amount of IL-2 produced by the patient TILs, untransduced and transduced TILs from the day of infusion were cultured for 4 days either in medium alone or stimulated with plate-bound anti-CD3 mAb (OKT3) in the presence of anti-Tac, which blocks the IL-2 receptor and thereby prevents uptake of IL-2 by the TILs. Results from the transduced TILs are listed in Table 2, and Fig. 2A shows a comparison of IL-2 secretion by untransduced and transduced TILs. Gene-modified TILs secreted significantly more IL-2 than did untransduced controls, especially in cohorts II and III when TILs were transduced during the first expansion.
FIG. 2.
IL-2-transduced TILs secrete large amounts of IL-2 and have prolonged survival after IL-2 withdrawal. (A) Patient (pt) TILs, either untransduced (UT) or transduced (Td) with the IL-2 gene from the day of infusion, were thawed and tested the next day in a 4-day assay for IL-2 production. Cells were cultured in medium alone and on anti-CD3 (OKT3 at 1 μg/ml)-coated plates, in the presence of anti-Tac to inhibit uptake of IL-2 by the TILs. IL-2 concentrations in the supernatants were determined by ELISA. Asterisks (*) denote that samples were not determined because of unavailability. (B) Untransduced (UT) and IL-2-transduced (Td) TILs were washed extensively on the day of infusion and placed in medium without IL-2. Viable cells were enumerated every 3–7 days by trypan blue exclusion and cell culture medium was refreshed weekly by replacing half of the spent medium with fresh medium.
To investigate whether IL-2-transduced TILs exhibited prolonged survival in vitro, IL-2 was withdrawn from untransduced and transduced TILs on the day of infusion followed by culture in medium without IL-2. Figure 2B shows that transduced TILs survived far longer than their untransduced counterparts after IL-2 withdrawal. Telomere lengths were also determined for most TILs at the time of infusion and ranged from 3 to 5.5 kb (Table 2).
Tumor specificity of the transduced TILs was confirmed by IFN-γ secretion after stimulation with autologous or HLA-matched and HLA-mismatched tumor cell lines (Table 2 and Fig. 3A). The transduction process did not alter the tumor specificity as transduced TILs secreted similar IFN-γ levels compared with their untransduced counterparts, confirming previous data (Liu and Rosenberg, 2001). Furthermore, it was relevant to investigate the IL-2 production and proliferation of transduced TILs on tumor stimulation as this would resemble the in vivo situation after adoptive transfer of the cells. The majority of transduced TIL samples (six of eight) secreted IL-2 specifically on autologous or matched tumor cell stimulation, whereas little or no IL-2 was produced on mismatched tumor stimulation (Fig. 3B). In contrast, only one of eight untransduced TIL samples was able to secrete IL-2 on matched tumor stimulation (Fig. 3B). Tumor stimulation induced proliferation in six of eight transduced TIL samples (stimulation index, >3) and three of eight untransduced TIL samples (Fig. 3C).
FIG. 3.
IL-2-transduced TILs secrete more IL-2 and proliferate more extensively on autologous or HLA-matched tumors. (A) Tumor specificity was confirmed 7 days before infusion in an overnight coculture of untransduced (UT) or transduced (Td) patient TILs together with autologous tumor cell lines (when available), HLA-matched tumor cell lines, or HLA-mismatched tumor cell lines. Supernatants were tested for IFN-γ by ELISA. (B) On the day of infusion, untransduced or transduced patient TILs were cocultured with autologous tumor cell lines (when available) or with HLA-matched tumor cell lines. As a negative control, HLA-mismatched tumor cell lines were used. All wells received anti-Tac to block IL-2 uptake by the TILs. After 4 days, supernatants were assayed for IL-2 by ELISA. (C) The same TILs as in (B) were tested in a proliferation assay after stimulation with identical tumor targets as in (B). After 48 hr, wells were pulsed with 1 μCi of [3H]thymidine for 18 hr and incorporation was measured as counts per minute.
Treatment and adverse events
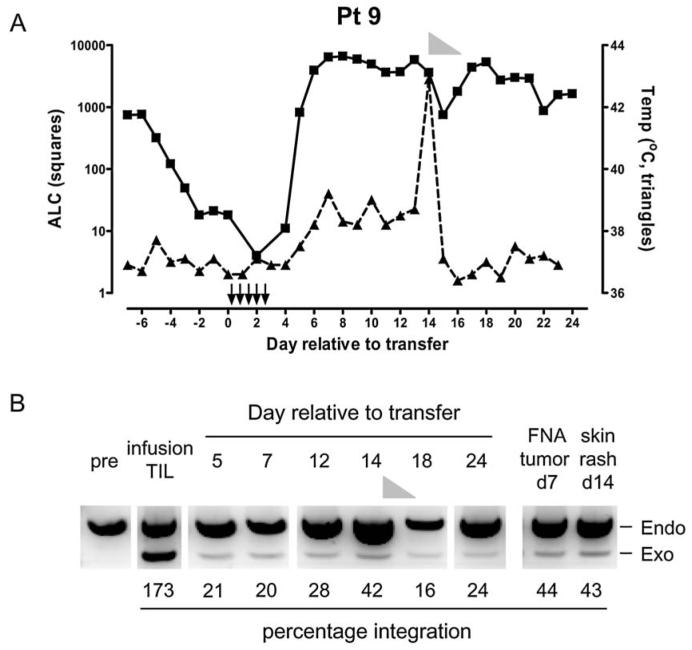
Transduced TILs were infused on day 14 or 15 after the last stimulation, on the day after the nonmyeloablative lymphodepleting regimen (Dudley et al., 2005). To assess the safety of these modified TILs, a dose escalation study was performed in which the first four patients received up to 1 × 1010 cells without additional IL-2 administration (mean, 7.7 × 109; range, 3–10 × 109) followed by four patients receiving up to 1 × 1011 TILs without IL-2 administration (mean, 4.9 × 1010; range, 1–10 × 1010). Patient 5 required treatment with steroids and an autologous hematopoietic stem cell (HSC) infusion on day 17 postinfusion because of the absence of hematological reconstitution and patient 8 developed sepsis shortly after infusion of TILs, to which the patient succumbed on day 8 postinfusion. This sepsis was due to a colonic ulcer, perhaps exacerbated by a previous anti-CTLA-4 treatment (Smith et al., 2007). In the remaining patients from cohorts I and II, infusion of the genetically modified TILs was well tolerated and no major adverse events were observed. The final five patients in cohort III (patients 9–13) received gene-modified TILs along with high-dose IL-2 administration to tolerance in order to give maximal support to the TILs (mean, 5 × 1010; range, 3–6.4 × 1010). An average of 8.2 doses of IL-2 were administered (range, 4–11; Table 2), and adverse events were transient in all patients and consistent with symptoms due to high-dose IL-2 infusion or the conditioning chemotherapy (Lotze et al., 1985; Dudley et al., 2002b, 2005). Patient 9 developed dyspnea during the second week and a high fever (42.9°C) as well as a skin rash on day 14 postinfusion without any indication of infection, requiring steroid treatment that completely resolved all symptoms.
Persistence of IL-2-transduced TILs in vivo
The persistence of transduced TILs within the reconstituted cells was determined by real-time quantitative TaqMan analysis. In patients who did not receive IL-2 administration, the IL-2-transduced TILs persisted at <0.5% in all patients from cohort I and at 1–2% for most patients in cohort II. In cohort II only one patient had levels ≥5% (Fig. 4A and B). In contrast, three of five patients in cohort III, receiving concomitant IL-2 administration, had ≥5% persistent IL-2-transduced TILs within the first 1–4 weeks (Fig. 4C), suggesting that the transduced cells did benefit from the additional IL-2 support. However, long-term persistence was observed only in the two patients who responded to the therapy (patients 5 and 9), and in those patients transduced TILs could be detected up to 4 months (latest time point available) at levels between 10 and 30% (Fig. 4D).
FIG. 4.
Persistence of IL-2-transduced TILs in vivo. DNA extracted from PBMCs was subjected to real-time quantitative TaqMan analysis to determine the percentage of transduced TILs in the circulation of patients at various time points after infusion. (A) Patient samples from cohort I. (B) Patient samples from cohort II. (C) Patient samples from cohort III. (D) Persistence of IL-2-transduced TILs in PBMC samples from the two responding patients, detected for at least 4 months. (E) IL-2 mRNA transcripts from vector-transduced TILs were determined by RT-PCR in PBMCs from patients with persistent TILs at various time points. β-Actin controls were determined for each sample and a representative β-actin is shown from the infusion TILs (1-month time point for patient 5). ND, not determined; PR, partial responder; NR, nonresponder.
To assess whether the transduced cells were actively transcribing mRNA from the inserted IL-2 gene, total RNA was extracted from several patient PBMC samples, including all patients with persistence ≥5% (except patient 11, for whom sufficient samples were not available). Figure 4E shows that vector-derived mRNA could be detected in all infusion TILs, as well as in postinfusion PBMCs from patients with persistent transduced TILs. In one patient (patient 12) with TIL persistence at 3–4%, a faint band was present up to 1 month, whereas in patients with <2% persistence no IL-2 mRNA could be detected. In the two responding patients (patients 5 and 9), the IL-2 transcripts remained unchanged in intensity between 1 and 4 months, indicating that the transduced cells were actively transcribing their transgene.
Serum samples from patients were analyzed for the presence of IL-2 protein. In patients without IL-2 administration, low IL-2 levels could be detected in the responding patient with TIL persistence (patient 5; highest level, 36 pg/ml on day 6) whereas IL-2 levels were undetectable in all other patients (see Supplementary Fig. 1A at www.liebertonline.com/hum). In patients from cohort III, high levels of IL-2 were detected during the first 3–4 days while IL-2 was administered (between 638 and 2,390 pg/ml), which declined rapidly to barely detectable levels in all patients (see Supplementary Fig. 1B at www.liebertonline.com/hum).
Recovery of endogenous lymphocytes
Analysis of the reconstitution of various subsets within the lymphocytic compartment showed that CD8+ T cell and natural killer (NK) cell recovery returned to normal by ∼1–2 months postinfusion, although CD4+ T cell and B cell reconstitution was delayed as noted previously (Dudley et al., 2005) (data not shown). Assessment of the frequency of T cells expressing FoxP3 (as a marker for regulatory T cells) after adoptive cell transfer showed that these cells were present at higher frequencies during the first 2 weeks posttransfer as compared with pretreatment samples, and returned to normal by 1 month postinfusion (Fig. 5A; all FoxP3+ cells were CD4+). However, the calculated numbers of FoxP3+ T cells in the early days after transfer were comparable in most patients to the preinfusion numbers (Fig. 5B). Patients who received high-dose IL-2 (Fig. 5, gray symbols) appeared to have higher frequencies and absolute numbers of FoxP3+ T cells than did patients receiving only IL-2-transduced TILs (Fig. 5, open symbols), indicating that IL-2 administration had a stronger impact on regulatory T cell recovery than the presence of IL-2-producing TILs.
FIG. 5.
Recovery of FoxP3-positive T cells after adoptive cell transfer with IL-2-transduced TILs. (A) Patient PBMCs were assessed by flow cytometry for the presence of FoxP3-positive T cells at various time points before transfer until 2 months post-transfer. Cells were stained with anti-CD3 and anti-CD4 surface markers, permeabilized, and stained for FoxP3 intracellularly. The frequency of FoxP3+ CD3+ T cells is shown. Patients who received transduced TILs without IL-2 are depicted as dashed gray lines with open symbols, whereas patients receiving concomitant IL-2 are represented as unbroken gray lines with gray symbols. The black line represents the mean of all patients. (B) The absolute number of FoxP3+ T cells was calculated for each patient on the basis of the absolute lymphocyte count (ALC) per microliter. Symbols are the same as in (A), and the black line represents the geometric mean.
IL-2-transduced TILs can traffic to lymph nodes as well as skin
A long-standing question after adoptive transfer of TILs has concerned the fate and trafficking of these cells. The unfortunate death due to sepsis of patient 8 on day 8 postinfusion enabled us to investigate the TIL distribution in several organs, because of their gene-marked phenotype. Table 3 shows the presence of IL-2-transduced TILs assessed by TaqMan analysis in autopsy samples from several secondary lymphoid organs, nonlymphoid organs, as well as some tumor metastases in this patient, who did not receive exogenous IL-2 administration. Surprisingly, even though infusion TILs were negative for CD62L and CCR7 (data not shown), transduced TILs were present in many lymph nodes, especially those draining the lung and retroperitoneum. Similarly, transduced TILs could be detected abundantly in the spleen and the lung, but were low in liver and small bowel specimens. Very low frequencies of transduced TILs were present in metastatic tumor samples from patient 8 days after transfer.
Table 3.
Percentage of IL-2 Transduced Tumor-Infiltrating Lymphocytes in Organs of Patient 8
| Organ | Percentagea | Organ | Percentage |
|---|---|---|---|
| Lymph nodes | |||
| Pararectal LN | 0.02 | Periduodenal LN | 0.53 |
| Pararectal LN | 0.21 | R hilar LN | 6.74 |
| R inguinal LN | 0.53 | R hilar LN | 0.82 |
| R supraclavicular LN | 0.14 | R hilar LN | 1.24 |
| Omental LN | 0.90 | L hilar LN | 10.46 |
| Periadrenal LN | 0.99 | L hilar LN | 7.77 |
| Periadrenal LN | 0.64 | L hilar LN | 11.30 |
| Periaortic LN | 0.18 | L hilar LN | 7.72 |
| LN at bifurcation | 0.31 | Carinal LN | 4.49 |
| Retroperitoneal LN | 0.93 | Carinal LN | 5.87 |
| Retroperitoneal LN | 7.63 | Carinal LN | 5.81 |
| Retroperitoneal LN | 0.51 | Peripancreatic LN | 0.49 |
| Para caval LN | 0.85 | Lesser curvature LN | 0.19 |
| Renal hilum LN | 0.33 | Lesser curvature LN | 0.77 |
| Periduodenal LN | 0.61 | Lesser curvature LN | 1.72 |
| Organs | |||
| Spleen | 4.11 | Transverse colon ulcer | 0.48 |
| Spleen | 7.63 | Small bowel | 0.24 |
| Liver | 0.43 | Small bowel | 0.47 |
| Lung | 33.63 | Small bowel | 0.35 |
| Pancreas | 0.04 | ||
| Tumor metastases | |||
| L adrenal nodule | 0.01 | Intragastric nodule | 0.54 |
| Intragastric nodule | 0.06 | Celiac axis nodule | 0.03 |
Abbreviations: L, left; LN, lymph node; R, right.
The percentage of IL-2-transduced TILs was determined on the basis of a patient-specific standard curve obtained from the infusion sample mixed into untransduced PBLs; samples with >0.5% IL-2-transduced TILs were considered positive and are depicted in boldface.
Additional information could be obtained regarding migration of the infused TILs from patient 9, who received transduced TILs with IL-2 administration, resulting in lymphocytosis between days 7 and 10 (Fig. 6A). This patient experienced a skin rash and a pulmonary infiltrate with dyspnea during the second week after transfer, which resolved after a 4-day course of methylprednisone. Tissues were obtained from this patient to investigate the presence of IL-2-transduced TILs. Figure 6B shows the presence of the exogenous IL-2 gene as determined by semiquantitative PCR in PBL samples postinfusion. More importantly, a distinct exogenous IL-2 band was also present in a fine needle aspirate from a subcutaneous tumor taken on day 7 postinfusion. Strikingly, a skin sample obtained from the cutaneous rash on day 14 showed the presence of transduced TILs to a similar extent as in the tumor sample. These data indicate that the transduced TILs were able to home to tumor sites as early as day 7 (in the presence of IL-2 administration), but that in this particular patient these cells homed to skin sites as well.
FIG. 6.
IL-2-transduced TILs can traffic to tumor as well as skin. (A) Patient 9 experienced lymphocytosis between days 7 and 10 (absolute lymphocyte count [ALC], <4700/μl; squares). During the second week after transfer this patient developed a fever (temperature [Temp] in degrees Celsius; triangles) that peaked on day 14 concomitant with a skin rash. Steroid treatment (methylprednisone) is shown as a gray triangle from day 14 to day 18. Arrows depict IL-2 administration (n = 11). (B) PBMC samples as well as a fine needle aspirate (FNA) from a subcutaneous tumor (on day 7) and a sample of skin rash (on day 14) were assayed for the presence of IL-2-transduced TILs. Semiquantitative PCR amplified the endogenous (endo) IL-2 and the exogenous (exo) IL-2 genes within these samples and the percentage integration was calculated. Steroid treatment is indicated as a gray triangle.
Clinical outcome
Of the 13 patients who were treated with IL-2-transduced TILs, 12 were evaluable for tumor response. In seven patients who did not receive any additional IL-2 administration, one partial responder (patient 5; response duration, 4 months) was observed with regression in tumors in the lung, subcutaneous tissues, and lymph nodes. The cohort of five patients receiving transduced TILs along with IL-2 administration resulted in one partial responder (patient 9; response duration, 4 months) with regression in subcutaneous lesions and two patients with mixed responses (patients 10 and 11). The patients with mixed responses had >30% reduction of metastases in the lung for 1 or 2 months whereas other lesions remained stable, followed by the appearance of new tumor metastases in the brain. The low frequency of response (17% overall) observed in this trial is in contrast with the ∼50% response rate in patients receiving non-modified TILs with high-dose IL-2 after lymphodepletion (Dudley et al., 2005). Because of the previously reported direct association between telomere length of the infused TILs and the likelihood of TIL persistence and patient clinical response, we compared the telomere length of the infused TILs in this clinical trial with the telomere lengths of TILs from a historical cohort divided into responding and nonresponding patients (Fig. 7). The mean telomere length ± SEM for IL-2-transduced TILs was 4.2 ± 0.3 kb, whereas responding patient TIL telomere lengths were 6.3 ± 0.3 kb and nonresponding patient TIL telomere lengths were 5.3 ± 0.3 kb (Zhou et al., 2005). The short telomeres in the IL-2-transduced cells provided a possible explanation for the low response rate in this clinical trial.
FIG. 7.

Comparison of telomere lengths of patient TILs. Telomere length of the infusion TILs was assessed for patients within the IL-2-transduced cohort (SBIL2) and compared with responding and nonresponding patients from the historical cohort of patients receiving nonmyeloablative chemotherapy and TILs (NMA R and NMA NR, respectively). The telomere length of the responding patient from the IL-2-transduced TIL cohort (patient 9; patient 5 could not be assessed) is shown as a solid symbol. Statistical analysis was performed by Wilcoxon rank sum test.
DISCUSSION
The study described in this paper investigated the efficacy of adoptive cell transfer therapy using genetically modified TILs engineered to produce IL-2. We hypothesized that TILs, by secreting their own growth factor, would be less likely to depend on exogenous cytokine support and therefore be superior effector cells with which to treat patients with metastatic melanoma. Transduced patient TILs produced high amounts of IL-2 on anti-CD3 and tumor stimulation in vitro. Furthermore, IL-2 withdrawal resulted in prolonged survival of transduced TILs, in some cases up to 4–6 months. In spite of these encouraging results in vitro, there was no increased efficacy of these cells in vivo when compared with unmanipulated TILs infused with high doses of IL-2 in a historical cohort (Dudley et al., 2005). As the number of patients in this trial was small, statistical analysis could not be performed; however, we did not think that results were encouraging enough to include additional patients.
The reasons to transduce the TILs with the IL-2 gene were manifold. IL-2 administration along with adoptive transfer has been shown to be a prerequisite for successful treatment in mouse models (Overwijk et al., 2003), and IL-2 has been shown to support TIL persistence in vivo (Yee et al., 2002). Because TILs are mainly CD8+ T cells, which have a much lower capacity for IL-2 secretion, the need for CD4+ T cell help (Janssen et al., 2003; Antony et al., 2005; Williams et al., 2006) might be circumvented in part by endowing the CD8+ T cell with IL-2 production. Finally, the persistence of TILs (as measured by TCR Vβ clonotype sequencing) in a cohort of patients receiving unmanipulated TILs with high-dose IL-2 was shown to correlate with objective clinical response (Robbins et al., 2004). In that study, 12 of 25 patients (48%) had persistent TILs clonotypes at levels >5% in the circulation at 1–2 months post-transfer. Responding patients had persistent clonotypes in 11 of 13 cases (85%), whereas nonresponding patients had nearly no persistent clonotypes (1 of 12, 4%).
In the current clinical trial with IL-2-transduced TILs, persistence of the cells at 1 month (as measured by IL-2 gene analysis) was observed in only one of seven of patients without IL-2 administration, and in two of five of patients who did receive exogenous IL-2 support. It thus did not appear that providing the TILs with the IL-2 gene improved their capacity to persist. It is interesting, however, that in this trial the two responding patients were the only patients who had persistence of TILs up to 4 months, further supporting our previous findings (Robbins et al., 2004). One question that arises with the insertion of a gene under the control of a retroviral promoter (in this vector the MMLV LTR promoter) is whether any silencing of the gene occurs. This phenomenon has been described in hematopoietic stem cells (Ellis and Yao, 2005), but few data are known for mature human T cells (Lamers et al., 2005; Morgan et al., 2006). Our data on mRNA levels of the introduced IL-2 gene indicated that no major silencing of the gene occurred in the two responding patients with persisting cells, as the intensities of the semiquantitative mRNA bands were similar between 1 and 4 months posttransfer.
The transduced TILs appeared to be able to traffic to lymph nodes as seen in patient 8, and to the tumor and skin in the presence of exogenous IL-2 as observed in patient 9. These data answered a long-standing question concerning whether TILs, which generally do not express any homing markers such as CCR7 or CD62L (Powell et al., 2005), would be able to traffic to lymph nodes (either actively or passively). One report described an alternative route for homing of effector T cells to activated lymph nodes by expression of the chemokine receptor CXCR3 (Guarda et al., 2007), which we also found to be expressed on the infusion TILs (data not shown).
The response rate of this clinical trial did not support our hypothesis that IL-2-transduced TILs would be more effective than unmanipulated TILs (Dudley et al., 2005). At present, we cannot be sure why this approach was unsuccessful, but the explanation is most likely multifactorial. First, it may be due in part to differences between the biodistribution and kinetics of the IL-2 secretion by the transduced TILs in vivo compared with exogenously administrated IL-2. Furthermore, the function of IL-2 is pleiotropic; IL-2 induces T cell proliferation and increases effector function by upregulating molecules such as granzymes and IFN-γ (Lord et al., 2000; Janas et al., 2005). However, IL-2 signaling pathways are also involved in maintaining peripheral tolerance by the induction of regulatory T cells as well as by inducing AICD (activation-induced cell death) in cells stimulated by autoantigens (Waldmann, 2006). Therefore, the IL-2-producing TILs may have stimulated the recovery of regulatory T cells, which constitutively express the high-affinity IL-2 receptor (Ahmadzadeh and Rosenberg, 2006), and thereby suppressed the function of the transferred TILs. However, results from the assessment of regulatory T cells in the circulation (as assessed by FoxP3 expression, because cell numbers were insufficient to perform functional suppression assays) did not support this theory. CD4+FoxP3+ T cells were present at higher frequencies early after TIL infusion, although this increase was associated mainly with the presence of exogenous IL-2 administration. Such an increase in FoxP3+CD4+ T cells has previously been observed in patients receiving IL-2 treatment after lymphodepletion, as well as after high-dose IL-2 administration in lymphoreplete hosts (Zhang et al., 2005; Ahmadzadeh and Rosenberg, 2006). In most patients, the absolute numbers of FoxP3+ T cells returned to normal within the first week after treatment. Furthermore, their frequency did not differ significantly from those in non-modified TILs from patients who also received high-dose IL-2 (data not shown).
More importantly, however, manipulation of the TILs for transduction appeared to have severely hampered the function of these cells in vivo. To transduce these cells with the IL-2 gene and subsequently generate sufficient cells for treatment, either a prolonged culture (for patients 1–3) or a secondary expansion (for patients 4–13) was necessary. Studies in mouse models have revealed the impact of multiple restimulations of T cells on the acquisition of effector function in vitro that paradoxically corresponded with reduced efficacy in vivo (Gattinoni et al., 2005b). Furthermore, infusion TILs from patients responding to TIL therapy were shown to have significantly longer telomere lengths than those from patients who failed to respond to therapy (Zhou et al., 2005). Telomere length is associated with the proliferative capacity of cells and is controlled by the enzyme telomerase, which is upregulated after activation of T cells (Hodes et al., 2002). Prolonged culture of TILs in the absence of restimulation significantly shortened their telomere length, and a second expansion generally reduced the telomere length by about ∼1 kb (J. Zhou, unpublished observations; and data not shown). This could explain the short telomere lengths (3–5.5 kb) that were observed in the TILs of patients in the current trial as compared with those in TILs of responding patients in previous studies (4.1–9.5 kb). These results indicate that the transduction process might have negatively impacted on the proliferative potential of TILs, thereby explaining the low in vivo efficacy of IL-2-transduced TILs.
This study demonstrates that administration of high numbers of IL-2-transduced TILs is a safe and well-tolerated treatment that does not cause additional toxicity as compared with those observed in patients receiving unmanipulated TILs and high-dose IL-2 injections. Alternative strategies will be needed to optimally use gene-modified TILs for improvement of current adoptive cell transfer protocols. Several options are feasible in the near future, including the use of alternative cytokines such as IL-15, which primarily promotes CD8+ memory T cell survival without the concomitant stimulation of regulatory T cells (Hsu et al., 2005; Waldmann, 2006), or the additional insertion of the human telomerase gene (hTERT) to counteract the loss of proliferative potential (Rufer et al., 2001). Alternatively, the use of lentiviral vectors might circumvent the prolonged culture and multiple stimulations of the cells, as they do not require cell proliferation for their transgene integration (Cavalieri et al., 2003). These manipulations can potentially be combined with the T cell receptor transfer technology, in which patient PBLs are engineered to become tumor reactive (Morgan et al., 2006), thus broadening the applicability of this approach for cancer patients with histologies other than melanoma.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the Intramural Research Program of the Center for Cancer Research, National Cancer Institute, National Institutes of Health. The authors thank all members of the TIL laboratory for expert cell culture of patient TILs, as well as the Surgery Branch immunotherapy clinical staff, fellows, and nurses for excellent patient care; Dr. John Wunderlich, Dr. Juhua Zhou, Dr. Jianping Huang, and Mona El-Gamil (Surgery Branch) for help and expertise; Don White for help with data management and statistical analysis; and Y. Tagaya (Metabolism Branch) for providing anti-Tac. The authors also thank K. Cornetta and the National Gene Vector Laboratory for production of the clinical-grade retroviral vector.
Footnotes
AUTHOR DISCLOSURE STATEMENT
No competing financial interests exist.
REFERENCES
- AHMADZADEH M, ROSENBERG SA. IL-2 administration increases CD4+ CD25hi Foxp3+ regulatory T cells in cancer patients. Blood. 2006;107:2409–2414. doi: 10.1182/blood-2005-06-2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ANTONY PA, PICCIRILLO CA, AKPINARLI A, FINKELSTEIN SE, SPEISS PJ, SURMAN DR, PALMER DC, CHAN CC, KLEBANOFF CA, OVERWIJK WW, ROSENBERG SA, RESTIFO NP. CD8+ T cell immunity against a tumor/self-antigen is augmented by CD4+ T helper cells and hindered by naturally occurring T regulatory cells. J. Immunol. 2005;174:2591–2601. doi: 10.4049/jimmunol.174.5.2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAVALIERI S, CAZZANIGA S, GEUNA M, MAGNANI Z, BORDIGNON C, NALDINI L, BONINI C. Human T lymphocytes transduced by lentiviral vectors in the absence of TCR activation maintain an intact immune competence. Blood. 2003;102:497–505. doi: 10.1182/blood-2003-01-0297. [DOI] [PubMed] [Google Scholar]
- DUDLEY ME, WUNDERLICH JR, ROBBINS PF, YANG JC, HWU P, SCHWARTZENTRUBER DJ, TOPALIAN SL, SHERRY R, RESTIFO NP, HUBICKI AM, ROBINSON MR, RAFFELD M, DURAY P, SEIPP CA, ROGERS-FREEZER L, MORTON KE, MAVROUKAKIS SA, WHITE DE, ROSENBERG SA. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002a;298:850–854. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUDLEY ME, WUNDERLICH JR, YANG JC, HWU P, SCHWARTZENTRUBER DJ, TOPALIAN SL, SHERRY RM, MARINCOLA FM, LEITMAN SF, SEIPP CA, ROGERS-FREEZER L, MORTON KE, NAHVI A, MAVROUKAKIS SA, WHITE DE, ROSENBERG SA. A phase I study of nonmyeloablative chemotherapy and adoptive transfer of autologous tumor antigen-specific T lymphocytes in patients with metastatic melanoma. J. Immunother. 2002b;25:243–251. doi: 10.1097/01.CJI.0000016820.36510.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUDLEY ME, WUNDERLICH JR, SHELTON TE, EVEN J, ROSENBERG SA. Generation of tumor-infiltrating lymphocyte cultures for use in adoptive transfer therapy for melanoma patients. J. Immunother. 2003;26:332–342. doi: 10.1097/00002371-200307000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUDLEY ME, WUNDERLICH JR, YANG JC, SHERRY RM, TOPALIAN SL, RESTIFO NP, ROYAL RE, KAMMULA U, WHITE DE, MAVROUKAKIS SA, ROGERS LJ, GRACIA GJ, JONES SA, MANGIAMELI DP, PELLETIER MM, GEA-BANACLOCHE J, ROBINSON MR, BERMAN DM, FILIE AC, ABATI A, ROSENBERG SA. Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J. Clin. Oncol. 2005;23:2346–2357. doi: 10.1200/JCO.2005.00.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELLIS J, YAO S. Retrovirus silencing and vector design: Relevance to normal and cancer stem cells? Curr. Gene Ther. 2005;5:367–373. doi: 10.2174/1566523054546233. [DOI] [PubMed] [Google Scholar]
- GATTINONI L, FINKELSTEIN SE, KLEBANOFF CA, ANTONY PA, PALMER DC, SPIESS PJ, HWANG LN, YU Z, WRZESINSKI C, HEIMANN DM, SURH CD, ROSENBERG SA, RESTIFO NP. Removal of homeostatic cytokine sinks by lymphodepletion enhances the efficacy of adoptively transferred tumor-specific CD8+ T cells. J. Exp. Med. 2005a;202:907–912. doi: 10.1084/jem.20050732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GATTINONI L, KLEBANOFF CA, PALMER DC, WRZESINSKI C, KERSTANN K, YU Z, FINKELSTEIN SE, THEORET MR, ROSENBERG SA, RESTIFO NP. Acquisition of full effector function in vitro paradoxically impairs the in vivo antitumor efficacy of adoptively transferred CD8+ T cells. J. Clin. Invest. 2005b;115:1616–1626. doi: 10.1172/JCI24480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUARDA G, HONS M, SORIANO SF, HUANG AY, POLLEY R, MARTIN-FONTECHA A, STEIN JV, GERMAIN RN, LANZAVECCHIA A, SALLUSTO F. L-selectin-negative CCR7− effector and memory CD8+ T cells enter reactive lymph nodes and kill dendritic cells. Nat. Immunol. 2007;8:743–752. doi: 10.1038/ni1469. [DOI] [PubMed] [Google Scholar]
- HODES RJ, HATHCOCK KS, WENG NP. Telomeres in T and B cells. Nat. Rev. Immunol. 2002;2:699–706. doi: 10.1038/nri890. [DOI] [PubMed] [Google Scholar]
- HSU C, HUGHES MS, ZHENG Z, BRAY RB, ROSENBERG SA, MORGAN RA. Primary human T lymphocytes engineered with a codon-optimized IL-15 gene resist cytokine withdrawal-induced apoptosis and persist long-term in the absence of exogenous cytokine. J. Immunol. 2005;175:7226–7234. doi: 10.4049/jimmunol.175.11.7226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JANAS ML, GROVES P, KIENZLE N, KELSO A. IL-2 regulates perforin and granzyme gene expression in CD8+ T cells independently of its effects on survival and proliferation. J. Immunol. 2005;175:8003–8010. doi: 10.4049/jimmunol.175.12.8003. [DOI] [PubMed] [Google Scholar]
- JANSSEN EM, LEMMENS EE, WOLFE T, CHRISTEN U, VON HERRATH MG, SCHOENBERGER SP. CD4+ T cells are required for secondary expansion and memory in CD8+ T lymphocytes. Nature. 2003;421:852–856. doi: 10.1038/nature01441. [DOI] [PubMed] [Google Scholar]
- LAMERS CH, GRATAMA JW, POUW NM, LANGEVELD SC, KRIMPEN BA, KRAAN J, STOTER G, DEBETS R. Parallel detection of transduced T lymphocytes after immunogene therapy of renal cell cancer by flow cytometry and real-time polymerase chain reaction: Implications for loss of transgene expression. Hum. Gene Ther. 2005;16:1452–1462. doi: 10.1089/hum.2005.16.1452. [DOI] [PubMed] [Google Scholar]
- LIU K, ROSENBERG SA. Transduction of an IL-2 gene into human melanoma-reactive lymphocytes results in their continued growth in the absence of exogenous IL-2 and maintenance of specific antitumor activity. J. Immunol. 2001;167:6356–6365. doi: 10.4049/jimmunol.167.11.6356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIU K, ROSENBERG SA. Interleukin-2-independent proliferation of human melanoma-reactive T lymphocytes transduced with an exogenous IL-2 gene is stimulation dependent. J. Immunother. 2003;26:190–201. doi: 10.1097/00002371-200305000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LORD JD, McINTOSH BC, GREENBERG PD, NELSON BH. The IL-2 receptor promotes lymphocyte proliferation and induction of the c-myc, bcl-2, and bcl-x genes through the trans-activation domain of Stat5. J. Immunol. 2000;164:2533–2541. doi: 10.4049/jimmunol.164.5.2533. [DOI] [PubMed] [Google Scholar]
- LOTZE MT, MATORY YL, ETTINGHAUSEN SE, RAYNER AA, SHARROW SO, SEIPP CA, CUSTER MC, ROSENBERG SA. In vivo administration of purified human interleukin 2. II. Half life, immunologic effects, and expansion of peripheral lymphoid cells in vivo with recombinant IL 2. J. Immunol. 1985;135:2865–2875. [PubMed] [Google Scholar]
- MORGAN RA, DUDLEY ME, WUNDERLICH JR, HUGHES MS, YANG JC, SHERRY RM, ROYAL RE, TOPALIAN SL, KAMMULA US, RESTIFO NP, ZHENG Z, NAHVI A, DE VRIES CR, ROGERS-FREEZER LJ, MAVROUKAKIS SA, ROSENBERG SA. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science. 2006;314:126–129. doi: 10.1126/science.1129003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OVERWIJK WW, THEORET MR, FINKELSTEIN SE, SURMAN DR, DE JONG LA, VYTH-DREESE FA, DELLEMIJN TA, ANTONY PA, SPIESS PJ, PALMER DC, HEIMANN DM, KLEBANOFF CA, YU Z, HWANG LN, FEIGENBAUM L, KRUISBEEK AM, ROSENBERG SA, RESTIFO NP. Tumor regression and autoimmunity after reversal of a functionally tolerant state of self-reactive CD8+ T cells. J. Exp. Med. 2003;198:569–580. doi: 10.1084/jem.20030590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POWELL DJ, JR., DUDLEY ME, ROBBINS PF, ROSENBERG SA. Transition of late-stage effector T cells to CD27+ CD28+ tumor-reactive effector memory T cells in humans after adoptive cell transfer therapy. Blood. 2005;105:241–250. doi: 10.1182/blood-2004-06-2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROBBINS PF, DUDLEY ME, WUNDERLICH J, EL GAMIL M, LI YF, ZHOU J, HUANG J, POWELL DJ, JR., ROSENBERG SA. Cutting edge: Persistence of transferred lymphocyte clonotypes correlates with cancer regression in patients receiving cell transfer therapy. J. Immunol. 2004;173:7125–7130. doi: 10.4049/jimmunol.173.12.7125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROSENBERG SA, LOTZE MT, YANG JC, AEBERSOLD PM, LINEHAN WM, SEIPP CA, WHITE DE. Experience with the use of high-dose interleukin-2 in the treatment of 652 cancer patients. Ann. Surg. 1989;210:474–484. doi: 10.1097/00000658-198910000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUFER N, MIGLIACCIO M, ANTONCHUK J, HUMPHRIES RK, ROOSNEK E, LANSDORP PM. Transfer of the human telomerase reverse transcriptase (TERT) gene into T lymphocytes results in extension of replicative potential. Blood. 2001;98:597–603. doi: 10.1182/blood.v98.3.597. [DOI] [PubMed] [Google Scholar]
- SMITH FO, GOFF SL, KLAPPER JA, LEVY C, ALLEN T, MAVROUKAKIS SA, ROSENBERG SA. Risk of bowel perforation in patients receiving interleukin-2 after therapy with anti-CTLA 4 monoclonal antibody. J. Immunother. 2007;30:130. doi: 10.1097/01.cji.0000211334.06762.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THERASSE P, ARBUCK SG, EISENHAUER EA, WANDERS J, KAPLAN RS, RUBINSTEIN L, VERWEIJ J, VAN GLABBEKE M, VAN OOSTEROM AT, CHRISTIAN MC, GWYTHER SG. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J. Natl. Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- WALDMANN TA. The biology of interleukin-2 and interleukin-15: Implications for cancer therapy and vaccine design. Nat. Rev. Immunol. 2006;6:595–601. doi: 10.1038/nri1901. [DOI] [PubMed] [Google Scholar]
- WILLIAMS MA, TYZNIK AJ, BEVAN MJ. Interleukin-2 signals during priming are required for secondary expansion of CD8+ memory T cells. Nature. 2006;441:890–893. doi: 10.1038/nature04790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YEE C, THOMPSON JA, BYRD D, RIDDELL SR, ROCHE P, CELIS E, GREENBERG PD. Adoptive T cell therapy using antigen-specific CD8+ T cell clones for the treatment of patients with metastatic melanoma: In vivo persistence, migration, and antitumor effect of transferred T cells. Proc. Natl. Acad. Sci. U.S.A. 2002;99:16168–16173. doi: 10.1073/pnas.242600099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHANG H, CHUA KS, GUIMOND M, KAPOOR V, BROWN MV, FLEISHER TA, LONG LM, BERNSTEIN D, HILL BJ, DOUEK DC, BERZOFSKY JA, CARTER CS, READ EJ, HELMAN LJ, MACKALL CL. Lymphopenia and interleukin-2 therapy alter homeostasis of CD4+CD25+ regulatory T cells. Nat. Med. 2005;11:1238–1243. doi: 10.1038/nm1312. [DOI] [PubMed] [Google Scholar]
- ZHOU J, SHEN X, HUANG J, HODES RJ, ROSENBERG SA, ROBBINS PF. Telomere length of transferred lymphocytes correlates with in vivo persistence and tumor regression in melanoma patients receiving cell transfer therapy. J. Immunol. 2005;175:7046–7052. doi: 10.4049/jimmunol.175.10.7046. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.