Abstract
We have previously shown in normal subjects that motor adaptation to imposed visual rotation is significantly enhanced when tested few days later. This occurs through a process of sleep-dependent memory consolidation. Here we ascertained whether patients with Parkinson’s disease (PD) learn, improve, and retain new motor skills in the same way as normal subjects. We tested 16 patients in early stages of PD and 21 control subjects over two days. All subjects performed reaching movements on a digitizing tablet. Vision of the limb was precluded with an opaque screen; hand paths were shown on the screen with the targets’ position. Unbeknownst to the subjects, the hand path on the screen was rotated by 30°. In experiment 1, patients taking dopaminergic treatment and controls adapted to rotation with targets appearing in an unpredictable order. In experiment 2, drug-naïve patients and controls adapted to rotation in a less challenging task where target’s appearance was predictable. Patients and controls made similar movements and adapted to rotation in the same way. However, when tested again over the following days, controls’ performance significantly improved compared to training, while patients’ performance did not. This lack of consolidation, which is present in the early stages of the disease and is independent from therapy, may be due to abnormal homeostatic processes that occur during sleep.
Keywords: reaching movements, motor learning, consolidation, sleep, Parkinson’s disease
1. Introduction
Impairment in the execution of automatic routines adds a significant burden to the daily activities and the quality of life of patients with Parkinson’s disease (PD) and their caregivers. The reasons for the inefficiency of automatic routines in PD are not clear. Contributing factors could be deficits in either memory acquisition or memory consolidation. Several studies have now shown that patients with PD are able to acquire new motor tasks: depending upon the type of task and the severity of disease, patients may require more practice than normal age-matched controls [1-3] or the same amount [4] but, in general, by the end of a training session they are able to achieve the same level of learning [5-7]. Recent studies seem to indicate that long-term retention for the learned task might be poorer [8-10] or virtually absent [5]. However, there are no systematic studies ascertaining whether consolidation of a motor skill is preserved in PD patients over the first few days after the original training.
Learning and consolidation of motor skills have been assessed with different paradigms in which subjects learn to adapt their movements either to new inertial configurations, or to viscoelastic forces or to novel visuomotor transformation [11-15]. In particular, to study interference and consolidation, we have used a task in which normal subjects adapted their movements to rotated visual displays [12,13,16]. This type of learning can be rather fast, occurs implicitly, without the subject’s awareness, and is dependent upon the activation of circumscribed areas in the posterior parietal lobe [12,16]. We have also found that consolidation of this learning, like other types of learning [17], is dependent on sleep [12]: when tested one or two days later, after sleep, adaptation is faster.
In the present study, we ascertained whether patients in the early stages of PD with and without dopaminergic therapy could successfully learn to adapt their movements to a 30° rotated visual display, and whether their performance improved when tested a few days later.
2. Subjects & Methods
2.1 Subjects
Sixteen patients with PD and 16 control subjects with normal neurological examination were tested in two separate experiments. All subjects were naïve to the motor adaptation tasks, were right-handed and had Mini-Mental State Examination scores of 27 or higher. All patients and eleven normal controls (experiment 2) underwent magnetic resonance imaging to exclude potential structural brain lesions (e.g. stroke, mass lesion, or hydrocephalus/atrophy). Written informed consent was obtained from all participants under a protocol approved by the institutional review board of the participating institutions.
Experiment 1
We recruited a group of 5 PD patients (4 men and 1 woman, mean age 60 years, SD 7.4, range: 49-68). Hoehn & Yahr stage ranged from 2 to 2.5, average disease duration was 8.4 ± 4.5 years (mean ± SD). All patients were optimally treated at the time of testing. All of them were taking levodopa (range from 200 to 800 mg/day) and pramipexole (range from 1.75 to 2.8 mg/day). One patient was taking biperidin 1 mg/day and another one was taking entacapone 800 mg/day. During the experiment, all patients maintained their regular medication schedule. Control subjects were five age-matched subjects (1 man and 4 women, mean age 61 years, SD 12.0, range 43-73) with normal neurological examination.
Experiment 2
Eleven patients with PD (8 men and 3 women) and 11 normal controls (7 men and 4 women) participated in this experiment. Patients had early idiopathic PD (Hoehn and Yahr Stage 1-2); their mean age was 57.9 years (SD 7.3, range: 45-68); average disease duration was 2.1 ± 3.1 years. At the time of the study, six of the patients were drug naïve, three were treated with deprenyl alone, and the remaining two were treated with both dopamine agonists and levodopa/carbidopa. During the experiment, all patients were drug-free for at least 12 hours. Normal controls had comparable age to the PD group (mean age 54.2 years, SD 8.6; range 43-67).
2.2 Experimental design
Details of the motor tasks have been described elsewhere [14,18]. Briefly, subjects moved a cursor on a digitizing tablet with their right hand. Movements were out and back from a central starting point to one of eight radial targets displayed on a computer screen. Targets appeared at 1-second intervals in synchrony with a tone. Instructions were to make out and back movements without corrections and to reverse sharply inside each target. Greying of the target circle indicated successful hits. A computer sampled hand positions at 200 Hz and controlled the experiments. All subjects learned to perform the basic motor task, i.e., without rotation, during a familiarization session. We define cycle as a group of 8 movements within each block.
Experiment 1
Performance was assessed in blocks of 48 seconds each with two tasks: RAN and RAN.ROT. The basic task was RAN: targets appeared randomly and subjects were instructed to wait for the target to appear and to reach it with a fast movement. In the RAN.ROT task, targets and instructions were as in RAN, but cursor display was rotated by 30° relative to the direction of the hand on the tablet. After a baseline RAN block, subjects performed three consecutive RAN.ROT blocks. These groups of subjects were tested 48 hours later as in the training session.
Experiment 2
Performance was assessed in trial blocks of 90 seconds each with two tasks: CCW and CCW.ROT. The basic task was CCW: targets appeared in a predictable counterclockwise order; subjects were instructed to reverse the movement inside each successive target in synchrony with the tone. Thus, they had to initiate movements before target appearance. In the CCW.ROT task, the direction of the screen cursor was rotated by 30°. Target presentation and instructions were as in CCW. All subjects performed a baseline CCW task and a block of CCW.ROT task. The six drug-naïve patients and six age-matched controls were also tested 24 h after training.
2.3 Data Analysis
As fully described previously [14,18], for each movement, we computed the following measures:
Onset time (CCW task) and Reaction time (RAN task), the time from target and tone presentation to movement onset.
Directional error at the peak velocity, the difference in degrees between the direction of the vector from the start to the target and that of the vector from the start to the movement peak velocity.
Movement time, the time between movement onset and end point.
Timing error (CCW task), the time from target and tone presentation to movement end.
Means were computed for each cycle of 8 movements and each complete block.
The rotation adaptation achieved in a trial block or in a movement cycle (8 movements) was also computed as a percentage with the formula: 100*[1-(Directional Error/30°)].
Mixed model analysis of variance (ANOVA) with post hoc comparisons were performed to assess the effects of group, cycles, and time of testing. All analyses were considered significant for p<0.05 corrected for multiple comparisons.
3. Results
3.1 Experiment 1
In this experiment, patients with stable PD and controls adapted to a rotated display by moving to targets that appeared randomly during a training session and 48 hours later.
During the initial session, they, first, performed a RAN task without rotation. In all subjects, movements were straight and directed to the appropriate target. Mean reaction and movement times were not different in the two groups as reported in Figure 1.
Figure 1.
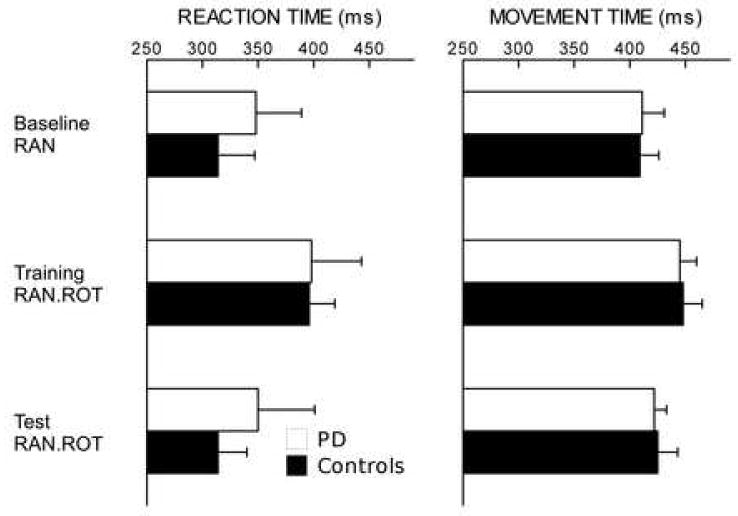
(experiment 1). Reaction and movement times (ms ± SE) during baseline, training RAN.ROT and test RAN.ROT do not differ between PD and controls (p=0.6 and p=0.9 respectively). Movement time increases during training RAN.ROT compared to baseline RAN in both PD and controls (p<0.04 and p<0.002 respectively). Also a slight increase in reaction time can be see, although this is not significant (PD: p=0.2, controls: p<0.08). Reaction and movement times show a decreasing trend toward baseline level during test RAN.ROT in both groups, anyway no significant difference can be found if compared to training RAN.ROT.
Then, all subjects adapted to rotation in the training RAN.ROT task: directional error decreased across movement cycles in the two groups of subjects (F[14,120]=13.38, p<0.0001) reflecting progressive adaptation to the rotated display. Average adaptation was similar in patients and controls (Figure 2A, p>0.7): during the last two cycles, patients reached an average adaptation of 53.7% (mean directional error ± SE: 13.9 ± 1.1°), while controls reached an average adaptation of 58.9% (mean directional error ± SE: 12.3 ± 2.2°). Mean movement time was increased during training RAN.ROT compared to the baseline RAN in both PD and controls (p<0.04 and p<0.002 respectively) (Figure 1).
Figure 2.
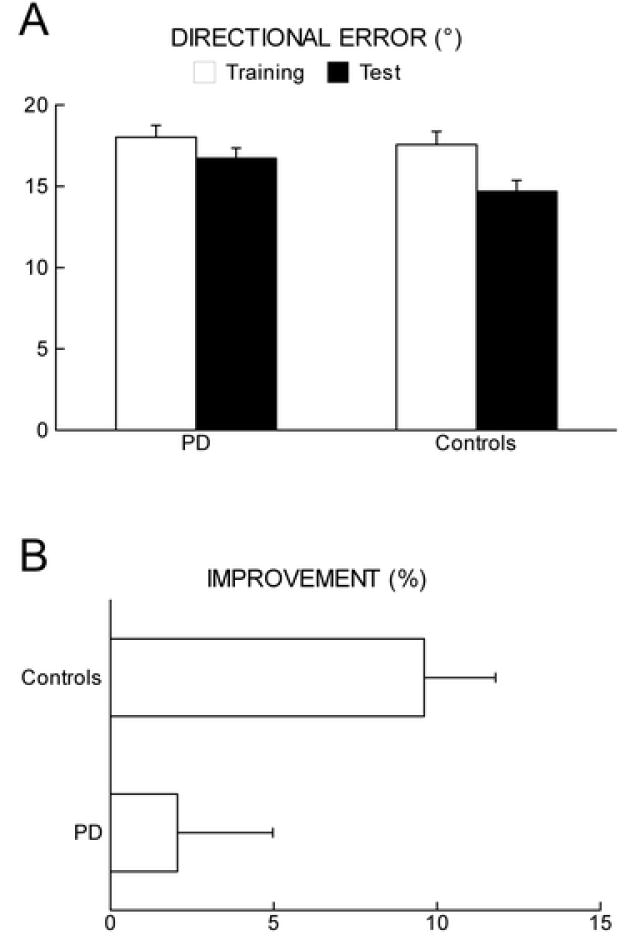
(experiment 1). (A) Mean directional error (degrees ± SE) during motor adaptation to a 30° rotated display in PD patients and age matched controls at training and test. During training, there is no difference between the two groups (p=0.7). At test compared to training, directional error is significantly reduced in normal controls (p<0.0001) but not in PD (p<0.1).
(B) Improvement in directional error reduction expressed in percentage (% ± SE) at test compared to training in the two groups. Only normal controls show a significant improvement (p<0.05).
All subjects were tested 48 hours later in the test session. Adaptation to 30° rotation at test RAN.ROT was faster than during training RAN.ROT (F[1,120]=20.7, p<0.0001). In the first block of testing, compared to the last block of training, adaptation in the control group significantly improved by 9.6 ± 2.2% (mean ± SE, p=0.0001), while PD patients showed only a modest, non significant, improvement of 2.1 ± 2.9% (mean ± SE, p=0.09) (Figure 2B). To be noticed, the adaptation to the rotated display during test was worse in PD patients than in age-matched controls (p=0.02).
At test, compared to training, movement and reaction times did not significantly decrease in the two groups (movement time: PD p=0.3, controls p=0.4; reaction time: PD p=0.3, controls p=0.087, Figure 1).
3.2 Experiment 2
The results of experiment 1 raised the possibility that motor task difficulty and dopaminergic therapy interfered with skill consolidation. Thus, we evaluated a group of patients in the early stages of the disease either drug-naïve or off dopaminergic therapy for twelve hours and a group of normal controls with CCW, a task where the target’s appearance is predictable. There were no differences in the performance of patients with right and left PD and there was no difference between the untreated and treated patients. Thus, the data of all patients were pooled and analyzed as a single group.
In the block of baseline CCW, movements were anticipatory, straight and accurate in both groups. Mean onset and movement times as well as timing errors of patients and controls were not significantly different (Figure 3), confirming prior results [18]. In the successive block where a 30° rotation was imposed (training CCW.ROT), adaptation was evident as a progressive decrease of the directional error (F[9,200]=15.4, p<0.0001) without significant difference between groups (groups: F[1,200]=3.3, p>0.1; cycles × groups: F[9,200]=0.2; p>0.9) (Figure 4A). Average adaptation in the last two cycles was 71.6% (mean directional error ± SE: 7.8 ± 3.6°) in the PD group and 70.0% (mean directional error ± SE: 7.9 ± 2.8°) in the control groups. (Please do not use respectively). The characteristics of the movements in training CCW.ROT were similar in the two groups. However, in both groups, mean onset times and timing errors increased compared to the baseline CCW (p<0.02), while movement time did not significantly change (p>0.1) (Figure 3).
Figure 3.
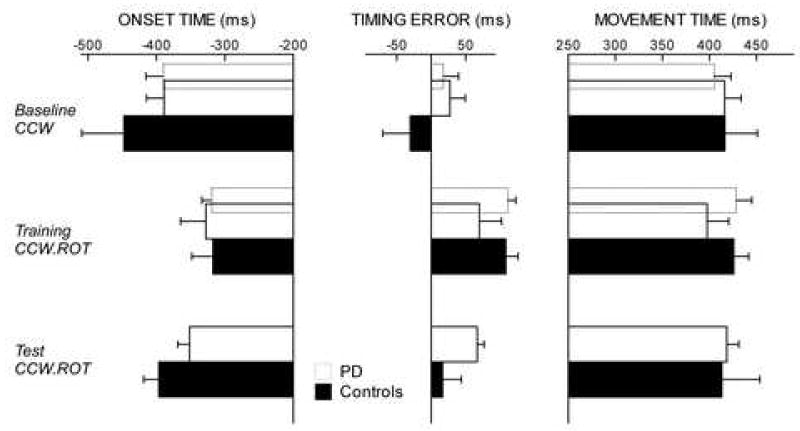
(experiment 2). The solid bars represent the 6 drug-naïve PD patients who were tested during baseline CCW, training CCW.ROT and test CCW.ROT (24h after training). The dotted bars represent all 11 patients (evaluated during baseline CCW and training CCW.ROT only). Onset time, timing error and movement time (ms ± SE) during baseline CCW, training CCW.ROT and test CCW.ROT do not differ between PD and controls. In both groups mean onset time and timing error increase during training CCW.ROT, while movement time do not significantly change (see text). Onset time and timing error return to baseline levels during test CCW.ROT.
Figure 4.
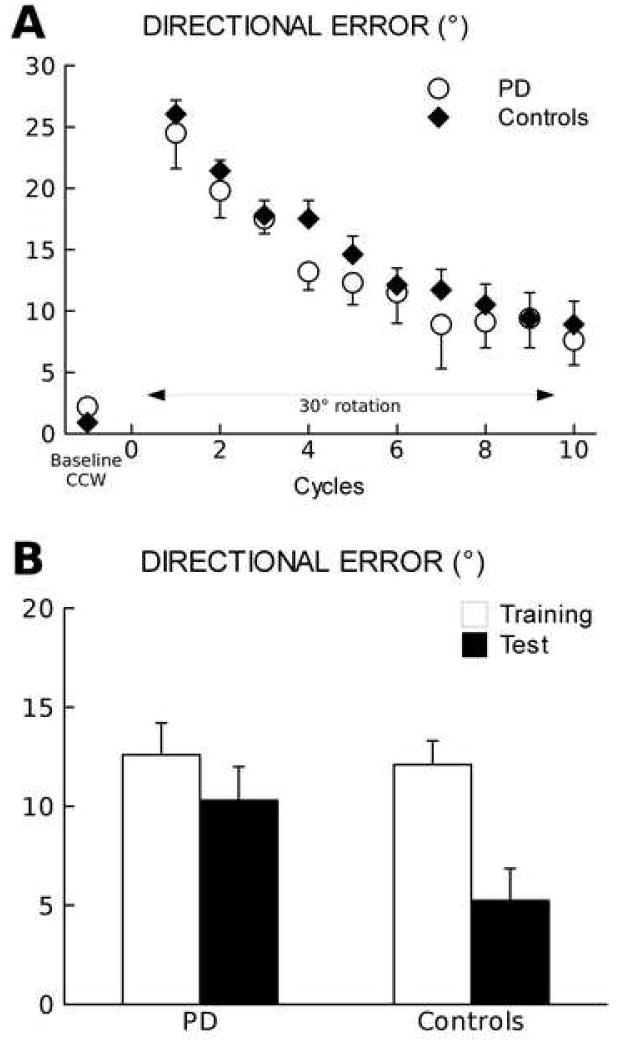
(experiment 2). (A) Directional error (degrees ± SE) significantly decreases across movement cycles in both PD and controls during training with a 30° rotated display (p<0.0001).
(B) Mean directional error (degrees ± SE) improvement during training and test in PD patients and controls. During test day, only controls show a significant improvement in adaptation compared to training (p=0.001).
In the subset of six drug-naïve patients and in six controls, rotation adaptation was tested 24 hours after training. In the testing bock, controls adapted to 30° rotation at a significantly faster rate (p=0.001) than during training (average improvement: 23.3 ± 4.1% corresponding to 6.9 ± 1.2° mean ± SE). In PD patients, instead, adaptation rate at test showed only a minimal, non-significant (p=0.37), improvement compared to training (average improvement: 7.6 ± 3.9%, corresponding to 2.3 ± 1.2° mean ± SE) (Figure 4B). At test, both groups had similar onset and movement times as well as timing errors with non-significant changes from the training session (p>0.2).
These results show that, when target appearance is predictable, patients in the early stages of PD adapt to rotated display in the same way as normal subjects by producing movements with characteristics similar to those of controls. However, while in normal subjects adaptation rate significantly improves at test 24 hour later, such improvement does not occur in patients with PD.
4. Discussion
The results of this paper show that patients in the early stages of PD are able to adapt their movements to a rotated display at the same speed of age-matched controls either when targets appear randomly and unpredictably, as in experiment 1 or when movements can be prepared in advance, as in experiment 2. However, PD patients lack the consolidation that normally occurs when subjects are tested after one or two night’s sleep [12].
4.1 Patients with PD adapt like normal subjects
We have found that adaptation to rotation in PD patients can occur at the same rate as in normal controls either when target location and time are predictable, as in CCW, or when target appearance is random and unpredictable, as in RAN. Although CCW and RAN entail the execution of similar movements, the two motor tasks have different processing requirements. In CCW, a timed-response paradigm [16], together with tone and target appearance subjects must predict movement duration. Parkinsonian patients can easily perform this task in the same way as normal subjects: they initiate and execute movements with normal temporal and spatial accuracy both with and without treatment [18]. This is probably due to the presence of constant external prompts -such as target and tone appearances- that promote temporal accuracy. In RAN, instead, targets are unpredictable, and instructions emphasize speed as well as accuracy: RAN is a choice reaction time task that allows for the exploration of stimulus-response processes. In other words, reaction times in such a context reflect the processes involved in stimulus identification and response preparation, thus providing information about attentional and working memory capacities. When a rotation is imposed, in PD patients, just as in age-matched controls, reaction times increased while directional errors decrease, although not as in CCW. Thus, the additional requirements in the adaptation task do not overload the working memory buffer of patients with PD in a disruptive way and learning occurs as in normal subjects. A possible explanation for the preservation of this type of learning is that subjects adapt to rotation implicitly, without impinging on working memory and attentional resources that can already be affected in the early stages of the disease [19]. Adaptation to rotated displays has been studied in patients with PD with different experimental paradigms and with conflicting results. For instance, studies with tasks in a prism-distorted visual environment [20] or involving visuomotor responses and visuoperceptual ability [21] showed that PD patients take the same time to adapt as normal controls. On the other hand, paradigms with explicit learning components, such as tasks of mental rotation, elicit abnormal results [22-24]. In a reaching task similar to ours, Contreras-Vidal and colleagues [25] studied the adaptation to a 90° rotated display in five patients with PD and found that adaptation was slower and incomplete in patients compared to controls. The learning of 90° rotation usually requires the use explicit mechanisms, as shown by the presence of frequent guessing and trial-and-error strategies as well as by considerable increases of reaction times [26]. In addition, this type of tasks can require the activity of dorsolateral prefrontal cortex, which can be deficient in PD [19], and could account for abnormal adaptation to 90° rotation. Our experimental paradigm, on the other hand, allows for a mostly implicit learning, as 30° rotation is hardly perceived, subjects adapt progressively and are not aware of it.
Finally, as shown in experiment 2, there were no differences in the adaptation between drug-naïve and treated patients. Our patients were in the early stages of PD. Although previous mental rotations studies have shown that PD stage and performance deterioration are not related [22,27], further studies are needed to ascertain whether with disease progression, adaptation rate also degrades.
4.2 Patients with PD lack normal consolidation
An important finding of this study is that, when tested the following day, unlike normal controls, PD patients did not improve their adaptation rate. Previous studies have hinted that PD might have deficient consolidation in tasks of implicit learning, such as the acquisition of a new visuomotor gain [5] or of novel different speed and accuracy requirements [8]. In those studies, small groups of patients were tested months later. During training, those patients failed to reach normal performance levels and, when tested months later, they showed no sign of improvement or consolidation [5,8,9]. However, these studies did not document whether those results stemmed from lack of consolidation or faster motor memory decay, as patients were not tested in the days immediately after initial training. Indeed, we found that patients in the early stages of PD, despite “normal” levels of initial learning, do not show ”normal” improvement when tested one or two days later. It is possible that that such improvement, which occurs in both normal young and age-matched controls [12,13], is achieved during sleep, but not during an equivalent period of wakefulness [12]. We discuss two possible neural mechanisms for the lack of improvement in PD. The first one is that PD affects, at some levels, the mechanisms inducing processes related to long-term potentiation (LTP). Indeed, recent studies have reported that in PD, the induction of LTP-like phenomena in the motor cortex might be impaired [28]: thus, learning might fail to trigger the appropriate mechanisms that are necessary to promote memory consolidation [29]. A second possibility is that, since sleep is normally required for this type of consolidation [12], lack of consolidation in PD could, in principle, be related to abnormal pattern in sleep microstructure. Indeed, sleep disturbances are frequent complaints in patients with PD also in the earliest stages of the disease [30], with prevalence varying from 40 to 98% [31-34]. Interestingly, a recent study examining power spectral changes [35] found a significant and selective decrease in slow wave activity (SWA, i.e., frequencies between 0.5 and 4.5 Hz) in both drug-naïve and treated patients compared to age-matched controls. A third possibility is that the two scenarios might coexist. According to a recent comprehensive hypothesis, the synaptic homeostasis hypothesis [36], during wakefulness, learning and its molecular counterpart, LTP, result in a net increase in synaptic strength in specific cortical circuits. This increase in synaptic strength promotes sleep SWA that, in turn, produces a post-sleep memory improvement. A deficit in LTP processes might fail to trigger the appropriate mechanisms to promote SWA and thus, memory consolidation. Thus, reduction in SWA could explain the present findings of abnormal consolidation in PD and provide a link between the deficit in memory consolidation and abnormal SWA in this disease.
Further studies are indeed warranted to confirm the presence of such consolidation deficits for this and other learning modalities. In addition, direct evidence is needed to ascertain whether deficiency in LTP mechanisms produces SWA abnormality in PD or primary degeneration of several structures and neurotransmitters implicated in the regulation of sleep and SWA [37,38] play a primary role in the motor memory consolidation deficits of PD.
Acknowledgments
We wish to thank Dr. Clara Moisello for help in data analysis and comments on the paper. We also thank Dr. Giulio Tononi for extensive, invaluable discussions on his hypothesis on the role of sleep. This work was supported by a grant from the National Parkinson Foundation (MFG, ADR), a grant from the McDonnell Foundation (MFG) and NIH R01 NS054864 (MFG). Part of the data were collected and analyzed with a custom-designed software by eTT, Genova, Italy.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Agostino R, Sanes JN, Hallett M. Motor skill learning in Parkinson’s disease. J Neurol Sci. 1996;139:218–26. [PubMed] [Google Scholar]
- 2.Krebs HI, Hogan N, Hening W, Adamovich SV, Poizner H. Procedural motor learning in Parkinson’s disease. Exp Brain Res. 2001;141:425–37. doi: 10.1007/s002210100871. [DOI] [PubMed] [Google Scholar]
- 3.Postle BR, Locascio JJ, Corkin S, Growdon JH. The time course of spatial and object learning in Parkinson’s disease. Neuropsychologia. 1997;35:1413–22. doi: 10.1016/s0028-3932(97)00054-7. [DOI] [PubMed] [Google Scholar]
- 4.Hufschmidt A, Lucking CH. Abnormalities of tracking behavior in Parkinson’s disease. Mov Disord. 1995;10:267–76. doi: 10.1002/mds.870100306. [DOI] [PubMed] [Google Scholar]
- 5.Smiley-Oyen AL, Worringham CJ, Cross CL. Motor learning processes in a movement-scaling task in olivopontocerebellar atrophy and Parkinson’s disease. Exp Brain Res. 2003;152:453–65. doi: 10.1007/s00221-003-1570-x. [DOI] [PubMed] [Google Scholar]
- 6.Appollonio I, Grafman J, Clark K, Nichelli P, Zeffiro T, Hallett M. Implicit and explicit memory in patients with Parkinson’s disease with and without dementia. Arch Neurol. 1994;51:359–67. doi: 10.1001/archneur.1994.00540160053008. [DOI] [PubMed] [Google Scholar]
- 7.Smiley-Oyen AL, Lowry KA, Kerr JP. Planning and control of sequential rapid aiming in adults with Parkinson’s disease. J Mot Behav. 2007;39:103–14. doi: 10.3200/JMBR.39.2.103-114. [DOI] [PubMed] [Google Scholar]
- 8.Mochizuki-Kawai H, Kawamura M, Hasegawa Y, Mochizuki S, Oeda R, Yamanaka K, et al. Deficits in long-term retention of learned motor skills in patients with cortical or subcortical degeneration. Neuropsychologia. 2004;42:1858–63. doi: 10.1016/j.neuropsychologia.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 9.Doyon J, Gaudreau D, Laforce R, Jr, Castonguay M, Bedard PJ, Bedard F, et al. Role of the striatum, cerebellum, and frontal lobes in the learning of a visuomotor sequence. Brain Cogn. 1997;34:218–45. doi: 10.1006/brcg.1997.0899. [DOI] [PubMed] [Google Scholar]
- 10.Cohen H, Pourcher E. Intact encoding, impaired consolidation in procedural learning in Parkinson’s disease. Exp Brain Res. 2007;179:703–8. doi: 10.1007/s00221-006-0827-6. [DOI] [PubMed] [Google Scholar]
- 11.Ghilardi MF, Gordon J, Ghez C. Learning a visuomotor transformation in a local area of work space produces directional biases in other areas. J Neurophysiol. 1995;73:2535–9. doi: 10.1152/jn.1995.73.6.2535. [DOI] [PubMed] [Google Scholar]
- 12.Huber R, Ghilardi MF, Massimini M, Tononi G. Local sleep and learning. Nature. 2004;430:78–81. doi: 10.1038/nature02663. [DOI] [PubMed] [Google Scholar]
- 13.Krakauer JW, Ghez C, Ghilardi MF. Adaptation to visuomotor transformations: consolidation, interference, and forgetting. J Neurosci. 2005;25:473–8. doi: 10.1523/JNEUROSCI.4218-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ghilardi MF, Alberoni M, Rossi M, Franceschi M, Mariani C, Fazio F. Visual feedback has differential effects on reaching movements in Parkinson’s and Alzheimer’s disease. Brain Res. 2000;876:112–23. doi: 10.1016/s0006-8993(00)02635-4. [DOI] [PubMed] [Google Scholar]
- 15.Shadmehr R, Brashers-Krug T. Functional stages in the formation of human long-term motor memory. J Neurosci. 1997;17:409–19. doi: 10.1523/JNEUROSCI.17-01-00409.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ghilardi M, Ghez C, Dhawan V, Moeller J, Mentis M, Nakamura T, et al. Patterns of regional brain activation associated with different forms of motor learning. Brain Res. 2000;871:127–45. doi: 10.1016/s0006-8993(00)02365-9. [DOI] [PubMed] [Google Scholar]
- 17.Karni A, Tanne D, Rubenstein BS, Askenasy JJ, Sagi D. Dependence on REM sleep of overnight improvement of a perceptual skill. Science. 1994;265:679–82. doi: 10.1126/science.8036518. [DOI] [PubMed] [Google Scholar]
- 18.Ghilardi MF, Eidelberg D, Silvestri G, Ghez C. The differential effect of PD and normal aging on early explicit sequence learning. Neurology. 2003;60:1313–9. doi: 10.1212/01.wnl.0000059545.69089.ee. [DOI] [PubMed] [Google Scholar]
- 19.Jahanshahi M, Jenkins IH, Brown RG, Marsden CD, Passingham RE, Brooks DJ. Self-initiated versus externally triggered movements. I. An investigation using measurement of regional cerebral blood flow with PET and movement-related potentials in normal and Parkinson’s disease subjects. Brain. 1995;118(Pt 4):913–33. doi: 10.1093/brain/118.4.913. [DOI] [PubMed] [Google Scholar]
- 20.Stern Y, Mayeux R, Hermann A, Rosen J. Prism adaptation in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1988;51:1584–7. doi: 10.1136/jnnp.51.12.1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boller F, Passafiume D, Keefe NC, Rogers K, Morrow L, Kim Y. Visuospatial impairment in Parkinson’s disease. Role of perceptual and motor factors. Arch Neurol. 1984;41:485–90. doi: 10.1001/archneur.1984.04050170031011. [DOI] [PubMed] [Google Scholar]
- 22.Amick MM, Schendan HE, Ganis G, Cronin-Golomb A. Frontostriatal circuits are necessary for visuomotor transformation: mental rotation in Parkinson’s disease. Neuropsychologia. 2006;44:339–49. doi: 10.1016/j.neuropsychologia.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 23.Dominey P, Decety J, Broussolle E, Chazot G, Jeannerod M. Motor imagery of a lateralized sequential task is asymmetrically slowed in hemi-Parkinson’s patients. Neuropsychologia. 1995;33:727–41. doi: 10.1016/0028-3932(95)00008-q. [DOI] [PubMed] [Google Scholar]
- 24.Lee AC, Harris JP, Calvert JE. Impairments of mental rotation in Parkinson’s disease. Neuropsychologia. 1998;36:109–14. doi: 10.1016/s0028-3932(97)00017-1. [DOI] [PubMed] [Google Scholar]
- 25.Contreras-Vidal JL, Buch ER. Effects of Parkinson’s disease on visuomotor adaptation. Exp Brain Res. 2003;150:25–32. doi: 10.1007/s00221-003-1403-y. [DOI] [PubMed] [Google Scholar]
- 26.Elliott T, Ghilardi MF, Mazzoni P, Ghez C. Time course of learning and generalization differ among visuomotor transformations of reaching movements. Soc Neurosci Abstr. 2000;26:179. [Google Scholar]
- 27.Wright WG, Gurfinkel V, King L, Horak F. Parkinson’s disease shows perceptuomotor asymmetry unrelated to motor symptoms. Neurosci Lett. 2007;417:10–5. doi: 10.1016/j.neulet.2007.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morgante F, Espay AJ, Gunraj C, Lang AE, Chen R. Motor cortex plasticity in Parkinson’s disease and levodopa-induced dyskinesias. Brain. 2006;129:1059–69. doi: 10.1093/brain/awl031. [DOI] [PubMed] [Google Scholar]
- 29.Hadipour-Niktarash A, Lee CK, Desmond JE, Shadmehr R. Impairment of retention but not acquisition of a visuomotor skill through time-dependent disruption of primary motor cortex. J Neurosci. 2007;27:13413–9. doi: 10.1523/JNEUROSCI.2570-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tandberg E, Larsen JP, Karlsen K. A community-based study of sleep disorders in patients with Parkinson’s disease. Mov Disord. 1998;13:895–9. doi: 10.1002/mds.870130606. [DOI] [PubMed] [Google Scholar]
- 31.Brotini S, Gigli GL. Epidemiology and clinical features of sleep disorders in extrapyramidal disease. Sleep Med. 2004;5:169–79. doi: 10.1016/j.sleep.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 32.Diederich NJaCCL. Sleep disturbances in Parkinson’s disease. In: C S, H W, Walters A, editors. Sleep and movement disorders. Elsevier Science; 2003. [Google Scholar]
- 33.Kumar S, Bhatia M, Behari M. Sleep disorders in Parkinson’s disease. Mov Disord. 2002;17:775–81. doi: 10.1002/mds.10167. [DOI] [PubMed] [Google Scholar]
- 34.Lees AJ, Blackburn NA, Campbell VL. The nighttime problems of Parkinson’s disease. Clin Neuropharmacol. 1988;11:512–9. doi: 10.1097/00002826-198812000-00004. [DOI] [PubMed] [Google Scholar]
- 35.Brunner H, Wetter TC, Hogl B, Yassouridis A, Trenkwalder C, Friess E. Microstructure of the non-rapid eye movement sleep electroencephalogram in patients with newly diagnosed Parkinson’s disease: effects of dopaminergic treatment. Mov Disord. 2002;17:928–33. doi: 10.1002/mds.10242. [DOI] [PubMed] [Google Scholar]
- 36.Tononi G, Cirelli C. Sleep and synaptic homeostasis: a hypothesis. Brain Res Bull. 2003;62:143–50. doi: 10.1016/j.brainresbull.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 37.Lai YY, Siegel JM. Physiological and anatomical link between Parkinson-like disease and REM sleep behavior disorder. Mol Neurobiol. 2003;27:137–52. doi: 10.1385/MN:27:2:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rye DB. The two faces of Eve: dopamine’s modulation of wakefulness and sleep. Neurology. 2004;63:S2–7. doi: 10.1212/wnl.63.8_suppl_3.s2. [DOI] [PubMed] [Google Scholar]


