Abstract
Purpose
To identify prognostic factors associated with survival beyond 4 years and overall response in patients with metastatic melanoma treated with high-dose bolus i.v. interleukin-2 (IL-2) given either alone or in combination with a variety of melanoma vaccines.
Study Design
684 consecutive patients with metastatic melanoma received high-dose bolus i.v. IL-2 either alone or in conjunction with a variety of melanoma vaccines. Treatments occurred between August 1, 1985 and January 1, 2006.
Results
The overall objective response rate was 13% for patients receiving IL-2 alone and 16% for patients who received IL-2 with vaccine. In patients treated with IL-2 alone (n = 305) and IL-2 with vaccine (n = 379), having an objective response was associated with survival beyond 4 years (P < 0.0001). No pretreatment factors could be identified that were strongly associated with increased rate of objective response or long-term survival in patients receiving IL-2 alone. In patients receiving IL-2 with vaccines, there were increased response rates in patients with s.c. or cutaneous disease only and lower response rates with visceral disease only. Patients who received the gp100:209-217(210M) peptide plus IL-2 showed a strong trend to increased objective responses compared with IL-2 alone (22% versus 12.8%; P = 0.01) and also compared with patients who received a variety of vaccines that did not include this immunogenic peptide (13.8%; P = 0.009).
Conclusion
IL-2 can produce a modest response rate in patients with metastatic melanoma including patients with durable complete responses. S.c. or cutaneous disease only and vaccination with gp100:209-217(210M) peptide was associated with significant increase in response rates.
Melanoma is the fifth most common cancer among men and the fourth most common cancer in women in the United States. For the year 2007, the American Cancer Society estimates there will be 59,940 new cases and 8,110 deaths from melanoma in the United States (1).
Dacarbazine is the only chemotherapeutic drug approved by the Food and Drug Administration for patients with metastatic melanoma and results in response rates between 10% and 15% (2); however, there are very few durable responses and most patients experience disease relapse after a few weeks or months. In 1998, the Food and Drug Administration approved high-dose bolus interleukin-2 (IL-2) based on its ability to mediate durable complete responses in patients with metastatic melanoma (3, 4).
Despite the extensive use of IL-2, no reliable prognostic variables have been identified that are associated with improved response rates or survival. The Surgery Branch of the National Cancer Institute has the largest single institution series of patients treated with high-dose IL-2 and this provided us with an opportunity to analyze prognostic and treatment-related factors in 684 consecutive patients with metastatic melanoma treated with high-dose IL-2 either alone or in conjunction with melanoma vaccines.
Patients and Methods
Patients
Consecutive patients (n = 684) with metastatic melanoma treated with high-dose bolus i.v. IL-2 (Novartis) alone (n = 305) or with vaccines (n = 379) in the Surgery Branch of the National Cancer Institute between August 1985 and January 2006 were included in the study. All patients signed informed consent before enrollment on the protocols. The protocols were approved by the Institutional Review Board at the National Cancer Institute. Median potential follow-up for all patients was 7.4 years (8.3 years for IL-2 alone and 7.3 years for IL-2 plus vaccine).
Patients treated with polyethylene glycol–modified IL-2, those treated with s.c. IL-2, or those treated with IL-2 before referral to the National Cancer Institute were excluded. Patients were also excluded if they received IL-2 along with chemotherapy, radiotherapy, monoclonal antibodies, other cytokines, or any cell-based therapy. Peptides, DNA, poxviruses, adenoviruses, vaccinia viruses, or a combination of these were used in the patients treated in the IL-2 plus vaccine cohort. Many of these trials have been reported separately, each with small numbers of patients (5–8).
Translational Relevance
High-dose IL-2 can produce a modest response rate in patients with metastatic melanoma including some patients with durable complete responses. This single institution study of patients treated with high-dose bolus i.v. IL-2 alone (n = 305) or in conjunction with vaccines (n = 379) represents the largest published series on the treatment of consecutive patients with metastatic melanoma with IL-2. The aim was to identify factors predictive of response or survival in patients treated with IL-2. No single pretreatment factor was predictive of response or survival in patients treated with IL-2 alone. In patients receiving IL-2 with vaccines, there were increased response rates in patients with s.c. or cutaneous disease only and lower response rates with visceral disease only. Patients treated with IL-2 plus the highly immunogenic gp209-217(210M) peptide vaccine showed improvement in the overall response rate compared with patients not receiving IL-2 with vaccines (22% versus 12.8% P = 0.01) and also compared with patients who received a variety of vaccines that did not include this immunogenic peptide (13.8%; P = 0.009). Although these data are highly suggestive, these require confirmation in a randomized trial.
Histologic confirmation of melanoma by the Pathology Department at the NIH was required before enrollment. Patients were required to have measurable metastatic disease. An interval of at least 1 month from any systemic treatment was required before enrollment. Patients with significant cardiac, respiratory, or kidney disease were not enrolled in the trials.
Treatment
Patients were treated with recombinant high-dose IL- 2 at a dose of 720, 000 IU/kg suspended in 50 mL of 5% albumin solution and given over 15 min. Dosing during each cycle of therapy was done every 8 h for a maximum of 12 to 15 doses. Dosing was stopped if there was development of grade 3/4 toxicity not readily reversed by supportive therapy, mental status changes, or for patient refusal.
The patients treated with IL-2 alone received the first dose of the second cycle ~14 days after the first dose of the first cycle. Patients who received IL-2 in conjunction with vaccine received treatment every 3 weeks; vaccine was administered before the first dose of IL-2 in each cycle. Two cycles of treatment constituted one course.
Patients were enrolled with the intention to complete two cycles and then have an evaluation 2 months after the start of therapy. Not all patients were able to complete a second cycle of therapy due to toxicity or disease progression, but all enrolled patients are included in this analysis. Patients with stable disease or evidence of tumor regression received additional courses of treatment.
Response evaluation
Before enrollment on protocol, metastatic disease was documented using computerized axial tomography or magnetic resonance imaging of the brain, chest, abdomen, and pelvis. Positron emission tomography, radionuclide bone scans, or photographs were done if deemed appropriate. Lactate dehydrogenase levels before enrollment were used to subcategorize patients according to the current American Joint Commission on Cancer classification (9).
Before 2005, response was determined by WHO criteria, in which the products of the maximum perpendicular diameters of all tumors before and following treatment were compared (10). An objective response partial response was defined as ≥50% decrease in the sum of the products of the maximum perpendicular diameters of all evaluable tumors lasting at least 1 month with no new or enlarging tumors. A complete response was defined as the disappearance of all evaluable lesions lasting >1 month.
Subsequently, the RECIST criteria was used, in which a partial response is defined as a ≥30% decrease in the sum of the longest diameters with no new lesions appearing (11). A complete response is defined as disappearance of all the evaluable lesions, lasting for >1 month from the time at which the disappearance was first documented.
Patients not meeting the criteria for partial or complete response were classified as nonresponders.
Patients who had a partial response to IL-2 therapy and subsequently had surgical resection of any residual disease as part of their management continued to be classified as partial responders despite no radiographic evidence of disease.
Statistical analysis
Analyses were done separately within IL-2 and IL-2 plus vaccine patient groups. Dichotomous variables were evaluated for their association with response or survival >4 years using a Fisher’s exact test. Continuously measured variables (or variables that could be treated as if they were continuous) were evaluated for their association with response or survival >4 years by a Wilcoxon rank-sum test. Because this was an exploratory analysis, and many tests are being done within IL-2 and IL-2 plus vaccine patient groups, a more stringent level for results was applied to consider a univariate association to be statistically significant. In addition, to determine whether a set of pretreatment variables may be taken together to predict whether a patient will respond clinically or survive beyond 4 years, a logistic regression model was used, incorporating baseline or pretreatment factors found in univariate analyses to be associated with at least a trend toward an association with the outcome (P < 0.10, approximately, separately for both endpoints, for IL-2 and IL-2 plus vaccine). That analysis was intended to suggest factors worth consideration for determining if a patient is likely to be responsive to IL-2 alone or to IL-2 plus vaccine or to live beyond 4 years.
Based on the observed pattern of overall survival, we were interested in exploring factors associated with survival in excess of the duration of survival for patients with a complete response. None of the patients who had a complete response experienced a relapse after 3 years; thus, to be conservative, survival beyond 4 years was chosen as the threshold to evaluate factors associated with long-term survival. From the 684 patients on study, 644 were usable for the analysis of long-term survival at 4 years; the other 40 were enrolled on trial after January 1, 2003 and were still alive as of December 2006; thus, their survival status at 4 years could not be determined.
Duration of response or survival was determined from the date patients received their first dose of IL-2.
Given the varying degrees of dependence and independence of the variables that were examined, no formal adjustments were done for multiple comparisons; however, P < 0.005 was felt to be sufficient to consider an association to be interpreted as being statistically significant. Instances in which 0.005 < P < 0.05 will result in that variable being considered as having a trend toward an association (with greater emphasis on the lower P values within that range). All P values are two-sided.
Results
Patient demographics
Three hundred five patients received IL-2 alone and 379 patients received IL-2 in combination with vaccines (Table 1). There were 432 men (63%) and 252 (37%) women. The mean age of the patients was 45 years; the range was 17 to 69 years. Eighty-three percent of patients had an Eastern Cooperative Oncology Group score of 0 and 15% had an Eastern Cooperative Oncology Group of 1. Twenty-four percent of the patients had received prior chemotherapy and 18% had received prior radiation therapy. Seventy percent of patients had received two or more prior therapies.
Table 1.
Patients: demographic characteristics
| Variable | Trait | IL-2, n (%) | IL-2 + vaccine, n (%) | Total |
|---|---|---|---|---|
| Total | Patients | 305 (100) | 379 (100) | 684 (100) |
| Sex | Male | 190 (62) | 242 (64) | 432 (63) |
| Female | 115 (38) | 137 (36) | 252 (37) | |
| Race | Asian | 1 (0) | 0 (0) | 1 (0) |
| Black | 3 (1) | 2 (1) | 5 (1) | |
| White | 301 (99) | 377 (99) | 678 (99) | |
| Age (y) | 11–20 | 5 (2) | 4 (1) | 9 (1) |
| 21–30 | 30 (10) | 29 (8) | 59 (9) | |
| 31–40 | 76 (25) | 74 (20) | 150 (22) | |
| 41–50 | 88 (29) | 131 (35) | 219 (32) | |
| 51–60 | 79 (26) | 101 (27) | 180 (26) | |
| 61–70 | 27 (9) | 39 (10) | 66 (10) | |
| ≥70 | 0 (0) | 1 (0) | 1 (0) | |
| Eastern Cooperative Oncology Group score | 0 | 242 (79) | 328 (87) | 570 (83) |
| 1 | 57 (19) | 49 (13) | 106 (15) | |
| 2 | 6 (2) | 2 (1) | 8 (1) | |
| Prior therapy | None | 2 (1) | 0 (0) | 2 (0) |
| Surgery | 300 (98) | 379 (100) | 679 (99) | |
| Chemotherapy | 69 (23) | 97 (26) | 166 (24) | |
| Radiotherapy | 50 (16) | 76 (20) | 126 (18) | |
| Hormonal | 4 (1) | 32 (8) | 36 (5) | |
| Immunotherapy | 185 (61) | 273 (72) | 458 (67) | |
| Any 2 or more | 214 (70) | 301 (79) | 515 (75) | |
| Any 3 or more | 72 (24) | 133 (35) | 205 (30) | |
| Response | Complete response | 12 (4) | 13 (3) | 25 (4) |
| Partial response | 27 (9) | 46 (12) | 73 (11) | |
| None | 260 (85) | 317 (84) | 577 (84) |
Objective responses and survival
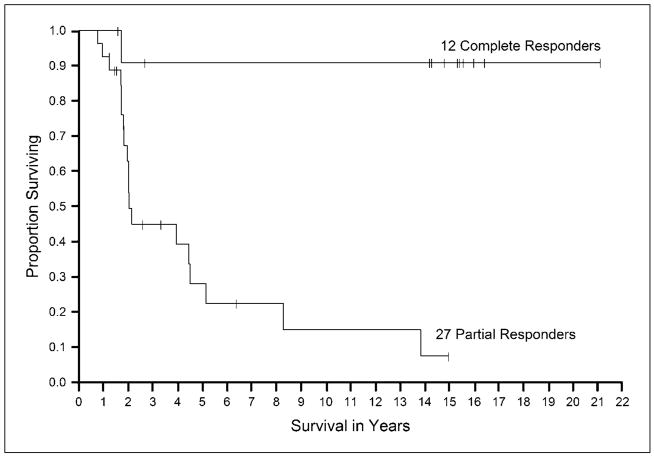
The objective response rates in patients who received IL-2 alone or in conjunction with vaccine were 13% and 16%, respectively (Table 2). Complete responses were seen in 4% and 3% of patients, respectively. In patients who received IL-2 alone, the median duration of partial and complete responses was 24 and >176 months, respectively. For patients treated with IL-2 plus vaccine, the median duration of partial and complete responses were 9.4 and 7.8 months. Eleven of 13 of patients who achieved a complete response to IL-2 alone had ongoing responses at 17 to 253 months compared with only 4 of 13 patients with ongoing responses in the IL-2 plus vaccine group (P = 0.015). This difference in durability of response rates may be due to the different schedules of IL-2 administration between the two groups. None of the patients in the IL-2 alone group who experienced a complete response relapsed after being disease free for 1.5 years.
Table 2.
Duration of response (months) in patients with metastatic melanoma treated using high-dose bolus IL-2
| Treatment | Total | Complete response | Partial response |
|---|---|---|---|
| IL-2 | 305 | n = 13 (4%) | n = 26 (9%) |
| 253+, 197+, 192+, 192+, 185+,181+, 172+, 171+, 70+, 31+, 17+, 16, 12 | 36, 32, 30, 23+, 22, 20, 19, 18+, 16, 15, 14+,13, 11, 11, 10, 9, 8, 8, 8, 8, 6, 6, 4, 4, 4, 3 | ||
| IL-2 + vaccine | 379 | n = 13 (3%) | n = 46 (12%) |
| 120+, 107+, 96+, 43+, 19, 17, 7, 7, 7, 6, 5, 5, 2 | 117, 84+, 75, 74+, 45, 36, 31, 23, 18, 17, 16, 15, 14, 14, 14, 13, 12, 12, 12, 11, 10, 9, 9, 9, 8, 7, 7, 6, 6, 6, 6, 5, 5, 5, 5, 5, 4, 4, 4, 4, 4, 4, 4, 4, 3, 2 |
NOTE: + indicates ongoing response as of August 1, 2006.
When results were evaluated according to categories based on the time of enrollment over the course of the 20-year period of the study, there was no effect on the period of enrollment and response (data not shown).
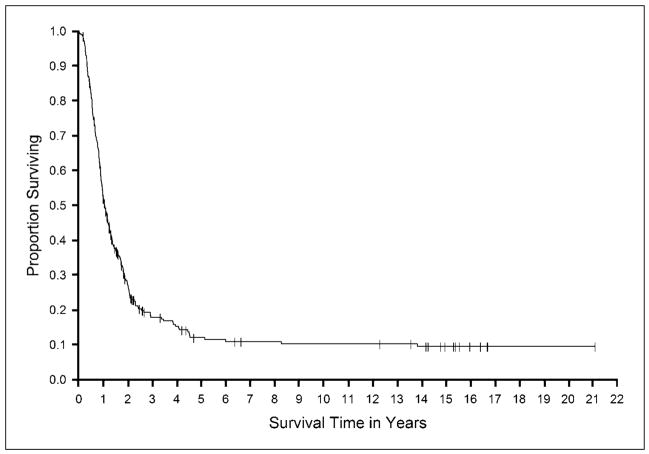
Figures 1 and 2 show the survival in all patients and in the responding patients, respectively, in the group treated with IL-2 alone. In patients treated with IL-2 plus vaccines, overall survival for the patients is shown in Fig. 3 and survival of the responding patients is shown in Fig. 4. The median survival in each of the groups was 12.8 and 14.2 months, respectively. The survival at 2 and 4 years was 27% and 16% for the IL-2 group and was 28% and 13% for the IL-2 plus vaccine group.
Fig. 1.
Kaplan-Meier curve of overall survival for all patients treated with IL-2 alone.
Fig. 2.
Kaplan-Meier curve of overall survival for all responding patients treated with IL-2 alone.
Fig. 3.
Kaplan-Meier curve of overall survival for all patients treated with IL-2 with vaccine.
Fig. 4.
Kaplan-Meier curve of over all survival of responding patients treated with IL-2 with vaccine.
Analysis of factors associated with survival beyond 4 years
Univariate analyses of dichotomous factors and their association with survival >4 years are presented separately for patients treated with IL-2 and IL-2 plus vaccine in Tables 3, Table 4 and 5. By logistic regression, in patients treated with IL-2 alone, having an objective response to IL-2 was associated with survival beyond 4 years [odds ratio (OR), 9.96; 95% confidence interval (95% CI), 4.74–20.93; P < 0.0001]. Other variables with at least a trend toward an association with survival included not having prior radiation (P = 0.038), having Eastern Cooperative Oncology Group status 0 (P = 0.034), higher eosinophil peak (P = 0.02), and higher platelet peak (P = 0.02). In patients treated with IL-2 plus vaccines, objective response (OR, 5.54; 95% CI, 2.69–11.38; P = 0.0001) and number of sites (OR, 0.57; 95% CI, 0.40–0.79; P = 0.0009) were associated with long survival in a logistic regression model. Peak eosinophils (P = 0.03), baseline versus peak eosinophils (P = 0.02), and baseline versus nadir WBC (P = 0.009) are also potentially associated with long-term survival.
Table 3.
Pretreatment factors: association with prognosis (two-tailed P values)
| IL-2 alone
|
IL-2 + vaccine
|
|||
|---|---|---|---|---|
| Response | Survival* | Response | Survival* | |
| Sex | ≥0.1 | ≥0.1 | ≥0.1 | ≥0.1 |
| Race | ≥0.1 | ≥0.1 | ≥0.1 | ≥0.1 |
| Eastern Cooperative Oncology Group score (0 vs 1–2) | ≥0.1 | 0.03 | ≥0.1 | ≥0.1 |
| Prior treatment | ||||
| Immunotherapy | ≥0.1 | ≥0.1 | ≥0.1 | ≥0.1 |
| IFN-α | ≥0.1 | ≥0.1 | ≥0.1 | ≥0.1 |
| Radiotherapy | ≥0.1 | 0.04 | 0.01† | ≥0.1 |
| Chemotherapy | ≥0.1 | ≥0.1 | ≥0.1 | ≥0.1 |
| Hormonal therapy | ≥0.1 | ≥0.1 | ≥0.1 | ≥0.1 |
| Baseline laboratory values | ||||
| WBC count | ≥0.1 | ≥0.1 | ≥0.1 | ≥0.1 |
| Lymphocytes count | ≥0.1 | ≥0.1 | 0.05† | ≥0.1 |
| Eosinophil count | 0.02† | ≥0.1 | 0.08 | ≥0.1 |
| Platelet count | ≥0.1 | ≥0.1 | ≥0.1 | ≥0.1 |
| Bilirubin | ≥0.1 | ≥0.1 | ≥0.1 | ≥0.1 |
| Creatinine | ≥0.1 | ≥0.1 | 0.04 | ≥0.1 |
| TSH | ≥0.1 | ≥0.1 | ≥0.1 | ≥0.1 |
| FT4 | ≥0.1 | ≥0.1 | ≥0.1 | ≥0.1 |
| No. metastatic sites | 0.02 | 0.06 | <0.0001 | <0.0001 |
Survival at 4 y.
Lower in responders.
Table 4.
| (A) Sites of metastases association with response rates
| |||||||
|---|---|---|---|---|---|---|---|
| IL-2 alone
|
IL-2 + vaccine
|
||||||
| Total | % Response | P* | Total | % Response | P* | ||
| Sites of disease | |||||||
| S.c. and/or cutaneous alone | 20 | 33 | ≥0.1 | 28 | 46 | 0.0005† | |
| S.c. and/or cutaneous alone and lymph node | 19 | 21 | ≥0.1 | 26 | 23 | ≥0.1 | |
| Lymph node alone | 29 | 21 | ≥0.1 | 37 | 19 | ≥0.1 | |
| Visceral alone | 70 | 11 | ≥0.1 | 69 | 14 | ≥0.1 | |
| Visceral and cutaneous | 45 | 13 | ≥0.1 | 55 | 11 | ≥0.1 | |
| Visceral and lymph nodes | 47 | 6 | ≥0.1 | 60 | 7 | 0.05 | |
| Viscera and s.c. | 32 | 12.5 | ≥0.1 | 57 | 9 | ≥0.1 | |
| Bone and any other site(s) | 29 | 10 | ≥0.1 | 28 | 21 | ≥0.1 | |
| Brain and any other site(s) | 13 | 0 | ≥0.1 | 20 | 10 | ≥0.1 | |
| Any visceral component | 194 | 21 | ≥0.1 | 241 | 10 | 0.00038‡ | |
| M stage by American Joint Commission on Cancer classification | |||||||
| M1 | 47 | 17 | ≥0.1 | 61 | 33 | 0.00063† | |
| M2 | 22 | 18 | 20 | 15 | |||
| M3 | 236 | 11 | 298 | 12 | |||
| (B) Laboratory values after starting IL-2: association with prognosis (two-tailed P values)
| |||||||
|
IL-2 alone
|
IL-2 + vaccine
|
||||||
| Response | Survival§ | Response | Survival§ | ||||
|
| |||||||
| WBC count | |||||||
| Peak | ≥0.1 | ≥0.1 | 0.06|| | ≥0.1 | |||
| Nadir | ≥0.1 | ≥0.1 | ≥0.1 | ≥0.1 | |||
| Change | ≥0.1 | ≥0.1 | ≥0.1 | ≥0.009 | |||
| Lymphocyte count | |||||||
| Peak | 0.006¶ | ≥0.1 | 0.03 | ≥0.1 | |||
| Nadir | ≥0.1 | ≥0.1 | ≥0.1 | ≥0.1 | |||
| Change | 0.05¶ | 0.09|| | ≥0.1 | ≥0.1 | |||
| Eosinophil count | |||||||
| Peak | ≥0.1 | ≥0.10 | 0.02¶ | 0.03 | |||
| Nadir | 0.02|| | 0.09 | ≥0.1 | ≥0.1 | |||
| Change | ≥0.1 | ≥0.1 | 0.04¶ | 0.02 | |||
| Platelet count | |||||||
| Peak | 0.05¶ | 0.02¶ | ≥0.1 | ≥0.1 | |||
| Nadir | ≥0.1 | ≥0.1 | 0.0008|| | ≥0.10 | |||
| Change | ≥0.1 | ≥0.1 | ≥0.1 | ≥0.1 | |||
| Creatinine | |||||||
| Peak | ≥0.1 | ≥0.1 | ≥0.10 | 0.08|| | |||
| Nadir | ≥0.1 | ≥0.1 | 0.07¶ | ≥0.1 | |||
| Change | ≥0.1 | ≥0.1 | ≥0.1 | 0.06|| | |||
| Bilirubin | |||||||
| Peak | ≥0.1 | ≥0.1 | 0.09¶ | ≥0.1 | |||
| Nadir | ≥0.1 | ≥0.1 | ≥0.1 | ≥0.1 | |||
| Change | ≥0.1 | ≥0.1 | ≥0.1 | ≥0.1 | |||
P values are for comparison between the fraction with the site of disease stated versus all patients who have disease in any other sites. The association between the three M stage classification groups and response was determined by a generalized version of Fisher’s exact test.
Higher response rate.
Lower response rate.
Survival at 4 y.
Lower in responders or survivors.
Higher in responders or survivors.
Table 5.
Association of vaccine type with rate of objective response in patients receiving high-dose IL-2
| Vaccine | Total | Objective response (%) | P | ||
|---|---|---|---|---|---|
| None (IL-2 alone) | 305 | 39 (12.8) |
|
|
|
| gp209-2M peptide | 0.01 | ||||
| Alone | 49 | 9 (25.0) | |||
| Plus other peptides | 65 | 11 (16.9) | |||
| In fowlpox virus | 36 | 9 (25.0) | |||
| Total | 150 | 34 (22.3) | |||
| 0.009 | |||||
| Peptides (non-HLA-A2) | 145 | 14 (10) | |||
| Adenocarcinoma and carcinoma | 57 | 10 (17.5) | |||
| Viruses encoding full-length | |||||
| MART-1 or gpl00 | |||||
| Total | 202 | 24 (13.8) |
There was no statistical association between long-term survival and the M stage classification for patients treated with high-dose IL-2 alone or in combination with vaccines (P > 0.1).
Analysis of factors associated with response
Univariate analyses of pretreatment and treatment factors for effect on response for patients treated with IL-2 and IL-2 plus vaccine are presented separately in Tables 3–5. In IL-2-treated patients, lower baseline eosinophils (P = 0.02), number of sites (P = 0.02), eosinophil nadir (P = 0.02), and peak lymphocytes (P = 0.006) were at least moderately well associated with response. There was no association between the M classification and response in the patients treated with IL-2 alone (P ≥0.1).
For patients treated with IL-2 and vaccine, having a s.c. site (P = 0.00005) and not having any visceral component to the site (P = 0.00038) were significantly associated with response as was fewer total number of sites (P < 0.0001). Also, patients with stage M1 disease treated with IL-2 with vaccine had an increased likelihood of response (P = 0.00064).
Not having received prior radiation (P = 0.013), lower baseline creatinine (P = 0.04), and peak eosinophil count (P = 0.02) were associated to a lesser degree with response in this group as well.
In a logistic regression model constructed to identify if factors may jointly affect response, lower baseline eosinophils (OR, 0.997; 95% CI, 0.89–1.0; P = 0.03) and fewer sites of disease (OR, 0.73; 95% CI, 0.54–0.99; P = 0.04) were jointly associated with response in patients treated with IL-2 alone. In patients treated with IL-2 plus vaccine, fewer sites of disease (OR, 0.72; 95% CI, 0.56–0.94; P = 0.02), lower baseline creatinine (OR, 5.7; 95% CI, 1.27–25.90; P = 0.02), lack of prior radiation (OR, 0.34; 95% CI, 0.13–0.93; P = 0.04), s.c. site of disease (OR, 2.68, 95% CI, 1.01–7.13; P = 0.05), and not having any visceral site of disease (OR, 0.45; 95% CI, 0.23–0.86; P = 0.02) were found to be jointly associated with response. After adjustment for the other factors in the model, the M classification did not have prognostic effect on the likelihood of response in patients treated with Il-2 and vaccine.
Prognostic effect of the number of IL-2 doses administered in the first course
For the IL-2-treated patients, analysis of the number of doses given in the first course showed a trend toward an association between responders (16.7 ± 0.65; median, 16; range, 9–30) and nonresponders (15.06 ± 0.28; median, 15; range, 3–31; P = 0.07).
In patients who received IL-2 plus vaccine, the number of doses administered in the first course showed a somewhat stronger association between responders (16.3 ± 0.33; median, 16; range, 10–22) and nonresponders (14.2 ± 0.23; median, 15; range, 3–24; P = 0.001).
Analysis of response related to the type of vaccine administered
The gp100:209-217(210M) peptide (called gp209-2M) is a synthetic peptide in which methionine replaces threonine at position 2 in the native gp209-2M peptide (12, 13). In previously published studies from the Surgery Branch, it was shown that immunization with the gp209-2M peptide (14–16) and gp209-2M fowlpox vaccines lead to the development of T cells reactive to this specific epitope (5).
Published reports from other trials in the Surgery Branch of the National Cancer Institute showed that other vaccines used in clinical trials within the Branch rarely caused the development of specific reactive T cells or produced objective tumor responses (8).
Analysis of response versus the type of vaccine administered showed that patients treated with IL-2 in addition to the gp209-2M vaccine (Table 5) had a response rate of 22.3% versus 12.8% in those treated with IL-2 alone (P = 0.01) or 13.8% in those patients who received vaccine regimens not containing the gp209-2M construct (P = 0.009).
Discussion
IL-2 was approved by the Food and Drug Administration in 1998 based on its ability to cause durable and complete responses in patients with metastatic melanoma (3, 17). Regressions of large volumes of metastatic disease in a variety of locations have been seen. The current approved regimen gives IL-2 at a dose of 600,000 to 720,000 IU/kg every 8 h for a maximum of 12 to 15 doses or to patient tolerance. Using this regimen, overall response rates of about 15% have been reported. Attempts at using low-dose i.v. regimens of IL-2 or alternate routes of administration of IL-2 (s.c. injection, inhalation, etc.) to abrogate the toxicities (18–22) associated with IL-2 have resulted in lower response rates.
Despite the small complete response rate, the complete responses can be durable. Nearly all the patients who remained disease-free beyond the first 1.5 years in patients treated with IL-2 alone and 3 years in those treated with IL-2 plus vaccine remained complete responders at >10 years.
Comparison of the Kaplan-Meier survival curves in the responding patients in the IL-2 alone and IL-2 with vaccine treated groups showed that complete responses in the IL-2 with vaccine group were not as durable as the complete responses in the IL-2 alone treated group. There were only four complete responses beyond 3 years in the patients treated with IL-2 in combination with vaccines. One factor that could account for this is the difference in dosing schedules between the IL-2 alone and the IL-2 with vaccine protocols. Patients treated with IL-2 and vaccines received the IL-2 cycles every 3 weeks as opposed to days 1 and 14 every 2 months in the IL-2 alone protocols. An alternate explanation for this is possibility that the vaccines could abrogate the effects IL-2.
The number of doses of IL-2 administered in the first course showed a trend toward significance in both IL-2 alone (P = 0.07) and IL-2 with vaccine (P = 0.001) groups. However, this did not have an effect on survival. Some patients were unable to complete the planned course of therapy due to disease progression and toxicity, so the significance of more doses of IL-2 on outcome is unclear.
In this single institution, review of 305 consecutive patients treated with IL-2 alone no single factor showed statistical significance (P < 0.005) in predicting the likelihood of having a response. The baseline eosinophil count, number of sites of disease, peak lymphocytes, and eosinophil nadir showed a trend toward response. Because the baseline eosinophil count and number of sites of disease are factors known before the onset of therapy, they were entered into a logistic regression model. In the final model, both factors remained; a lower baseline eosinophil count and a smaller number of sites of disease were associated with a weak trend toward increased likelihood of responding to IL-2 alone.
A reduction in the lymphocyte count typically occurs in patients during treatment with IL-2 (9). This is typically followed by a rebound lymphocytosis at the conclusion of therapy. A peak in the lymphocyte count typically occurs 2 to 5 days after the cessation of IL-2. The peak lymphocytosis showed a trend toward significance in responders versus nonresponders in patients treated with IL-2 alone (P = 0.0059). Similar results have been shown in previously published papers (23, 24).
In the 379 consecutive patients treated with IL-2 with vaccine, the presence of s.c. or cutaneous disease only were significantly associated with the likelihood of having a response (46% versus 13%; P = 0.00005). Also not having visceral disease increased the likelihood of having a response (24% versus 10%; P = 0.00038). A prior study from this institution also showed statistical significance for patients who had s.c. and/or cutaneous disease only (10). Data from that study included patients treated with both IL-2 alone and IL-2 with vaccines. In this review, the presence of s.c. or cutaneous disease only in patients treated with IL-2 alone did not have an improved response rate.
For the patients treated with IL-2 with vaccines, one of the strongest predictors of the likelihood of having a response was treatment with the gp209-2M peptide vaccine alone or in combination with other vaccines. Patients treated with this vaccine were twice as likely to achieve an overall objective response than other patients in the study. Although these data are highly suggestive, it must be emphasized that this was a subgroup analysis and would require confirmation in a randomized trial.
Two previously reported immune mediated phenomena associated with treatment with IL-2 are the development of vitiligo and hypothyroidism (23, 25, 26). Vitiligo was not included as a variable for review in this study, as the reporting on the data for vitiligo was incomplete in the study population. Baseline thyroid function or the development of hypothyroidism during treatment did not show an association with response in the study population for IL-2 alone or IL-2 with vaccine treated patients (data not shown).
The toxicities associated with IL-2 span a large spectrum of well-characterized symptoms and clinical signs. Chills, rigors, malaise, nausea, vomiting, diarrhea, erythroderma, fluid retention, hypotension, and mental status changes are the most common side effects associated with IL-2 therapy (27). The toxicities are thought to be due to a combination of the capillary leak syndrome and lymphocyte infiltration of tissues and have been widely reported.
The common side effects can be safely treated with appropriate monitoring, prophylactic antibiotic coverage, analgesics, antiemetic drugs, and judicious fluid resuscitation (27). Over time, experience in the administration of IL-2 has resulted in a significant decrease in the number of grade 3 and 4 toxicities associated with treatment with high-dose IL-2. The mean age of the 684 patients in this study was lower than that reported in other trials of stage IV melanoma. This could have some bearing on the results from the trial.
The mortality from high-dose IL-2 therapy in this Surgery Branch experience was 0.73% (5 of the 684 patients).
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 2.Balch CM, Atkins MB, Sober AJ. Cutaneous melanoma. In: Devita VT, Hellman S, Rosenberg SA, editors. Cancer. Principles & practice of oncology. 7. Philadelphia: Lippincott-Raven Publishers; 2005. p. 1754. [Google Scholar]
- 3.Atkins MB, Lotze MT, Dutcher JP, et al. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. J Clin Oncol. 1999;17:2105–16. doi: 10.1200/JCO.1999.17.7.2105. [DOI] [PubMed] [Google Scholar]
- 4.Rosenberg SA, Yang JC, Topalian SL, et al. Treatment of 283 consecutive patients with metastatic melanoma or renal cell cancer using high-dose bolus interleukin 2. JAMA. 1994;271:907–13. [PubMed] [Google Scholar]
- 5.Rosenberg SA, Yang JC, Schwartzentruber DJ, et al. Recombinant fowlpox viruses encoding the anchor-modified gp100 melanoma antigen can generate antitumor immune responses in patients with metastatic melanoma. Clin Cancer Res. 2003;9:2973–80. [PMC free article] [PubMed] [Google Scholar]
- 6.Rosenberg SA, Yang JC, Sherry RM, et al. Inability to immunize patients with metastatic melanoma using plasmid DNA encoding the gp100 melanoma-melanocyte antigen. Hum Gene Ther. 2003;14:709–14. doi: 10.1089/104303403765255110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosenberg SA, Zhai Y, Yang JC, et al. Immunizing patients with metastatic melanoma using recombinant adenoviruses encoding MART-1or gp100 melanoma antigens. J Natl Cancer Inst. 1998;90:1894–900. doi: 10.1093/jnci/90.24.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nat Med. 2004;10:909–15. doi: 10.1038/nm1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Balch CM, Soong SJ, Atkins MB, et al. An evidence-based staging system for cutaneous melanoma. CA Cancer J Clin. 2004;54:131–49. doi: 10.3322/canjclin.54.3.131. quiz 82–4. [DOI] [PubMed] [Google Scholar]
- 10.Miller AB, Hoogstraten B, Staquet M, Winkler A. Reporting results of cancer treatment. Cancer. 1981;47:207–14. doi: 10.1002/1097-0142(19810101)47:1<207::aid-cncr2820470134>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 11.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 12.Parkhurst MR, Salgaller ML, Southwood S, et al. Improved induction of melanoma-reactive CTL with peptides from the melanoma antigen gp100 modified at HLA-A*0201-binding residues. J Immunol. 1996;157:2539–48. [PubMed] [Google Scholar]
- 13.Kawakami Y, Robbins PF, Wang X, et al. Identification of new melanoma epitopes on melanosomal proteins recognized by tumor infiltrating T lymphocytes restricted by HLA-A1, -A2, and -A3 alleles. J Immunol. 1998;161:6985–92. [PubMed] [Google Scholar]
- 14.Rosenberg SA, Yang JC, Schwartzentruber DJ, et al. Immunologic and therapeutic evaluation of a synthetic peptide vaccine for the treatment of patients with metastatic melanoma. Nat Med. 1998;4:321–7. doi: 10.1038/nm0398-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salgaller ML, Marincola FM, Cormier JN, Rosenberg SA. Immunization against epitopes in the human melanoma antigen gp100 following patient immunization with synthetic peptides. Cancer Res. 1996;56:4749–57. [PubMed] [Google Scholar]
- 16.Kammula US, Lee KH, Riker AI, et al. Functional analysis of antigen-specific T lymphocytes by serial measurement of gene expression in peripheral blood mononuclear cells and tumor specimens. J Immunol. 1999;163:6867–75. [PubMed] [Google Scholar]
- 17.Rosenberg SA, Yang JC, White DE, Steinberg SM. Durability of complete responses in patients with metastatic cancer treated with high-dose interleukin-2: identification of the antigens mediating response. Ann Surg. 1998;228:307–19. doi: 10.1097/00000658-199809000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anderson PM, Sorenson MA. Effects of route and formulation on clinical pharmacokinetics of interleukin-2. Clin Pharmacokinet. 1994;27:19–31. doi: 10.2165/00003088-199427010-00003. [DOI] [PubMed] [Google Scholar]
- 19.Atzpodien J, Korfer A, Evers P, et al. Low-dose subcutaneous recombinant interleukin-2 in advanced human malignancy: a phase II outpatient study. Mol Biotherapy. 1990;2:18–26. [PubMed] [Google Scholar]
- 20.Atkins MB. Interleukin-2: clinical applications. Semin Oncol. 2002;29:12–7. doi: 10.1053/sonc.2002.33077. [DOI] [PubMed] [Google Scholar]
- 21.Karp SE. Low-dose intravenous bolus interleukin-2 with interferon-α therapy for metastatic melanoma and renal cell carcinoma. J Immunother. 1998;21:56–61. doi: 10.1097/00002371-199801000-00007. [DOI] [PubMed] [Google Scholar]
- 22.Tagliaferri P, Barile C, Caraglia M, et al. Daily low-dose subcutaneous recombinant interleukin-2 by alternate weekly administration: antitumor activity and immunomodulatory effects. Am J Clin Oncol. 1998;21:48–53. doi: 10.1097/00000421-199802000-00011. [DOI] [PubMed] [Google Scholar]
- 23.Phan GQ, Attia P, Steinberg SM, White DE, Rosenberg SA. Factors associated with response to high-dose interleukin-2 in patients with metastatic melanoma. J Clin Oncol. 2001;19:3477–82. doi: 10.1200/JCO.2001.19.15.3477. [DOI] [PubMed] [Google Scholar]
- 24.Royal RE, Steinberg SM, Krouse RS, et al. Correlates of response to IL-2 therapy in patients treated for metastatic renal cancer and melanoma. Cancer J Sci Am. 1996;2:91–8. [PubMed] [Google Scholar]
- 25.Krouse RS, Royal RE, Heywood G, et al. Thyroid dysfunction in 281patients with metastatic melanoma or renal carcinoma treated with interleukin-2 alone. J Immunother EmphasisTumor Immunol. 1995;18:272–8. doi: 10.1097/00002371-199511000-00008. [DOI] [PubMed] [Google Scholar]
- 26.Kruit WH, Goey SH, Lamers CH, et al. High-dose regimen of interleukin-2 and interferon-α in combination with lymphokine-activated killer cells in patients with metastatic renal cell cancer. J Immunother. 1997;20:312–20. doi: 10.1097/00002371-199707000-00008. [DOI] [PubMed] [Google Scholar]
- 27.Schwartzentruber DJ. Guidelines for the safe administration of high-dose interleukin-2. J Immunother. 2001;24:287–93. doi: 10.1097/00002371-200107000-00004. [DOI] [PubMed] [Google Scholar]